Abstract
Objectives
Liver transplantation (LT) in Milan Criteria (MC) hepatocellular carcinoma (HCC) has excellent outcomes. Pre-transplant loco-regional therapy (LRT) has been used to downstage HCC to meet the MC. However, its benefit in patients with a brief waiting time to transplant remains unclear. This study evaluated outcomes in patients with short waitlist times to LT for MC-compliant HCC.
Methods
Patients undergoing LT for MC HCC at either of two transplant centres between 2002 and 2009 were retrospectively evaluated for outcome. Patients for whom post-transplant follow-up amounted to <12 months were excluded.
Results
A total of 225 patients were included, 93 (41.3%) of whom received neoadjuvant LRT. The median waiting time to transplant was 48 days. Mean post-transplant follow-up was 32.2 months. Overall and disease-free survival at 1 year, 3 years and 5 years were 93.1%, 82.4% and 72.6%, and 91.3%, 79.3% and 70.6%, respectively. There was no difference in overall (P = 0.94) and disease-free survival (P = 0.94) between groups who received and did not receive pre-LT LRT. There were also no disparities in survival or tumour recurrence among categories of patients (with single tumours measuring <3 cm, with single tumours measuring 3–5 cm, with multiple tumours).
Conclusions
Loco-regional therapy followed by rapid transplantation in MC HCC appears not to have an impact on post-transplant outcome.
Keywords: liver transplantation, survival, hepatocellular carcinoma, HCC, outcomes, Milan Criteria, waiting time, loco-regional therapy, mortality
Introduction
Liver transplantation (LT) holds the potential for cure in selected patients with hepatocellular carcinoma (HCC) complicating cirrhosis.1,2 Patients with tumours within the Milan Criteria (MC) (single lesions measuring 2–5 cm or up to three lesions measuring <3 cm each) exhibit post-transplant 5-year disease-free survival of 75% and tumour recurrence rates of 8–12%.3 Therefore, efforts to maintain HCCs within these criteria have driven the implementation of neoadjuvant loco-regional therapy (LRT) among transplant centres.4
Although no post-transplant survival benefits of neoadjuvant LRT have been observed, patients in whom advanced tumours are unsuccessfully downstaged have exhibited worse outcomes.5,6 As a result, a good response to LRT has served as a surrogate for favourable tumour biology and has become a pivotal factor in the selection of patients for transplant when HCC is beyond the MC.7,8
Before the implementation of the Model for End-stage Liver Disease (MELD) liver allocation system in 2002, waitlist times for MC HCC patients were significantly longer. As a result, tumour progression had a negative impact on waitlist dropout rates and on patient survival.9 Because the current UNOS allocation system enables MELD exception points for HCC patients within MC, waitlist times are shorter and rates of dropout for reasons of tumour progression have improved.10
Based on data suggesting that HCC lesions can double in size within 6 months, current guidelines support the treatment of patients with small tumours when waitlist time exceeds this period.1 However, some transplant clinicians may consider the use of neoadjuvant LRT even in the setting of short waitlist times, arguing that induced tumour necrosis decreases tumour burden, which can prevent vascular invasion and may reduce the risk for tumour recurrence.
As the MC embraces a heterogeneous group of HCC patients, there may be a role for neoadjuvant LRT in multifocal disease or in single tumours measuring >3 cm in the setting of short waitlist time. This potential is further emphasized by reports that tumour burden is underestimated in 20–40% of cases by current cross-sectional imaging technology.11–13 This postulate follows the general oncologic principle of treating the disease in its early stages; however, it holds some caveats. Although there are no large randomized controlled trials (RCTs), available data reveal that neoadjuvant LRT for small tumours in patients with short waitlist times does not offer survival benefits or reduce tumour recurrence rates.14 Secondly, the absence of accurate biomarkers predicting tumour behaviour makes time after treatment the sole factor capable of unveiling tumour biology.
In line with this, researchers have been able to identify patients with HCC beyond the MC with post-transplant outcomes similar to those of patients with MC-compliant HCC. In these cases, patients who were successfully downstaged by neoadjuvant LRT and exhibited no tumour progression over a particular length of time (≥3 months) were selected for transplantation.4
More recently, the similar concept of ‘ablating and waiting’ has been proposed for MC-compliant HCC patients in an effort to reduce, although modestly, the risk for tumour relapse after transplantation in this group of patients.15 Roberts et al.15 and Yao et al.4 have raised concerns with regard to rapid transplantation (within 3 months of LRT) in patients with HCC within and beyond the MC, respectively. In an era of severe organ shortage, such endeavours are well accepted and efforts to expand graft and patient survival should be continuously fostered.
