Abstract
Objectives
There are few reports on the efficacy of hepatectomy for hepatocellular carcinoma (HCC) in patients with renal dysfunction (RD). This study aimed to clarify the validity of hepatectomy for treating HCC in RD patients, and to compare postoperative courses in RD and non-RD patients.
Methods
The clinical features of 722 HCC patients who underwent curative hepatectomy between 1986 and 2009 were retrospectively reviewed. Seventeen patients (2.4%) with preoperative serum creatinine levels of >2.0 mg/dl were defined as the RD group, and, of these, seven who did not receive preoperative haemodialysis were defined as borderline patients. Clinicopathological characteristics and postoperative outcomes were compared between the RD group (n = 17) and the non-RD group (n = 705). The postoperative courses of borderline patients were reviewed in detail.
Results
Overall survival (P = 0.177) and disease-free survival (P = 0.942) after hepatectomy did not differ significantly between the groups. Incidences of massive ascites (35.3% vs. 14.3%; P = 0.034) and pleural effusion (52.9% vs. 17.6%; P = 0.001), defined as massive effusion (ME), were significantly higher in the RD group than in the non-RD group. Hypoalbuminaemia (≤2.8 g/dl; P = 0.031), heavy blood loss (≥1000 ml; P = 0.012) and intraoperative blood transfusion (P = 0.007) were risk factors for ME. Among the borderline patients, serum creatinine values were not increased immediately after surgery and four patients underwent haemodialysis.
Conclusions
Preoperative hypoalbuminaemia, heavy blood loss and blood transfusion are independent risk factors for ME in RD patients. Preoperative improvement of anaemia and reduction of blood loss by meticulous surgical techniques may prevent ME in RD patients who require hepatectomy for HCC.
Keywords: hepatocellular carcinoma, renal dysfunction, end-stage renal failure, liver resection, hepatectomy, haemodialysis, ascites, pleural effusion
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide and its incidence is increasing.1,2 The majority of asymptomatic HCC patients are diagnosed at an advanced stage; therefore, their prognosis is not favourable.3–5 The number of patients with chronic kidney disease who eventually submit to end-stage renal failure and require haemodialysis is rising in line with an increase in lifestyle-related diseases such as diabetes and metabolic syndrome.6 Some reports have demonstrated that the presence of renal dysfunction (RD) in patients with malignancy may have a negative prognostic impact, such as an increased risk for tumour recurrence,7,8 massive ascites as liver dysfunction,9–11 worsening of hepatitis,12,13 and other complications such as surgical site infections.14,15 Only three studies have demonstrated the results of hepatectomy in HCC patients with RD.16–18 All of them reported that liver resection for HCC was justified in selected patients with end-stage renal failure as a result of improvements in techniques and knowledge of RD; however, this has not been demonstrated in borderline patients with RD in whom haemodialysis is not initiated preoperatively.16–18 Surgeons who are concerned about the postoperative induction of haemodialysis are sometimes unsure whether hepatectomy should be performed. The current report describes a retrospective study of patients with curatively resectable HCC, with and without RD, in whom liver resection was performed, and compares outcomes in patients with and without RD to determine whether liver resection for HCC is justified in the subset of patients with RD. The study also examined clinical findings in borderline patients with RD who did not receive preoperative haemodialysis and determined the risk factors for postoperative complications in these patients.
Materials and methods
Patients and indications for hepatectomy
Between January 1986 and August 2009, a total of 722 consecutive patients underwent curative hepatectomy for primary HCC at the Department of Surgery and Science, Kyushu University Hospital, Fukuoka, Japan. During this period, hepatic resection, as the therapy of choice for primary HCC, was performed to eradicate the disease. Of these patients, 17 (2.4%) showed preoperative serum creatinine (Cre) values >2.0 mg/dl and were classified as the RD group. The remaining 705 patients were classified as the non-RD group. Additionally, of the 17 patients in the RD group, seven patients who did not undergo preoperative haemodialysis were defined as borderline patients.
Tumour-specific evaluation, including abdominal ultrasonography, abdominal and thoracic computed tomography (CT), hepatic angiography with CT, magnetic resonance imaging of the abdomen, bone scintigraphy, and determination of alpha-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), was performed in all patients admitted for liver resection. Liver function was evaluated preoperatively according to the Child–Pugh system of classification and indocyanine green retention test at 15 min (ICG R15), as reported previously.19 The surgical procedure was selected according to the following criteria: trisegmentectomy was performed in patients with an ICG R15 result of <15%; bisegmentectomy was performed in patients with an ICG R15 result of 15–25%; monosegmentectomy was performed in patients with an ICG R15 result of 26–35%, and subsegmentectomy was performed in patients with an ICG R15 result of 36–45%. The presence of ascites and an ICG R15 result of >45% were considered as absolute contraindications for resection. Operative indications in the RD group did not differ from those in the non-RD group.
