Abstract
Introduction
A pancreaticoduodenectomy is the reference treatment for a resectable pancreatic head ductal adenocarcinoma. The probability of 5-year survival in patients undergoing such treatment is 5–25% and is associated with relatively high peri-operative morbidity and mortality. The objective of the present study was to evaluate risk factors predictive of outcome for patients undergoing a pancreaticoduodenectomy for a pancreatic adenocarcinoma.
Methods
This retrospective analysis incorporated data from the Vancouver General Hospital and the British Columbia Cancer Agency (BCCA) from 1999–2007.
Results
The 5-year survival of 100 patients was 12% with a median survival of 16.5 months. Ninety-day mortality was 7%. Predictors of 90-day mortality included age ≥ 80 years (P < 0.001) and an American Society of Anesthesiologists (ASA) score = 3 (P = 0.012) by univariate analysis and age ≥80 years (P < 0.001) by multivariate analysis. The identifiable predictive factor for poor 5-year survival was an ASA score = 3 (P = 0.043) whereas a Dindo–Clavien surgical complication grade ≥ 3 was associated with a worse outcome (P = 0.013). Referral to the BCCA was associated with a favourable 5-year survival (P = 0.001).
Conclusions
The present study identifies risk factors for patient selection to enhance survival benefit in this patient population.
Keywords: pancreatic neoplasia, resection < pancreatic neoplasia, pancreaticoduodenectomy, Whipple resection
Introduction
Adenocarcinoma of the pancreas is a formidable therapeutic challenge. The incidence of pancreatic cancer is essentially equivalent to the death rate.1 The majority of the patients present with systemic disease and have a survival rate of less than 4% at 5 years.1,2 Approximately 15% of patients presenting with a pancreatic adenocarcinoma have apparently localized disease at the time of diagnosis and are candidates for surgical resection such as a pancreaticoduodenectomy (Whipple resection).3 The 5-year survival for patients undergoing surgery for apparently curable disease is low, ranging from 5–25%.4,5 In spite of these results, in the absence of effective stand-alone systemic therapy, surgical resection remains the only option for long-term survival.6,7
In most high-volume institutions, a pancreaticoduodenectomy is associated with mortality rates of less than 6%, but morbidity can range from 30–60%.5,8 Further, the resource utilization for pancreatic resections, especially a pancreaticoduodenectomy, is demanding especially as it relates to pre-operative work-up, operating room requirements and length of hospital stay.9 Given the suboptimal survival data in conjunction with peri-operative mortality and morbidity, and resource requirements associated with a pancreaticoduodenectomy for a pancreatic adenocarcinoma, it is essential to evaluate patient selection to optimize overall outcomes.
The objective of the present study was to identify variables that may help predict the outcome for patients undergoing a pancreaticoduodenectomy for a pancreatic ductal adenocarcinoma at the Vancouver General Hospital (VGH), an academic teaching hospital of the University of British Columbia.
Methods
A retrospective chart review at VGH was undertaken to identify patients who underwent a pancreaticoduodenectomy with curative intent for a ductal adenocarcinoma of the pancreas from 1 December 1999 to 31 December 2007. The date of final follow-up was 25 April 2010. Data were extracted and cross-referenced from the VGH Decision Support patient database, surgeon patient files and the British Columbia Cancer Agency (BCCA) Information Systems and Cancer Registry. Only patients with a pathologically confirmed diagnosis of a pancreatic head ductal adenocarcinoma undergoing a pancreaticoduodenectomy were included. Patients were excluded if the pathological diagnosis could not be confirmed or if patients underwent a pancreaticoduodenectomy for other disease processes including but not limited to an acinar cell carcinoma, a cholangiocarcinoma, a duodenal carcinoma, carcinoma of the Ampulla of Vater or neuroendocrine tumours.
