Abstract
Malignant airway obstruction and hemoptysis are common in lung cancer patients. Recently, airway stent is commonly used to preserve airway in malignant airway obstruction. Hemoptysis can be managed through various methods including conservative treatment, endobronchial tamponade, bronchoscopic intervention, embolization and surgery. In our case studies, we sought to investigate the effectiveness of airway stents for re-opening the airway as well as tamponade effects in four patients with malignant airway obstruction and bleeding caused by tumors or lymph node invasions.
Keywords: Bronchial stent, Malignant airway obstruction, Hemoptysis, Lung cancer
INTRODUCTION
Large airway invasion is common among patients diagnosed with lung cancer. These patients may develop dyspnea, stridor, intractable cough, hemorrhage, atelectasis, or postobstructive pneumonia, or a combination of the aforementioned manifestations because of tracheobronchial tumor extension (1). These patients are poor surgical candidates judged on the basis of either physiological or oncological criteria. The optimal management of patients with central airway obstruction includes radiotherapy, laser therapy, photodynamic therapy, cryotherapy and airway stenting, which can be done either with or without bronchoscopic guidance. Airway stent can especially achieve immediate and durable airway patency resulting in an improved quality and length of life span in patients with lung cancer (2).
Hemoptysis particularly occurs in about 30% of all the lung cancer patients, and can be lethal to them. The treatment options of hemoptysis are pharmacology, bronchoscopic intervention, vascular embolization and surgical resection (3). In selected cases, endobronchial tamponade was used to block bleeding and protect airway of contralateral lung. Endobronchial tamponade is obtained by double-lumen endobronchial tube or endobronchial balloon catheter. However, these measures only offer transient control of bleeding and need prolonged mechanical ventilation (4).
In the present report, we have described three cases of selective bronchial stent placement in patients with uncontrolled hemoptysis from lung cancer.
CASE REPORTS
Procedure Technique
Between September 2009 and July 2010, 3 men (mean age 65.3 ± 17.0 years) with uncontrolled hemoptysis from lung cancer underwent bronchial stent insertion. Patients who had dyspnea and recurrent or prolonged hemoptysis despite continued conservative management were selected for the airway stenting.
All the procedures were performed by following the same method. All the patients were monitored closely for their vital signs during the procedure. All bronchial stent placements were performed in the angiography room under fluoroscopic and bronchoscopic guidance. A 0.035 hydrophilic guidewire was advanced to the distal portion of stenotic and bleeding segment of bronchus under bronchoscopic guidance. A 5 Fr angiographic catheter was then inserted and consecutively a small amount of non-ionic contrast material was injected into the airway to obtain bronchogram. A stiff guidewire was exchanged. Then, a covered retrievable stent (Hercules airway stent, S&G Biotech, Seongnam, Korea) was deployed in the target area under fluoroscopic guidance. Finally, follow-up bronchoscopy was performed immediately after stenting to confirm the location and patency of the inserted stent.
Case 1
A 78 year-old male was diagnosed with central non-small cell lung cancer 12 months earlier. The primary lung cancer was located in the proximal right upper lobar bronchus associated with postobstuctive atelectasis of the right upper lobe. However, at the time of diagnosis, the patient refused all the curative means. He had exhibited cough and increasing blood tinged sputum in the previous 4 months. We performed CT imaging on outpatient clinics, which showed a completely obstructed right upper lobar bronchus by a tumor with postobstructive pneumonia of the right upper lobe. The wall of the right main bronchus and bronchus intermedius was thickened and the lumen of the right main bronchus was almost narrowed by tumor infiltrations. Metastatic lymphadenopathy was observed in the right lower paratracheal and subcarinal area (Fig. 1A). Nine days after the CT scan, the patient was hospitalized and underwent bronchoscopy. A bronchoscopic examination demonstrated that orifice of the right main bronchus was nearly completely obstructed by the endoluminal mass and there was diffuse bleeding from the right main bronchus. The patient received urgent external beam radiotherapy to control bleeding from the friable endobronchial tumor and bleeding gradually decreased for several days. However, eleven days after the radiation therapy, a sudden onset of dyspnea developed. Chest radiography showed that the right lung had totally collapsed and bronchoscopic examination revealed complete obstruction of the right main bronchus with diffuse bleeding.