The diagnosis of HCC was confirmed by pathological findings after hepatectomy. Curative resection was defined as complete macroscopic and microscopic removal of the tumour, excluding radiofrequency ablation and ethanol injection therapy. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration. Informed consent in writing for participation in this study was obtained from each patient.
Clinical factors
The clinical factors analysed included: age; sex; hepatitis B surface antigen (HBs-Ag); hepatitis C virus antibody (HCV-Ab); preoperative complete blood count; biochemical analyses [Cre, blood urea nitrogen (BUN), albumin, total bilirubin (T-bil), prothrombin time (PT), ICG R15]; preoperative tumour markers (serum AFP and DCP); tumour diameter and number; Child–Pugh class; tumour stage; pathological findings (tumour differentiation, microvascular invasion, intrahepatic metastasis, degree of chronic hepatitis in non-cancerous regions); intraoperative and postoperative outcomes (operation time, blood loss, type of resection, resected liver weight, blood transfusion, hospital stay after hepatectomy, complications), and survival. The size of the largest tumour was measured at its greatest dimension if the patient had two or more tumours. Two categories of HCC were distinguished according to whether the disease manifested as a single tumour or as multiple tumours. Major hepatectomy was defined as involving no fewer than three segments. Massive ascites was defined as ascites sustained until postoperative day 7; pleural effusion was defined in a similar manner. In addition, massive effusion (ME) was defined as massive ascites and/or pleural effusion.
Histological examination
All of the resected specimens were cut into serial slices of 5–10 mm in thickness and fixed in 10% formalin. After macroscopic examination, the slice with the greatest tumour dimension was trimmed, embedded in a paraffin block, and cut into 4-µm microscopic sections. The sections were stained with haematoxylin and eosin. Tumour differentiation, microvascular invasion and intrahepatic metastasis were examined. Tumour–node–metastasis (TNM) staging and other pathological findings were defined according to the Liver Cancer Study Group of Japan.20 The extent of chronic hepatitis in non-cancerous regions was classified according to the degree of necroinflammatory activity (graded from A0 to A4) and the degree of fibrosis (staged from F0 to F4).21
Follow-up and treatment of HCC recurrence
The clinical follow-up of patients who underwent hepatectomy for HCC was conducted according to a strict protocol that did not change during the study period. Liver function and renal function tests were performed every day until 1 week postoperatively and subsequently bi-weekly during the first month and then monthly for 6 months. Patients underwent ultrasound scans and enhanced CT scans at 6-month intervals. Hepatic angiography, bone scintigraphy or a thoracic CT scan was also performed when recurrence was suspected. The median follow-up period after initial hepatectomy was 7.1 years.
Patients with recurrent HCC that could be surgically treated for a cure underwent resection or ablation of tumours. All patients who were considered to be unsuitable for surgical treatment were referred for palliative care by radiotherapy, transarterial catheter chemoembolization or systemic chemotherapy using 5-fluorouracil and cisplatin.
Statistical analysis
All statistical analyses were performed using jmp Version 7.01 (SAS Institute, Inc., Cary, NC, USA). Continuous variables with normal distribution are expressed as the mean ± standard deviation (SD); those without normal distribution are expressed as medians and ranges. These variables were compared with independent samples using the non-parametric Wilcoxon test or with dependent samples using the parametric paired t-test. Categorical data were compared using Fisher's test and the chi-squared test. Logistic regression analysis was performed to identify independent risk factors for postoperative complications after hepatectomy. P-values of <0.05 were considered to indicate statistical significance.
Results
Clinical features of HCC patients with RD
The median age of patients in the RD group was 58 years (range: 31–82 years); 14 of the 17 patients were men. The median value of serum Cre was 6.9 mg/dl (range: 2.3–12.2 mg/dl) and the most common aetiology for RD was related to diabetes mellitus (n = 8). Data for longterm outcomes showed that eight patients had recurrences and the median recurrence-free survival period was 18.0 months (range: 5.8–38.5 months). Ten patients died during the follow-up period; the median length of survival was 42.4 months (range: 2.6–69.9 months).
Comparison of baseline characteristics
Baseline characteristics including background and laboratory data for the RD group were compared with those for the non-RD group (Table 1).
Table 1.