Demographic, clinical, radiological and pathological data were assessed. This included patient demographics: age, gender, surgical wait-times, co-morbidities; presenting symptoms: abdominal pain, back pain, cholangitis, jaundice; radiological findings: tumour size, a suspected lymphadenopathy, tumour relation to vascular structures, metastases; surgical management: primary surgeon, classic or pylorus-preserving resection; R status of resection (R0–R2 as documented by the pathology report indicating status of tumour margins relative to resection margin), vascular resection, peri-operative complications as defined by the Dindo–Clavien classification,10 transfusion requirements; pathology: intra-operative frozen sections, lymphovascular invasion, perineural spread and TNM stage.11
Vital status for all patients was obtained from VGH Decision Support Information Systems, BCCA Information Systems, surgeons' files, referring physicians and/or family physicians. Some data from patient charts older than 7 years were unavailable because of their removal from the database. Cause and date of death were verified from the BC Cancer Registry.
Ninety-day mortality was defined as death occurring less than 90 days from the date of surgery. Fisher's exact test was used to test for a relationship between each of the variables and 90-day mortality. As a result of the small number of events, a logistic regression with Firth's Penalized Likelihood was used for the 90-day mortality multivariate analysis. Survival curves were calculated using the Kaplan–Meier method and the log-rank test used as the test of association for 5-year survival. Cox regression analysis was used for the 5-year survival multivariate analysis. Variables were considered for inclusion in the multivariate models if the P-value in the univariate analysis was <0.1. A two-tailed 5% significance level was used for the multivariate analysis. All analyses were performed using SAS 9.2 (Vancouver, BC, Canada).
Results
One hundred and two patients from four surgical practices were identified who met the inclusion criteria. Two patients were excluded owing to a lack of sufficient follow-up data. One hundred patients were available for analysis, with 16 (16%) patients surviving beyond the last follow up date of 25 April 2010. Of the 84 patients who died, 75 (89%) died as a consequence of recurrent pancreatic cancer. The causes of death for the remaining nine (11%) are unknown but were not cancer related. Overall, the median survival was 16.5 months [95% confidence interval (CI): 13, 19]; 5-year survival was 12.1%, whereas the 90-day mortality was 7%. Overall survival is shown in Fig. 1.
Figure 1.
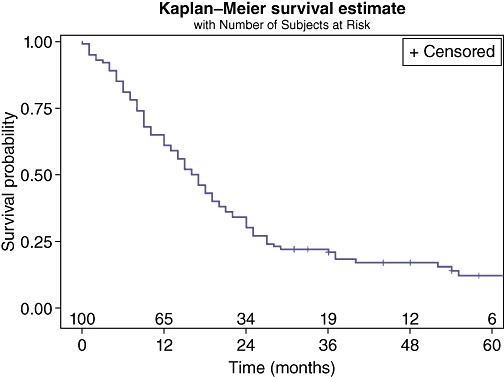
Survival of all patients undergoing a Whipple resection for pancreatic head ductal adenocarcinomas
Patient characteristics evaluated for 90-day mortality are presented in Table 1. Factors predictive of 90-day mortality included age ≥ 80 years (P < 0.001) or ASA score = 3 (P = 0.012). With specific reference to age ≥ 80 years, three deaths occurring within 90 days were in this patient population (n = 7) compared with four deaths in patients < 80 years (n = 93) (P < 0.001). From a logistic regression, a patient who was <80 years was 34 times more likely to survive 90 days past surgery compared with a patient who was 80 years or over (95% CI: 4.4, 250.0). In the multivariate analysis, age ≥ 80 years remained a statistically significant factor (P < 0.001) whereas ASA score = 3 after adjusting for the effect of age was no longer statistically significant (P = 0.068).
Table 1.