In order to expand existing data on this issue, this study reports data for 93 patients with waitlist times of <6 months who received pre-transplant LRT for MC-compliant HCC, and 132 similar patients who did not receive pre-transplant LRT, during the period from January 2002 to December 2009.
Materials and methods
This study was approved by the Cleveland Clinic and University Hospitals Institutional Review Boards. Medical records for patients who underwent LT for HCC from January 2002 to December 2009 in Cleveland, Ohio at two institutions, Cleveland Clinic and the University Hospital of Case Western Reserve University, were reviewed from a prospectively collected database. Patients who died within 1 month after transplantation or who underwent LT within 12 months of the review period (for whom follow-up data were inadequate) were excluded. Patients with HCC considered to fall within the MC were included in the study. All patients were initially evaluated by members of a dedicated team of hepatologists, hepatobiliary and LT surgeons, oncologists and interventional radiologists with an interest in HCC. The diagnosis of HCC was determined based on radiological criteria according to published guidelines1,16 or by histology. The management of LRT was based on a consensus agreement among the treating physicians. Loco-regional therapy interventions offered included radiofrequency ablation (RFA), transarterial chemoembolization (TACE), bland embolization, drug (doxorubicin) eluting bead (DEB) embolization, and Y90 radioembolization with Therasphere® or SIR-Spheres®.
Data collection
Data collected included patient demographics, aetiology of liver disease, MELD score, number and sizes of HCC lesions, loco-regional treatments, time to LT, donor organ types (cadaver donors including cardiac death donors, living donors), histopathology of explanted livers, recurrence of HCC and patient death.
Radiological response was evaluated at 1–3 months after treatment using triphasic computed tomography (CT) or magnetic resonance imaging (MRI). Need for subsequent treatment was defined by consensus agreement among the treating physicians. Complete response was considered in the absence of residual tumour enhancement.
At the time of transplantation, the size, number and distribution of HCC lesions, as well as the presence of tumour vascular invasion and tumour differentiation (good, moderate, poor) were determined by experienced liver pathologists. In patients who had undergone LRT, the percentage of tumour necrosis was graded as complete (100%), partial (50–99%) or poor (0–49%).
Data and statistical analysis
These variables were compared in patients whose HCC fulfilled the MC, and who did and did not receive LRT, respectively.
For analysis of continuous variables in the two groups, Student's t-test or, if appropriate, non-parametric Wilcoxon rank sum test was used. For comparison among multiple groups, analysis of variance (anova) was used. Categorical variables were analysed using Pearson's chi-squared test or Fisher's exact test.
Survival analysis was performed to assess post-transplant survival in each group. Kaplan–Meier plots were constructed and log-rank tests were used to compare the groups. Post-transplant follow-up was defined as the time from transplant to either death or the last follow-up visit. A P-value of <0.05 was considered to indicate statistical significance. All analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Between January 2002 and December 2009, 288 patients diagnosed with HCC underwent LT at the Cleveland Clinic and University Hospitals. Six patients died as a result of postoperative complications within 1 month of transplantation. Follow-up at the time of this data evaluation was insufficient (<12 months) for seven patients. These 13 patients were excluded from the study. Of the remaining 275 patients, 225 (81.8%) were identified as having HCC within the MC at the initial radiological evaluation and were included in the study.
Demographics and baseline characteristics
The majority of the patients were male (n = 180, 80.0%). The mean ± standard deviation (SD) age of the patients was 57.8 ± 8.4 years. Hepatitis C was the most common aetiology of liver disease (62.2%). Fourteen 14 (6.2%) patients had liver disease of more than one aetiology. A total of 93 (41.3%) patients had received LRT prior to LT and 132 (58.7%) patients underwent LT without prior LRT. There were no significant differences in patient demographics and aetiology of liver disease between the study groups (Table 1). Mean ± SD follow-up time was 32.2 ± 28 months and mean ± SD waiting time to transplantation was 93.7 ± 124 days. Mean waiting time to transplant was similar in both groups (86.1 days vs. 99.1 days; P = 0.44). Median waiting time to transplant was 48.0 days [confidence interval (CI) 25%: 20.5, 75%: 110] in the entire study population and was similar in the two groups (54.0 days vs. 43.5 days; P = 0.44).
Table 1.