Baseline characteristics of hepatocellular carcinoma patients with and without renal dysfunction (RD) who underwent liver resection
| Variable | RD group (n = 17) | Non-RD group (n = 705) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 58 ± 2 | 62 ± 1 | 0.024 |
| Gender, male, n (%) | 14 (82.4) | 560 (79.4) | 0.053 |
| HBs-Ag positive, n (%) | 3 (17.6) | 125 (17.7) | 0.814 |
| HCV-Ab positive, n (%) | 10 (58.8) | 392 (55.6) | 0.792 |
| Child–Pugh class, A/B/C, n (%) | 10/7/0 (58.8/41.2/0) | 564/127/14 (80.0/18.0/2.0) | 0.438 |
| Diabetes, n (%) | 8 (47.1) | 187 (26.5) | 0.044 |
| Hypertension, n (%) | 10 (58.8) | 187 (26.5) | 0.010 |
| Alcohol abuse, n (%) | 6 (35.3) | 345 (48.9) | 0.439 |
| Serum Cre, mg/dl, mean ± SD | 6.9 ± 0.1 | 0.8 ± 0.1 | 0.001 |
| Serum BUN, mg/dl, mean ± SD | 58.8 ± 1.2 | 14.4 ± 0.2 | 0.001 |
| Serum albumin, g/dl, mean ± SD | 3.8 ± 0.1 | 3.6 ± 0.1 | 0.023 |
| Serum T-bil, mg/dl, mean ± SD | 0.4 ± 0.1 | 0.8 ± 0.1 | 0.001 |
| PT, %, mean ± SD | 88.0 ± 0.5 | 91.8 ± 3.4 | 0.858 |
| ICG R15, %, mean ± SD | 10.7 ± 2.3 | 16.9 ± 0.3 | 0.004 |
| Platelet count, 104/µl, mean ± SD | 8.8 ± 2.1 | 9.2 ± 0.3 | 0.436 |
| Hb, g/dl, mean ± SD | 9.4 ± 2.1 | 13.9 ± 0.3 | 0.018 |
SD, standard deviation; HBs-Ag, hepatitis B surface antigen; HCV-Ab, hepatitis C virus antibody; Cre, creatinine; BUN, blood urea nitrogen; T-bil, total bilirubin; PT, prothrombin time; ICG R15, indocyanine green retention rate at 15 min; Hb, haemoglobin.
Comparison of pathological parameters of resected HCC and operative findings
The pathological parameters of resected HCC and operative findings are shown in Table 2.
Table 2.
Pathological parameters of resected hepatocellular carcinoma and operative findings in patients with and without renal dysfunction (RD)
| Variable | RD group (n = 17) | Non-RD group (n = 705) | P-value |
|---|---|---|---|
| Tumour size, cm, mean ± SD | 4.1 ± 0.9 | 3.9 ± 0.1 | 0.418 |
| Multiple tumours, n (%) | 3 (17.6) | 176 (25.0) | 0.287 |
| TNM stage, I + II/III + IV, n (%) | 7/10 (41.2/58.8) | 477/228 (67.7/32.3) | 0.080 |
| Differentiation, good + moderate/poor, n (%) | 9/8 (52.9/47.1) | 474/231 (67.2/32.8) | 0.404 |
| Microvascular invasion, n (%) | 6 (35.3) | 251 (35.6) | 0.721 |
| Intrahepatic metastasis, n (%) | 3 (35.3) | 202 (28.7) | 0.310 |
| Serum AFP, ng/ml, median (range) | 10 (1.5–42 720) | 21 (1.5–693 700) | 0.433 |
| Serum DCP, mAU/l, median (range) | 30 (2–19 280) | 22 (2–318 000) | 0.442 |
| Operation time, min, mean ± SD | 309 ± 29 | 320 ± 4 | 0.320 |
| Blood loss, g, mean ± SD | 905 ± 402 | 1323 ± 61 | 0.124 |
| Major hepatectomy (Hr2), n (%) | 0 | 17 (2.4) | 0.359 |
| Resected liver weight, g, mean ± SD | 291 ± 76 | 288 ± 12 | 0.450 |
| Blood transfusion, n (%) | 10 (58.8) | 208 (29.5) | 0.014 |
SD, standard deviation; TNM, tumour–node–metastasis; AFP, alpha-fetoprotein; DCP, des-γ-carboxyl prothrombin; Hr2, hepatic resection of two segments.
Comparison of overall and disease-free survival rates
Overall and disease-free survival curves are shown in Fig. 1. Overall survival rates after hepatectomy in the RD and non-RD groups, respectively, were 74.1% and 74.6% at 3 years, and 53.3% and 58.7% at 5 years, and thus did not differ significantly between the groups (P = 0.177). Disease-free survival rates after hepatectomy in the RD and non-RD groups, respectively, were 45.8% and 43.7% at 3 years, and 34.4% and 29.8% at 5 years, and thus did not differ significantly between the groups (P = 0.942).
Figure 1.