Patient characteristics and 90-day mortality factors
| Variable | Level | Survival <90 days | Survival ≥90 days | Fisher's P-value |
|---|---|---|---|---|
| Overall | 7 | 93 | ||
| Age Group | <80 | 3 | 90 | <0.001 |
| 80+ | 4 | 3 | ||
| Gender | Female | 2 | 41 | 0.696 |
| Male | 5 | 52 | ||
| Wait time to surgery | Missing | 5 | 20 | 1.000 |
| Less than 1 month | 1 | 27 | ||
| More than 1 month | 1 | 46 | ||
| Jaundice | No | 1 | 12 | 1.000 |
| Yes | 6 | 81 | ||
| Surgeon | 1 | 3 | 57 | 0.112 |
| 2 | 4 | 16 | ||
| 3 | 14 | |||
| 4 | 6 | |||
| Weight loss | No | 7 | 67 | 0.185 |
| Yes | 26 | |||
| Back pain | No | 6 | 80 | 1.000 |
| Yes | 1 | 13 | ||
| Anorexia | No | 7 | 65 | 0.186 |
| Yes | 28 | |||
| Symptom duration | Missing | 3 | 14 | 1.000 |
| Less than 2 months | 1 | 32 | ||
| More than 2 months | 3 | 47 | ||
| Number of symptoms | 0, 1 | 3 | 26 | 0.703 |
| 2 | 3 | 41 | ||
| 3, 4, 5 | 1 | 26 | ||
| Tumour size | Missing | 1 | 6 | 0.677 |
| ≤2 | 4 | 43 | ||
| >2 | 2 | 44 | ||
| Positive nodal status | Missing | 1 | 6 | 0.194 |
| No | 2 | 56 | ||
| Yes | 4 | 31 | ||
| ASA score | Missing | 4 | 0.012 | |
| 1, 2 | 48 | |||
| 3 | 7 | 41 | ||
| Complications | Missing | 13 | 0.131 | |
| 0, 1 | 1 | 37 | ||
| 2, 3, 4 | 6 | 43 | ||
| Arterial involvement | Missing | 1 | 9 | 1.000 |
| No | 6 | 76 | ||
| Yes | 8 | |||
| Venous involvement | Missing | 1 | 11 | 1.000 |
| No | 6 | 73 | ||
| Yes | 9 | |||
| Metastases | Missing | 2 | 13 | 1.000 |
| No | 5 | 78 | ||
| Yes | 2 | |||
| Pre-operative biopsy | Missing | 1 | 0.674 | |
| No | 4 | 64 | ||
| Yes | 3 | 28 |
All missing values are excluded from P-values.
Patient characteristics evaluated for 5-year survival are presented in Table 2. Factors associated with poor survival at 5 years included ASA score = 3 (P = 0.043) (Fig. 2), or development of complication grade 3/4 (P = 0.013) (Fig. 3). Patients with an ASA score of 1 or 2 had a median survival of 19.5 months (95% CI: 15.0, 24.0) compared with patients with an ASA score of III who had a median survival of 12.0 months (95% CI: 7.0, 16.0). This was the only identifiable pre-operative predictive factor for a worse outcome. Post-operatively, patients with no or minimal post-operative complications had a median survival of 19.5 months (95% CI: 15.0, 25.0) whereas patients with more significant complications requiring invasive interventions has a median survival of 12 months (95% CI: 9.0, 17.0). Referral to BCCA was also associated with an improved 5-year survival rate (P = 0.001) (Fig. 4) with a median survival of 18.5 months (95% CI: 15.0, 24.0) compared with a median survival of 8.0 months (95% CI: 2.0, 12.0) for non-referred patients. Neither chemotherapy nor radiation therapy had any significant impact on 5-year survival. Apart from the ASA score, there were no other pre-operative predictive factors available for multivariate analysis as surgical complications and BCCA referral and intervention were considered post-operative events.
Table 2.
Patient characteristics and 5-year mortality factors
| Variable | KM Log-rank P-value 5 years |
|---|---|
| Age group (80+) | 0.157 |
| Anorexia | 0.995 |
| Arterial involvement | 0.241 |
| ASA | 0.044a |
| Back pain | 0.524 |
| Biopsy | 0.267 |
| Referral to BCCA | 0.002a |
| Gender | 0.976 |
| Hepatic resection | 0.109 |
| Jaundice | 0.364 |
| Metastases | 0.268 |
| Nodal Status | 0.280 |
| Number of symptoms | 0.978 |
| Positive node group | 0.087 |
| Complications | 0.009a |
| Tumour size | 0.347 |
| Surgeon | 0.531 |
| Surgical wait time | 0.441 |
| Symptom duration | 0.111 |
| Venous involvement | 0.773 |
| Weight loss | 0.330 |
Denotes significance at 0.05.
Figure 2.