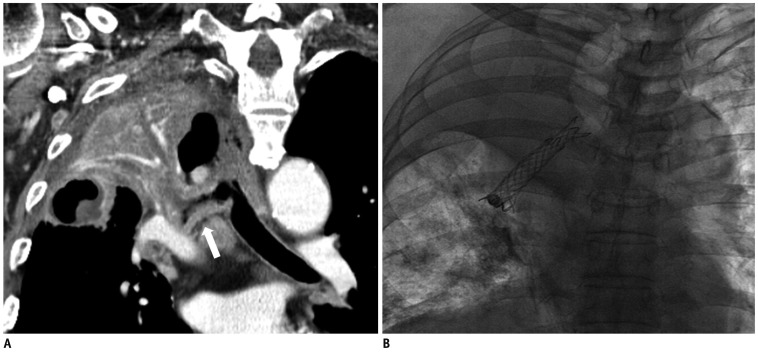
Fig. 1.
77-year-old male (patient 1) with non-small cell lung cancer.
A. Multi-planar reformatted oblique coronal CT image shows bronchial wall thickening with narrowing in right main bronchus and bronchus intermedius (not shown) and total atelectasis of right upper lobe due to obstruction of right upper lobar bronchus. B. Fluoroscopic image shows bronchial stent and aerated right basal lung.
Bronchial artery embolization could not be considered as a treatment option for hemoptysis because bleeding conditions were not because of active arterial bleeding and there was no prominent contrast enhancement of the mass as seen on the CT scan. We decided that bronchial stent insertion in the right main bronchus was necessary to both, re-open the airway and to control the bleeding of tumor through the tamponade effect. After the bronchogram, which was performed to identify the airway on a fluoroscopic image, we decided to sacrifice superior segment of the right lower bronchus to preserve basal segments of the right lower lobe. The 12 mm - 4.3 cm self-expanding silicone-covered nitinol stent (Hercules airway stent, S & G Biotech, Seongnam, Korea) was deployed in the right lower lobar bronchus to the right main bronchus. However, proximal portion of the right main bronchus was not completely covered due to its insufficient length. For this reason, another 12 mm - 5.3 cm self-expanding silicone-covered nitinol stent was reinserted, overlapping the first stent.
At the end of the procedure, an immediate improvement in the aeration of the right basal lung was observed on fluoroscopy and hemoptysis was subsided (Fig. 1B). Nevertheless, the patient died two days later due to lack of expectoration that resulted in whole lung aspiration pneumonitis or pneumonia.
Case 2
A 72 year-old man, with a history of tuberculosis ten years ago and an exposure to asbestosis, was diagnosed with squamous cell carcinoma on the left lower lobe about 20 months earlier. The patient underwent left lower lobectomy after a month of the diagnosis and had been treated with postoperative chemotherapy, but it failed to prevent recurrence and progression of the disease. The CT scan, which was performed on his visit, demonstrated an extremely stenotic left main bronchus by metastatic lymphadenopathy in mediastinum and both the hila (Fig. 2A). Fresh bloody sputum developed on the fourth day of admission and frequency of hemoptysis gradually increased up to three times per hour. Shortly afterwards, shortness of breath developed. Respiration rates were raised up to a 40/min and the hemoglobin level dropped from 10.1 g/dL to 7.1 g/dL. He was immediately intubated for mechanical ventilation. Chest radiography at the time showed total atelectasis of the left lung.
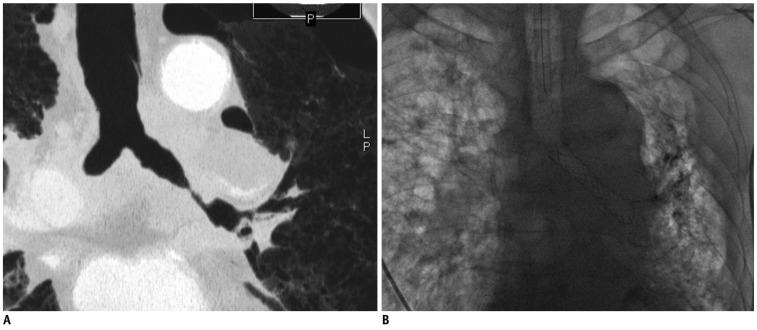
Fig. 2.
72-year-old male (patient 2) with left main bronchial stricture non-small cell lung cancer.
A. Multi-planar reformatted oblique coronal CT image shows extremely stenotic left main bronchus. B. Fluoroscopic image shows bronchial stent and immediately improved aeration in left lung.
We decided to insert the covered bronchial stent into the left main bronchus. On the fifth day after admission, 12 mm - 5.3 cm self-expanding silicone-covered nitinol stent (Hercules airway stent: S & G Biotech, Seongnam, Korea) was deployed in the left main bronchus under bronchoscopic and fluoroscopic guidance. Aeration of the left lung improved soon after bronchial stent placement (Fig. 2B). Bleeding through the endotracheal tube gradually decreased and stopped within two days after the procedure.