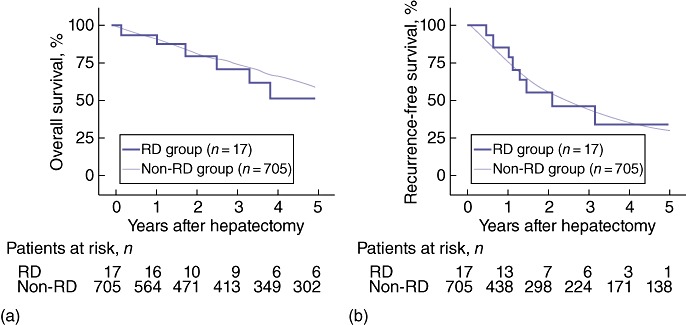
(a) Overall survival rates in renal dysfunction (RD) (n = 17) and non-RD (n = 705) patients with hepatocellular carcinoma (HCC). Overall survival rates in the RD and non-RD groups, respectively, were 74.1% and 74.6% at 3 years, and 53.3% and 58.7% at 5 years (P = 0.177). (b) Recurrence-free survival rates in RD (n = 17) and non-RD (n = 705) patients with HCC. Recurrence-free survival rates in the RD and non-RD groups, respectively, were 45.8% and 43.7% at 3 years, and 34.4% and 29.8% at 5 years (P = 0.942)
Comparison of postoperative clinical courses
Table 3 shows data for the postoperative clinical course in all patients; postoperative data for borderline patients are shown in Table 4. None of the seven patients with preoperative borderline RD showed an increase in serum Cre values immediately after surgery and three of these patients did not have haemodialysis; however, in four patients, haemodialysis was initiated postoperatively. The median time from hepatectomy to the initiation of haemodialysis was 8.5 months (range: 2.5–19.5 months). Of the two patients who underwent anatomical resection of the liver, haemodialysis was initiated in one at 19 months postoperatively and not at all in the other patient. Data for longterm outcomes showed that five patients remained alive during the follow-up period with a median survival of 25.2 months (range: 18.9–42.2 months).
Table 3.
Postoperative clinical course in patients with hepatocellular carcinoma with and without renal dysfunction (RD)
| Variable | RD group (n = 17) | Non-RD group (n = 705) | P-value |
|---|---|---|---|
| Hospital stay, days, mean ± SD | 41.0 ± 8.1 | 28.4 ± 1.2 | 0.026 |
| Complications, n (%) | 11 (64.7) | 319 (45.2) | 0.268 |
| SSI, n (%) | 3 (17.6) | 80 (11.3) | 0.450 |
| Massive ascites, n (%) | 6 (35.3) | 101 (14.3) | 0.034 |
| Pleural effusion, n (%) | 9 (52.9) | 124 (17.6) | 0.001 |
| Postoperative bleeding, n (%) | 0 | 14 (2.0) | 0.412 |
| Bile leakage, n (%) | 1 (5.8) | 36 (5.1) | 0.888 |
| Gastrointestinal bleeding, n (%) | 0 | 17 (2.4) | 0.365 |
| Liver failure, n (%) | 2 (11.8) | 23 (3.3) | 0.131 |
SD, standard deviation; SSI, surgical site infection.
Table 4.
Characteristics of patients with borderline renal dysfunction preoperatively (n = 7)
| Case | Age, years | Gender | Aetiology | Surgical method | Serum Cre, mg/dla | Duration from FD to Hx, months | Duration from Hx to HI, months | Survival (months after Hx) |
|---|---|---|---|---|---|---|---|---|
| 1 | 82 | M | HT, RS | Partial | 2.33/1.55/1.87/1.93/2.12 | 120 | (–) | Alive (18.9) |
| 2 | 61 | M | DM | Partial | 3.01/2.11/2.03/2.26/2.89 | 36 | (–) | Dead (13.2) |
| 3 | 58 | M | DM | Seg | 3.02/3.05/3.03/3.18/3.81 | 36 | 19 | Alive (19.9) |
| 4 | 72 | M | DM | Partial | 3.39/5.06/3.91/4.51/4.88 | 12 | 2 | Alive (42.2) |
| 6 | 57 | M | DM | Lobe | 5.62/5.91/5.87/5.47/5.98 | 24 | (–) | Dead (22.0) |
| 7 | 67 | M | DM | Partial | 5.76/6.02/5.99/5.78/8.76 | 6 | 8 | Alive (19.1) |
| 10 | 65 | F | HT | Partial | 7.28/3.69/6.81/6.73/7.44 | 24 | 5 | Alive (25.8) |
Creatinine values are: preoperative/1 week postoperatively/2 weeks postoperatively/1 month postoperatively/1 years postoperatively.