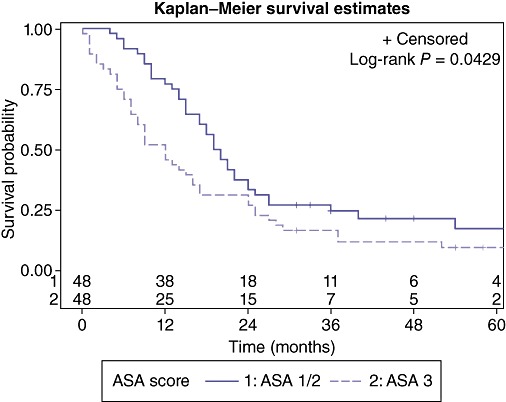
Effect of the American Society of Anesthesiologists (ASA) score on overall survival
Figure 3.
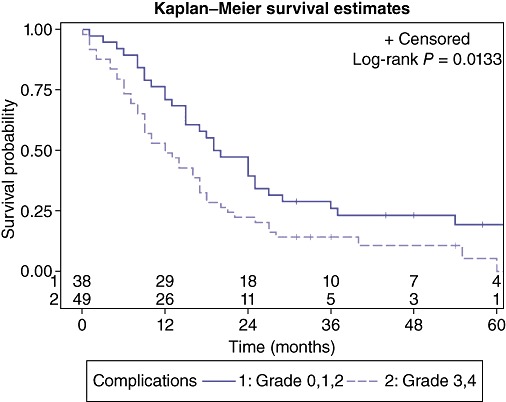
Effect of post-operative complications on overall survival
Figure 4.
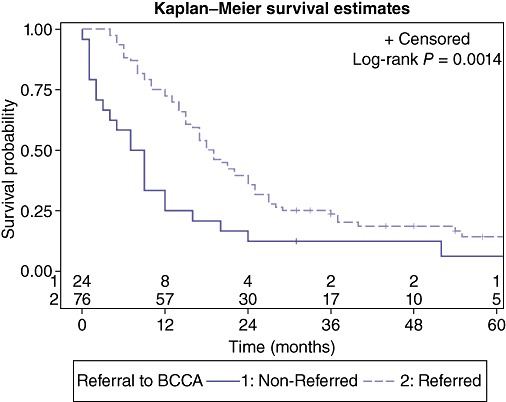
Effect of referral to the British Columbia Cancer Agency (BCCA) on survival
Biological factors (TNM stage, tumour differentiation, lymphovascular or perineural invasion) did not result in significant differences in 5-year survival although the presence of metastatic lymphadenopathy approached significance (P = 0.07) (Fig. 5) with a median survival for node negative patients: 22.0 months (95% CI: 12.0, 29.0); and node positive patients: 15.0 months (95% CI: 9.0, 18.0).
Figure 5.
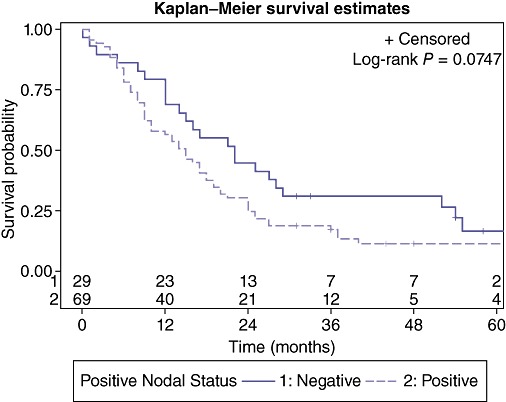
Effect of lymph node metastases on survival
Resection for curative intent (R0, R1) had a median survival of 17.0 months (95% CI: 14.0, 22.0). Median survival for patients undergoing R2 resection was 13.5 months (95% CI: 1.0, 19.0) and trended lower than those undergoing a resection with curative intent, with two patients surviving to 1 year and no patients surviving beyond 36 months. Resection of liver metastases had a median survival of 9.0 months with one patient surviving 30 months (Fig. 6).
Figure 6.