However, his blood pressure and urination decreased. He required continuous renal replacement therapy (CRRT) due to acute renal failure by hypotension. Sixteen days after the bronchial stent placement, he expired because of multi-organ failure.
Case 3
A 46 year-old male was diagnosed with non-small cell lung cancer 30 months earlier. The primary cancer was located in the orifice of the left upper lobe bronchus without metastasis. He was treated with left pneumonectomy after neoadjuvant chemotherapy along with postoperative radiation therapy and systemic chemotherapy. Eleven months after the surgery, chest CT scans showed recurring mass at the stump of the left main bronchus, which was also confirmed by bronchoscopic biopsy. The patient underwent second and third line systemic chemotherapy, but it was unable to stop the disease from progressing. Approximately four months prior to this visit, he was admitted due to presence of blood-tinged sputum. At that time, a bronchoscopic examination showed that the bleeding was from recurred mass in the left main bronchus.
He visited again within several weeks due to dysphagia and recurrent blood-tinged sputum. We performed CT imaging, which depicted progression of a recurred mass at the stump of the left main bronchus. It also conglomerated metastatic lymph nodes in the subcarinal and both the paratracheal areas. CT scan also showed narrowing of distal trachea and right proximal main bronchus (Fig. 3A). In addition, it showed invasion of adjacent esophagus by the recurred mass with conglomerated lymphadenopathy.
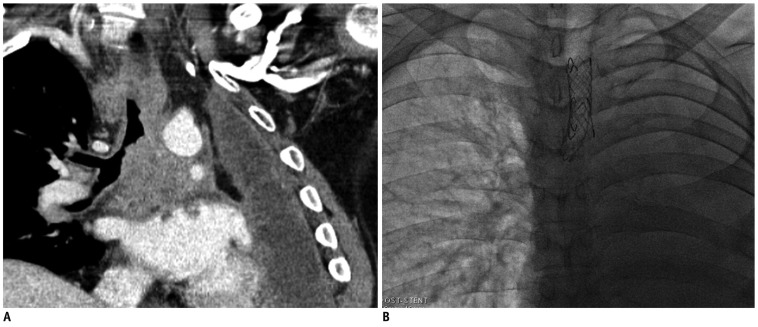
Fig. 3.
46-year-old male (patient 3) with non-small cell lung cancer. Patient has had history of left pneumonectomy 30 months earlier.
A. Multi-planar reformatted oblique coronal CT image shows narrowing of distal trachea and right main bronchus by recurred mass and conglomerated metastatic lymph nodes in subcarinal and paratracheal areas. B. Fluoroscopic image shows two overlapped bronchial stents.
On the eighth day of admission, he coughed up approximately 500 mL of fresh blood and the patient was transferred to the intensive care unit. Moreover, we decided to perform airway stenting. Two covered airway stents were inserted under fluoroscopic guidance. First, a 18 mm - 4 cm self-expanding silicone-covered nitinol stent (Hercules airway stent: S & G Biotech, Seongnam, Korea) was deployed in the distal trachea and another 12 mm - 3 cm self-expanding silicone-covered nitinol stent was deployed in the distal trachea to the right main bronchus, overlapping the former one (Fig. 3B).
After the procedure, there was a gradual decrease in the hemoptysis except for an episode of scanty bleeding that halted after conservative treatment. However, the patient needed daily bronchoscopic toileting because of lack of self-expectoration and retained mucus secretion. The patient expired 3 months after the procedure due to disease progression.
DISCUSSION
Hemoptysis is a potentially life-threatening manifestation of underlying lung disease that requires prompt diagnosis and management. The etiology of hemoptysis varies; bronghogenic carcinoma is one of the most common causes of hemoptysis with 19-32% of occurrence rate (5). The bleeding results from necrosis of a tumor, the rupture of small blood vessels in the area, or a tumor invading one of the pulmonary blood vessels. It is normally associated with mild to moderate amounts. However, massive bleeding can occur if the tumor erodes into one of the large pulmonary vessels and can signify a serious situation, where rapid treatment is crucial.
Management of hemoptysis aims to stop hemorrhage, replace blood loss, and treat underlying etiologies. Many therapeutic options are available to isolate and treat the site of bleeding. Maintenance of airway patency is of the utmost importance. The patients should be placed on lateral decubitus on the involved side. In addition, they should be selectively intubated with a double-lumen endotracheal tube to prevent aspiration to the unaffected contralateral lung and preserve gas exchange. Endobronchial tamponade using a balloon tamponade catheter is an alternate site-specific therapy through the bronchoscopy (6).