Cre, creatinine; FD, first diagnosis of renal dysfunction; Hx, hepatectomy; HI, haemodialysis induction; M, male; F, female; HT, hypertension; RS, renal sclerosis; DM, diabetes mellitus; Partial, partial hepatectomy; Seg, segmentectomy; Lobe, lobectomy.
Risk factors related to postoperative ME
Risk factors related to postoperative ME in patients with preoperative RD are shown in Table 5.
Table 5.
Univariate analysis for risk factors related to postoperative massive effusion (ME) in patients with renal dysfunction (n = 17)
| Variable | Non-ME (n = 11 ) | ME (n = 6) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 57 ± 4 | 58 ± 6 | 0.947 |
| Gender, male, n (%) | 1 (0.9) | 2 (33.3) | 0.220 |
| HBs-Ag positive, n (%) | 4 (36.4) | 0 | 0.086 |
| HCV-Ab positive, n (%) | 5 (45.5) | 5 (83.3) | 0.116 |
| Child–Pugh class, A/B + C, n (%) | 9/2 (81.8/18.2) | 4/2 (66.7/33.3) | 0.488 |
| Diabetes, n (%) | 5 (45.5) | 3 (50.0) | 0.858 |
| Hypertension, n (%) | 5 (45.5) | 5 (83.3) | 0.116 |
| Alcohol abuse, n (%) | 4 (36.4) | 1 (16.7) | 0.380 |
| Serum albumin ≤2.8 g/dl, n (%) | 0 | 2 (33.3) | 0.031 |
| Serum T-bil, mg/dl, mean ± SD | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.072 |
| PT, %, mean ± SD | 92 ± 5 | 91 ± 7 | 0.910 |
| ICG R15, %, mean ± SD | 11.4 ± 2.0 | 9.1 ± 2.9 | 0.531 |
| Platelet count, 104/µl, mean ± SD | 17.5 ± 3.3 | 14.4 ± 4.4 | 0.578 |
| Hb, g/dl, mean ± SD | 9.3 ± 0.6 | 9.7 ± 0.8 | 0.653 |
| Tumour size, cm, mean ± SD | 3.9 ± 0.9 | 3.6 ± 1.0 | 0.855 |
| Multiple tumours, n (%) | 2 (18.2) | 3 (50.0) | 0.121 |
| TNM stage, I + II/III + IV, n (%) | 6/5 (54.5/45.5) | 2/4 (33.3/66.7) | 0.531 |
| Tumour differentiation, good + moderate/poor, n (%) | 6/5 (54.5/45.5) | 4/2 (66.7/33.3) | 0.769 |
| Microvascular invasion, n (%) | 2 (18.2) | 2 (33.3) | 0.531 |
| Intrahepatic metastasis, n (%) | 2 (18.2) | 3 (50.0) | 0.181 |
| Serum AFP, ng/ml, mean ± SD | 4761 ± 3171 | 356 ± 4294 | 0.422 |
| Serum DCP, mAU/l, mean ± SD | 3470 ± 1647 | 134 ± 2127 | 0.235 |
| Operation time, min, mean ± SD | 281 ± 35 | 353 ± 47 | 0.239 |
| Blood loss ≥1000 ml, n (%) | 1 (0.9) | 4 (66.7) | 0.012 |
| Major hepatectomy (Hr2), n (%) | 2 (18.2) | 2 (33.3) | 0.488 |
| Resected liver weight, g, mean ± SD | 256 ± 115 | 313 ± 156 | 0.782 |
| Blood transfusion, n (%) | 1 (0.9) | 5 (83.3) | 0.007 |
SD, standard deviation; HBs-Ag, hepatitis B surface antigen; HCV-Ab, hepatitis C virus antibody; T-bil, total bilirubin, PT, prothrombin time; ICG R15, indocyanine green retention rate at 15 min; Hb, haemoglobin; TNM, tumour–node–metastasis; AFP, alpha-fetoprotein; DCP, des-γ-carboxyl prothrombin; Hr2, hepatic resection of two segments.
Discussion
The current study investigated the short- and longterm effects of hepatectomy in RD patients with HCC. This retrospective study is inherently disadvantaged by its status as a single-centre, non-randomized study including a small number of Japanese patients. However, it represents the first review of clinical findings in borderline patients with serum Cre values of >2.0 mg/dl who did not undergo preoperative haemodialysis. It also investigated risk factors related to postoperative complications in RD patients. Overall and disease-free survival rates did not differ between the RD and non-RD groups. However, the incidence of ME, defined as massive ascites and/or pleural effusion, was significantly higher in the RD group than in the non-RD group, and low serum albumin values, heavy blood loss and need for intraoperative blood transfusion were identified as risk factors for postoperative ME in patients with RD.