Demographic characteristics of patients
| All (n = 225) | Within Milan Criteria | P-value | ||
|---|---|---|---|---|
| LRT group (n = 93) | Non-LRT group (n = 132) | |||
| Age, years, mean ± SD | 57.8 ± 8.4 | 58.6 ± 9.4 | 57.2 ± 7.6 | 0.24 |
| Sex | ||||
| Male, n (%) | 180 (80.0) | 73 (78.5) | 107 (81.1) | 0.64 |
| Female, n (%) | 45 (20.0) | 20 (21.5) | 25 (18.9) | 0.64 |
| Diagnosis, n (%) | 0.28 | |||
| Hepatitis C | 140 (62.2) | 59 (63.4) | 81 (61.4) | |
| Alcohol | 36 (16.0) | 13 (14.0) | 23 (17.4) | |
| Non-alcoholic fatty liver disease | 24 (10.7) | 10 (10.8) | 14 (10.6) | |
| Hepatitis B | 16 (7.1) | 4 (4.3) | 12 (9.1) | |
| Others | 23 (10.2) | 9 (9.7) | 14 (10.6) | |
| More than one diagnosis | 14 (6.2) | 2 (2.2) | 12 (9.1) | |
| MELD score at listing (without exception points), mean ± SD | 13 ± 6.1 | 10.7 ± 3.6 | 14.5 ± 7 | <0.0001 |
| MELD score at transplant (without exception points), mean ± SD | 14.6 ± 6 | 12.6 ± 4.5 | 16 ± 6.6 | 0.052 |
| Transplant waiting time, days, mean ± SD | 93.7 ± 124 | 86.1 ± 121 | 99.1 ± 127 | 0.44 |
| Transplant waiting time, median, 25–75% CI | 48 (20.5–110) | 54 (29.5–87) | 43.5 (15–147.5) | 0.44 |
| Follow-up time, months, mean ± SD | 32.2 ± 28.2 | 30.1 ± 34.1 | 33.7 ± 23.1 | 0.38 |
LRT, loco-regional therapy; SD, standard deviation; MELD, Model for End-stage Liver Disease; CI, confidence interval.
MELD scores at listing without exception points were lower in the treated group (10.7 vs. 14.5; P < 0.0001). This difference was less noticeable at transplantation (12.6 vs. 16.0; P = 0.052).
The most frequent LRT modality was TACE, offered to 58 (62.4%) patients, followed by RFA in 28 (30.1%) patients. The other modalities offered were DEB in nine (9.7%) patients, bland embolization in two (2.2%) patients, and Y90 radioembolization in two (2.2%) patients. Six (6.5%) patients received a combination of therapies. Overall, 86 (92.5%) patients received only one session of therapy prior to LT and seven (7.5%) patients received more than one session, one of whom received four sessions of therapy prior to LT. The mean ± SD number of therapies prior to LT in the treated group was 1.2 ± 0.6.
Tumour characteristics by imaging and histopathology
Radiological diagnosis and staging revealed no significant difference between the groups in the mean number of liver lesions (1.6 vs. 1.3; P = 0.19). However, tumours was larger (2.8 cm vs. 2.2 cm; P = 0.002) in the group receiving LRT (Table 2). Similarly, evaluation of explanted livers showed that lesions were larger in the group undergoing LRT (3.3 cm vs. 2.6 cm; P = 0.001), but the mean number of lesions (2.2 vs. 1.8) did not differ significantly (P = 0.32).
Table 2.
Tumour characteristics
| All (n = 225) | Within Milan Criteria | P-value | ||
|---|---|---|---|---|
| LRT group (n = 93) | Non-LRT group (n = 132) | |||
| Pre-transplant tumour characteristics (imaging) | ||||
| Number of lesions, mean ± SD | 1.4 ± 1 | 1.6 ± 0.9 | 1.3 ± 1 | 0.19 |
| Size of the largest lesion, mean ± SD | 2.4 ± 1.3 | 2.8 ± 1.3 | 2.2 ± 1.2 | 0.002 |
| Single lesions, n (%) | 108 (48.0) | 37 (40.0) | 71 (66.3) | 0.28 |
| Post-transplant tumour characteristics (on explant) | ||||
| Number of lesions, mean ± SD | 2 ± 2.4 | 2.2 ± 3.5 | 1.8 ± 1.5 | 0.32 |
| Size of the largest lesion, mean ± SD | 2.9 ± 1.5 | 3.3 ± 1.8 | 2.6 ± 1.2 | 0.001 |
| Single lesions, n (%) | 118 (52.4) | 43 (46.2) | 75 (56.8) | 0.29 |
| Margin invasion presence, n (%) | 4 (1.8) | 2 (2.2) | 2 (1.5) | 0.09 |
| Vascular invasion presence, n (%) | 56 (24.9) | 20 (21.5) | 36 (27.3) | 0.36 |
| Well differentiated tumour, n (%) | 42 (18.7) | 9 (11.8) | 31 (23.4) | 0.001 |
| Pathology beyond MC, n (%) | 52 (23.1) | 24 (25.8) | 28 (21.2) | 0.30 |
SD, standard deviation; MC, Milan Criteria.