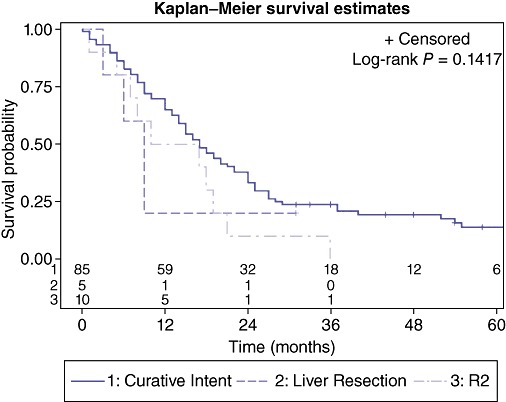
Effect of R2 resection or resection of liver metastases on survival
Discussion
Considering the mortality of an unresected pancreatic head ductal adenocarcinoma and a lack of an effective alternative therapy as stand-alone treatment, a pancreaticoduodenectomy remains the gold standard baseline treatment for this disease. However, careful patient selection that minimizes short- and intermediate-term mortality and morbidity is required to ensure appropriate survival benefit. The present study identified risk factors in 100 patients undergoing a pancreaticoduodenectomy that are associated with increased short-term (90 days) and long-term (5 years) mortality rates. By identifying and modifying these risk factors, the utility of a pancreaticoduodenectomy in this patient population may be increased.
Patient physiology is an important factor to consider before recommending surgery, even in the presence of technically resectable disease. In the present study, an ASA score of 3 is associated with a higher 90-day and 5-year mortality rate. While age itself should not be considered a contraindication for a pancreaticoduodenectomy,12,13 octogenarians should be approached cautiously, especially if they have any associated comorbidities resulting in an ASA score of 3.
Careful patient selection and consideration of their ability to sustain the physiological stress of a pancreaticoduodenectomy is in part related to the likelihood of development of post-operative complications. Intuitively, post-operative complications should affect peri-operative mortality, but these complications also impact 5-year survival rates.14,15 This may in part be secondary to an associated immunologically compromised state associated with development of more severe complications which increase the risk of cancer recurrence.16 Minimization of post-operative complications also emphasizes the necessity of meticulous and precise intra-operative judgment and technical practices to reduce the incidence and severity of complications. These operator-dependent factors appear to influence both the peri-operative outcomes as well as 5-year survival rates.17–19 While every effort should be made to attempt resection of the tumour, these efforts should be tempered by risk vs. benefit assessment. Individual surgeons can only make an informed decision by knowing their own data.
The concept of the borderline resectable pancreatic cancer patient is an emerging state of disease primarily defined by CT imaging criteria.20,21 In this series, the patients could not be specifically classified by these criteria. There was insufficient data available to determine if involvement of major vascular structures by imaging reports affected the 5-year survival rates. Similarly, resection of vascular structures or R resection status could not be shown to influence intermediate- or long-term survival. However, the numbers of R2 resections could suggest encroachment of the tumour onto adjacent vascular structures and an inability to resect all macroscopic disease. These are often patients that require vascular resections and reconstruction leading to an increased risk of post-operative complications. If pre-operative imaging is suspicious for vascular involvement, then a pancreaticoduodenectomy for high-risk patients (based upon age and ASA score or significant co-morbidities) may not be advisable. Further, patients who had a concomitant liver resection for metastatic disease (n = 5) had a significantly worse outcome with only one patient surviving to 1 year. Resection of a liver metastasis in this setting is clearly beyond the borderline-resectable criteria and should not be performed unless there are extenuating circumstances. What is not known is whether patients undergoing R2 resections or concomitant liver resections had an improved quality of life.
A multidisciplinary and systematic approach to cancer therapy has previously been shown to be beneficial in cancer care.22–24 The BCCA is a provincial agency providing a province-wide, population-based cancer control programme for the residents of British Columbia and the Yukon. The BCCA's mandate covers the spectrum of cancer care, from prevention and screening, to diagnosis, treatment and through to rehabilitation. The BCCA's policy in the management of pancreatic cancers has not, until recently, included routine consideration of adjuvant chemotherapy. Radiation therapy is administered only on a case-by-case basis. Referrals to the BCCA had a positive effect on intermediate and long-term survival in spite of the insignificant survival benefit of chemotherapy or radiation therapy. This may be secondary to the coordinated, systematic medical and psychosocial therapy available from BCCA that may be more efficient and more available to referred patients than to those not referred for management.