Data for the borderline patients showed no evidence of an increase in the initiation of haemodialysis after major surgery or of worse overall survival. Patients with RD are subject to several types of risk in major surgery, even without a requirement for haemodialysis,22 and the most critical issue in curative hepatectomy in RD patients with HCC is that the procedure is performed safely in order to prevent the deterioration of renal function and avoid the need for haemodialysis. According to the Renal Data Registry Committee of the Japanese Society for Dialysis Therapy, a survey of over one million Japanese patients found that the mean age of new patients on dialysis was 67.2 years23 and the time from first diagnosis of RD to haemodialysis therapy was reported as in the range of 14.2–27.9 months.24–26 In the present report, four of seven borderline patients underwent haemodialysis postoperatively. Their mean age was 66.1 years (range: 59–72 years) and the mean time from first diagnosis of RD to haemodialysis therapy was 28.0 months (range: 14.0–55.5 months). These data suggest that hepatectomy did not strongly affect the issue of whether or not postoperative haemodialysis was initiated in borderline patients.23–26 In terms of prognosis, RD is known to represent a severe risk factor for cardiovascular dysfunction and cardiorenal anaemia syndrome,27 and 5-year survival in RD patients who do not undergo major surgery has been reported as 60.1%.23 In the present study, the overall survival rate after hepatectomy was 53.3% at 5 years in RD patients. Indeed, there was no strong evidence that hepatectomy resulted in the deterioration of renal function and survival rates and it did not increase the initiation of haemodialysis in borderline RD patients after surgery for HCC.
Cardiorenal anaemia syndrome is the main cause of deterioration in postoperative cardiovascular dysfunction and often results in other postoperative complications.27 In this syndrome, activation of the renin–angiotensin–aldosterone system, imbalance of reactive oxygen species, and irritation of the sympathetic nervous system result in the acceleration of coronary sclerosis, impairment of cardiac microcirculation, and hypertension.28 Ohno et al. demonstrated that onset of sodium retention is strongly related to a critical threshold of hepatic function after hepatectomy in a rodent model.29 The underlying mechanisms of ME after surgery remain unclear. One of the mechanisms of ME is an impaired haemodynamic state postoperatively.30,31 A hypervolemic state exists in patients after hepatectomy, caused by intraoperative excess infusion or return from the ‘third space’, even in patients with normal renal function. Fluid in the peritoneal cavity and peripheral vasodilatation then contribute to a decreased effective blood volume, which further compromises renal blood volume and the hypervolemic state.32,33 In patients with RD who tend to be in a hypervolemic state, these mechanisms are likely to aggravate or at least maintain ME development and sustain the vicious cycle that causes ME. Activation of vasoconstrictor systems triggered by liver dysfunction or inflammatory cytokines released from leukocytes may at least in part induce the development of peripheral vasodilatation and impaired effective blood volume.34,35
Ishizawa et al.36 demonstrated that large blood loss and low platelet count are independent risk factors for massive ascites after hepatectomy and discussed how to manage insufficient urinary output indicating RD caused by portal hypertension in the early postoperative period. Their findings are consistent with those of the present study; in fact, large blood loss and hypoalbuminaemia indicating liver dysfunction were independent risk factors for ME in the present study and these results do not contradict the finding of elevated portal hypertension after hepatectomy. It is clear that liver dysfunction causes an increase in portal hypertension that exists prior to hepatectomy.37,38 Some studies have reported that virus titres such as that for HCV decreased in patients after dialysis.39,40 These data indicated that improvements in liver function in RD patients compared with those in non-RD patients may be affected by the reduction of HCV load by dialysis; however, any direct cause–effect relationship between a reduction in HCV titre and dialysis remains unknown.
Some studies on attempts to treat cardiorenal anaemia syndrome have reported that the administration of erythropoietin often improves renal anaemia, as well as renal function and survival after the initiation of haemodialysis.41–43 Previous reports16–18 have demonstrated the effects of hepatectomy in RD patients receiving haemodialysis and have concluded that careful operative techniques and perioperative care are crucial to achieving lower morbidity and mortality in HCC patients with end-stage renal failure; however, it is not clear how RD patients with HCC who require hepatectomy should be managed. The preoperative administration of erythropoietin in RD patients may be very important for preventing renal anaemia and postoperative complications such as ME, but further data are required to confirm the efficacy of erythropoietin in improving postoperative outcome.41–44
In conclusion, RD patients with HCC experience a higher frequency of postoperative complications such as ME. Hypoalbuminaemia, massive blood loss and a high frequency of intraoperative blood transfusion are independent risk factors for ME, defined as massive ascites and/or pleural effusion, in RD patients after hepatectomy. Although it may be possible to safely manage ME that is mainly caused by renal anaemia, step-by-step trials are essential to improve postoperative outcomes.
Conflicts of interest
None declared.