However, endobronchial tamponade only offers temporary relief and prolonged mechanical ventilation is necessary (4). Surgery removes the source of bleeding and is the most definitive form of therapy for patients with hemoptysis. However, a large proportion of patients are not the suitable candidates for surgery due to their advanced condition of pulmonary diseases with poor pulmonary reserves and preexisting co-morbidities. In addition, mortality rates of up to 40% have been reported following emergency surgical interventions (7).
Bronchial artery embolization is considered a successful procedure to control hemoptysis in various pulmonary diseases and is the most effective non-surgical treatment available for massive hemoptysis (7). However, prognosis remains poor in the patients with tumor-related hemoptysis (8).
Airway stents are traditionally used in the palliation of lung cancer to maintain patency of reopened airways, to secure patency of a collapsed or extrinsically compressed central airway, and to seal fistulas (1, 9). There have been two case reports that described airway stent placement to treat pulmonary hemorrhage from lung cancer (3). Airway stent placement has been regarded as a successful treatment option with not only tamponade and isolation of bleeding source but also for the maintenance of airway.
In the three patients mentioned in our series, the main indication for bronchial stent placement was airway obstruction with hemoptysis in advanced stages of lung cancer. Hemoptysis gradually decreased or ceased in all the patients. Immediate re-opening of airway with improvement of aeration on fluoroscopic images was also observed in all the cases. One patient died due to disease progression while surviving for three months after the procedure. The remaining two patients died within 16 days after the procedure due to non-procedural related complications like, multi-organ failure or aspiration pneumonitis. The retention of mucous secretion occurred in one stent placement and required daily bronchoscopic toileting. So, patient's life expectancy and general condition should be considered before selecting the patients for treatment.
The retention of mucous secretions is a common complication resulting from airway stent placement. Covered airway stents or plastic stents prevent normal clearance of secretions and may lead to obstruction and infection. Stent migration is another common complication. The risk with a covered stent is much higher than with a bare one (10). Covered airway stents have decreased risks of tumor in-growth and granulation tissue formation. However, granulation tissue developed at the end because of irritation by the stent or the migration of a stent may block the bronchus (1, 10). Other complications such as, breakage of the stent and fistula between bronchus and major vessels can also occur.
In conclusion, using airway stenting for endobronchial tamponade may be considered as a treatment option for hemoptysis arising from lung cancer that cannot be successfully dealt with other treatments. However, identifying the patient's underlying condition and morbidity is essential for patient's selection. Airway stent placement also resulted in preservation of airway patency in patients with obstructive airway lesions.
References
- 1.Chin CS, Litle V, Yun J, Weiser T, Swanson SJ. Airway stents. Ann Thorac Surg. 2008;85:S792–S796. doi: 10.1016/j.athoracsur.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 2.Saji H, Furukawa K, Tsutsui H, Tsuboi M, Ichinose S, Usuda J, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11:425–428. doi: 10.1510/icvts.2010.238196. [DOI] [PubMed] [Google Scholar]
- 3.Chung IH, Park MH, Kim DH, Jeon GS. Endobronchial stent insertion to manage hemoptysis caused by lung cancer. J Korean Med Sci. 2010;25:1253–1255. doi: 10.3346/jkms.2010.25.8.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med. 1999;20:89–105. doi: 10.1016/s0272-5231(05)70129-5. [DOI] [PubMed] [Google Scholar]
- 5.Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112:440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- 6.Lee SM, Kim HY, Ahn Y. Parallel technique of endobronchial balloon catheter tamponade for transient alleviation of massive hemoptysis. J Korean Med Sci. 2002;17:823–825. doi: 10.3346/jkms.2002.17.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando HC, Stein M, Benfield JR, Link DP. Role of bronchial artery embolization in the management of hemoptysis. Arch Surg. 1998;133:862–866. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- 8.Wang GR, Ensor JE, Gupta S, Hicks ME, Tam AL. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol. 2009;20:722–729. doi: 10.1016/j.jvir.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Lund ME, Garland R, Ernst A. Airway stenting: Applications and practice management considerations. Chest. 2007;131:579–587. doi: 10.1378/chest.06-0766. [DOI] [PubMed] [Google Scholar]
- 10.Zakaluzny SA, Lane JD, Mair EA. Complications of tracheobronchial airway stents. Otolaryngol Head Neck Surg. 2003;128:478–488. doi: 10.1016/S0194-59980300002-0. [DOI] [PubMed] [Google Scholar]