As management of technically resectable pancreatic head adenocarcinomas continue to evolve, the criteria for selection of patients for a pancreaticoduodenectomy requires continued review. With an aging population and more sophisticated imaging techniques available, the number of patients presenting for a potential resection will increase. Careful evaluation of factors that may result in a high likelihood of post-operative complications and assessment, overall patient physiological must be undertaken to ensure appropriate application of a physiologically stressful and resource intensive procedure. Each institution should review its own peri-operative morbidity and mortality data comparing the risk of the procedure to its long-term results. Octogenarians with a ASA score of 3 require a high degree of scrutiny to determine if the risks of a pancreaticoduodenectomy justify the risks, especially if there is any concern regarding the possibility of a borderline resectable cancer. Individual patient outcomes and institutional results could be improved if the patients' peri-operative risks justify the long-term outcomes. As a corollary, the reduction of peri-operative complications would significantly improve these outcomes.
Acknowledgments
Statistical support was provided by the BCCA Surgical Oncology Network.
Author contributions
G.E., MD (Acquisition of data; revision of manuscript; final approval); N.C., MBBS (Acquisition of data; revision of manuscript; final approval); C.McG., MSc (Study design, statistical and data analysis; revision of manuscript; final approval); F.K., MSc (Statistical analysis; revision of manuscript; final approval); A.B. MD, MSc, FRCSC (Data contribution; revision of manuscript; final approval); C.S., MD, MSc, FRCSC (Data contribution; revision of manuscript; final approval); G.W., MD, MSc, FRCSC (Data contribution; revision of manuscript; final approval); S.C., MD, PhD, FRCSC (Study conception & design, data contribution & analysis; drafting of manuscript).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4: survival of cancer patients diagnosed in 1995–1999: results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Batidas JA, Poen JC, Niederhuber JE. Pancreas. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical Oncology. 2nd. Philadelphia, PA: Churchill-Livingston; 2000. pp. 1749–1783. [Google Scholar]
- 4.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Stojadinovic A, Brooks A, Hoos A, Jaques DP, Conlon KC, Brennan MF. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg. 2003;196:954–964. doi: 10.1016/S1072-7515(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Cunningham D, Friess H, Bassi C, Stocken DD, Tait DM, et al. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol. 2003;14:675–692. doi: 10.1093/annonc/mdg207. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. for the Groupe Tumeurs Digestives of Unicancer and the PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell: Oxford; 2009. [Google Scholar]
- 12.Garden OJ. Liver and pancreatic resection in the elderly. HPB Surg. 1997;10:259–261. doi: 10.1155/1997/84160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis DJ, Hsu A, Brand MI, Saclarides TJ. Morbidity and mortality in octogenarians and older undergoing major intestinal surgery. Dis Colon Rectum. 2009;52:59–63. doi: 10.1007/DCR.0b013e31819754d4. [DOI] [PubMed] [Google Scholar]
- 14.de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz RS. Immunodeficiency, immunosuppression, and susceptibility to neoplasms. J Natl Cancer Inst. 2001;28:5–9. doi: 10.1093/oxfordjournals.jncimonographs.a024257. [DOI] [PubMed] [Google Scholar]
- 16.Kressner U, Graf W, Mahteme H, Pahlman L, Glimelius B. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316–321. doi: 10.1007/s10350-004-6174-4. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topal B, Van de Sande S, Fieuws S, Penninckx F. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg. 2007;94:1377–1381. doi: 10.1002/bjs.5861. [DOI] [PubMed] [Google Scholar]
- 20.Lall CG, Howard TJ, Skandarajah A, DeWitt JM, Aisen AM, Sandrasegaran K. New concepts in staging and treatment of locally advanced pancreatic head cancer. AJR Am J Roentgenol. 2007;189:1044–1050. doi: 10.2214/AJR.07.2131. [DOI] [PubMed] [Google Scholar]
- 21.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Langer B. Role of volume outcome data in assuring quality in HPB surgery. HPB (Oxford) 2007;9:330–334. doi: 10.1080/13651820701611234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fennell ML, Das IP, Clauser S, Petrelli N, Salner A. The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;2010:72–80. doi: 10.1093/jncimonographs/lgq010. [DOI] [PMC free article] [PubMed] [Google Scholar]