References
- 1.Shirabe K, Toshima T, Taketomi A, Taguchi K, Yoshizumi T, Uchivama H, et al. Hepatic aflatoxin B1-DNA adducts and TP53 mutations in patients with hepatocellular carcinoma despite low exposure to aflatoxin B1 in southern Japan. Liver Int. 2011;31:1366–1372. doi: 10.1111/j.1478-3231.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- 2.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma – with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 3.Shirabe K, Kajiyama K, Harimoto N, Tsugita E, Wakiyama S, Maehara Y. Risk factors for massive bleeding during major hepatectomy. World J Surg. 2010;34:1555–1562. doi: 10.1007/s00268-010-0495-3. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 5.Shirabe K, Kajiyama K, Harimoto N, Masumoto H, Fukuya T, Ooya M, et al. Prognosis of hepatocellular carcinoma accompanied by microscopic portal vein invasion. World J Gastroenterol. 2009;15:2632–2637. doi: 10.3748/wjg.15.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, et al. Beneficial effect of autologous blood transfusion on infectious complications and colorectal cancer surgery. Lancet. 1993;342:1328–1333. doi: 10.1016/0140-6736(93)92247-q. [DOI] [PubMed] [Google Scholar]
- 8.Jagoditsch M, Pozgainer P, Klingler A, Tschmelitsch J. Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum. 2006;49:1116–1130. doi: 10.1007/s10350-006-0573-7. [DOI] [PubMed] [Google Scholar]
- 9.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Huang YH, Su CW, Lin HC, Chiang JH, Lee PC, et al. Renal failure in patients with hepatocellular carcinoma and ascites undergoing transarterial chemoembolization. Liver Int. 2010;30:77–84. doi: 10.1111/j.1478-3231.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 11.Montoliu S, Ballesté B, Planas R, Alvarez MA, Rivera M, Miguel M, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616–622. doi: 10.1016/j.cgh.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in haemodialysis patients. Am J Kidney Dis. 2010;56:371–378. doi: 10.1053/j.ajkd.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Butt AA, Skanderson M, McGinnis KA, Ahuja T, Bryce CL, Barnato AE, et al. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. J Viral Hepat. 2007;14:688–696. doi: 10.1111/j.1365-2893.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 14.Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: a retrospective analysis of 1446 patients. Arch Surg. 2006;141:1014–1018. doi: 10.1001/archsurg.141.10.1014. [DOI] [PubMed] [Google Scholar]
- 15.Weber WP, Zwahlen M, Reck S, Misteli H, Rosenthal R, Buser AS, et al. The association of preoperative anaemia and perioperative allogeneic blood transfusion with the risk of surgical site infection. Transfusion. 2009;49:1964–1970. doi: 10.1111/j.1537-2995.2009.02204.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng SB, Yeh DC, Shu KH, Wu CC, Wen MC, Liu TJ, et al. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. 2006;93:273–278. doi: 10.1002/jso.20465. [DOI] [PubMed] [Google Scholar]
- 17.Yeh CN, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma in end-stage renal disease patients: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:2067–2071. doi: 10.3748/wjg.v11.i14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orii T, Takayama T, Haga I, Fukumori T, Amada N. Efficacy of a liver resection for hepatocellular carcinoma in patients with chronic renal failure. Surg Today. 2008;38:329–334. doi: 10.1007/s00595-007-3634-1. [DOI] [PubMed] [Google Scholar]
- 19.Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. doi: 10.1046/j.1365-2168.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 20.Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 2nd English edn. Tokyo: Kanehara; 2003. pp. 22–23. [Google Scholar]
- 21.Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, et al. New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun. 1996;6:112–119. [Google Scholar]
- 22.Mori S, Sawada T, Hamada K, Kita J, Shimoda M, Tagaya N, et al. Gastrectomy for patients with gastric cancer and non-uremic renal failure. World J Gastroenterol. 2007;13:4589–4592. doi: 10.3748/wjg.v13.i34.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakai S, Suzuki K, Masakane I, Wada A, Itami N, Ogata S, et al. Overview of regular dialysis treatment in Japan. Ther Apher Dial. 2010;14:505–540. doi: 10.1111/j.1744-9987.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Nakata H, Yoshihara F, Kamide K, Horio T, Nakahama H, et al. Effect of early nephrology referral on the initiation of haemodialysis and survival in patients with chronic kidney disease and cardiovascular diseases. Circ J. 2007;71:511–516. doi: 10.1253/circj.71.511. [DOI] [PubMed] [Google Scholar]
- 25.Devins GM, Mendelssohn DC, Barre PE, Binik YM. Predialysis psychoeducational intervention and coping styles influence time to dialysis in chronic kidney disease. Am J Kidney Dis. 2003;42:693–703. doi: 10.1016/s0272-6386(03)00835-7. [DOI] [PubMed] [Google Scholar]
- 26.Inaguma D, Tatematsu M, Shinjo H, Suzuki S, Mishima T, Inaba S, et al. Effect of an educational programme on the predialysis period for patients with chronic renal failure. Clin Exp Nephrol. 2006;10:274–278. doi: 10.1007/s10157-006-0439-2. [DOI] [PubMed] [Google Scholar]
- 27.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 28.Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WN. Association of high serum creatinine and anaemia increases the risk of coronary events: results from the prospective community-based Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919–2925. doi: 10.1097/01.asn.0000092138.65211.71. [DOI] [PubMed] [Google Scholar]
- 29.Ohno T, Sabra R, Branch RA. Sodium retention and hepatic function after two-thirds hepatectomy in the rat. Hepatology. 1991;14:511–517. [PubMed] [Google Scholar]
- 30.Fernández-Rodriguez CM, Prieto J, Zozaya JM, Quiroga J, Guitián R. Arteriovenous shunting, haemodynamic changes, and renal sodium retention in liver cirrhosis. Gastroenterology. 1993;104:1139–1145. doi: 10.1016/0016-5085(93)90285-k. [DOI] [PubMed] [Google Scholar]
- 31.Kalambokis G, Economou M, Fotopoulos A, Al Bokharii J, Pappas C, Katsaraki A. The effects of chronic treatment with octreotide versus octreotide plus midodrineon systemic haemodynamics and renal haemodynamics and function in non-azotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879–885. doi: 10.1111/j.1572-0241.2005.40899.x. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:157–170. doi: 10.1016/s0168-8278(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 33.Henriksen JH, Fuglsang S, Bendtsen F, Moller S. Arterial hypertension in cirrhosis: arterial compliance, volume distribution, and central haemodynamics. Gut. 2006;55:380–387. doi: 10.1136/gut.2005.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sansoé G, Silvano S, Mengozzi G, Todros L, Smedile A, Touscoz G, et al. Inappropriately low angiotensin II generation: a factor determining reduced kidney function and survival in patients with decompensated cirrhosis. J Hepatol. 2004;40:417–423. doi: 10.1016/j.jhep.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 35.La Villa G, Barletta G, Pantaleo P, Del Bene R, Vizzutti F, Vecchiarino S, et al. Haemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosis. Hepatology. 2001;34:19–27. doi: 10.1053/jhep.2001.25756. [DOI] [PubMed] [Google Scholar]
- 36.Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;16:46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]
- 37.Solà E, Lens S, Guevara M, Martín-Llahí M, Fagundes C, Pereira G, et al. Hyponatraemia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertension. Hepatology. 2010;52:1783–1790. doi: 10.1002/hep.23893. [DOI] [PubMed] [Google Scholar]
- 38.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodelling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Fabrizi F, Martin P, Dixit V, Brezina M, Cole MJ, Vinson S, et al. Biological dynamics of viral load in haemodialysis patients with hepatitis C virus. Am J Kidney Dis. 2000;35:122–129. doi: 10.1016/S0272-6386(00)70310-6. [DOI] [PubMed] [Google Scholar]
- 40.Martins RS, Filho OA, Gonçales NS, Del Castillo DM, Silva LD, Faria LC, et al. Kinetics of hepatitis C virus load and haemodialysis: is there any influence of the reuse of dialysis membrane on HCV viraemia? Scand J Infect Dis. 2012;44:190–196. doi: 10.3109/00365548.2011.627377. [DOI] [PubMed] [Google Scholar]
- 41.Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anaemia by erythropoietin therapy retards the progression of chronic renal failure, especially in non-diabetic patients. Nephron. 1997;77:176–185. doi: 10.1159/000190270. [DOI] [PubMed] [Google Scholar]
- 42.Kamar N, Reboux AH, Cointault O, Esposito L, Cardeau-Desangles I, Lavayssière L, et al. Impact of very early high doses of recombinant erythropoietin on anaemia and allograft function in de novo kidney-transplant patients. Transpl Int. 2010;23:277–284. doi: 10.1111/j.1432-2277.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 43.Nagarajan S, Mansfield E, Hsieh S, Liu R, Hsieh F, Li L, et al. Transplant reno-vascular stenoses associated with early erythropoietin use. Clin Transplant. 2007;21:597–608. doi: 10.1111/j.1399-0012.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 44.Muirhead N, Cattran DC, Zaltzman J, Jindal K, First MR, Boucher A, et al. Safety and efficacy of recombinant human erythropoietin in correcting the anaemia of patients with chronic renal allograft dysfunction. J Am Soc Nephrol. 1994;5:1216–1222. doi: 10.1681/ASN.V551216. [DOI] [PubMed] [Google Scholar]