There was no difference between the groups in the presence of vascular invasion (21.5% vs. 27.3%; P = 0.36). There was less representation of well-differentiated tumours (11.8% vs. 23.4%; P = 0.001) in the group that underwent LRT (Table 2).
A total of 52 (23.1%) patients in whom HCC was staged by imaging as MC-compliant were re-staged as outside the MC on examination of explanted livers. This occurred with equal frequency in both groups (Table 2). In these patients, the mean ± SD number of lesions was higher (1.8 ± 1.2 vs. 1.3 ± 0.9; P = 0.03), tumour diameter was greater (3.2 ± 1.3 vs. 2.1 ± 1.1 cm; P≤ 0.001) and vascular invasion was more frequent (53.8% vs. 18.0%; P = 0.0002) than in the group in which HCC was not re-staged as non-compliant with the MC on explant evaluation.
There were no differences in levels of tumour markers [alpha-fetoprotein (AFP)] prior to LT between the groups (data not shown). There was no difference in the source of organ donors between the groups.
Survival analysis
Overall survival at 1 year, 3 years and 5 years in the whole study population was 93.1%, 82.4% and 72.6%, respectively. Disease-free survival in the entire cohort at 1 year, 3 years and 5 years was 91.3%, 79.3% and 70.6%, respectively. Analysis of overall survival at 1 year, 3 years and 5 years in the groups receiving LRT (93.5%, 84.4% and 64.9%, respectively) and not receiving LRT (92.9%, 81.4% and 76.2%, respectively) revealed no statistical difference (P = 0.94) (Fig. 1). Similarly, analysis of disease-free survival at 1 year, 3 years and 5 years in the treated (90.7%, 81.8% and 64.2%, respectively) and non-treated (91.3%, 78.2% and 74.3%, respectively) groups showed no statistical difference (P = 0.94) (Fig. 2).
Figure 1.
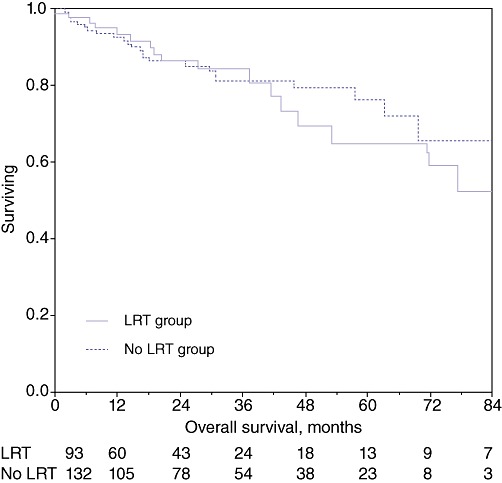
Kaplan–Meir survival curves: overall survival in the treated and non-treated groups (P = 0.94). LRT, loco-regional therapy
Figure 2.
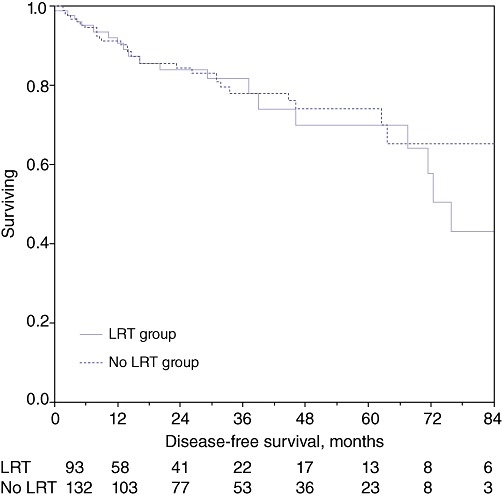
Kaplan–Meir survival curves: disease-free survival in the treated and non-treated groups (P = 0.94). LRT, loco-regional therapy
Accordingly, the treated and non-treated groups showed similar rates of tumour recurrence (11.8% vs. 9.8%; P = 0.64) and mortality (19.4% vs. 18.2%; P = 0.82) during the mean follow-up period of 32.2 months.
Subgroup analysis based on treatment response
In the LRT group, in patients who demonstrated a complete radiological response, 1-, 3- and 5-year overall survival rates (90.0%, 85.9% and 73.6%, respectively) and 1-, 3- and 5-year recurrence-free survival rates (90.0%, 79.7% and 66.4%, respectively) were comparable with rates of overall survival (P = 0.79) and recurrence-free survival (P = 0.85) in patients who did not demonstrate a complete radiological response. No patient or tumour characteristics were indicated as significantly predicting a higher likelihood of achieving a complete radiological response following LRT (data not shown).
Analysis of data for 16 patients who achieved complete tumour necrosis in the explanted liver showed better 1-, 3- and 5-year overall survival (100%, 100% and 100%, respectively) and 1-, 3- and 5-year recurrence-free survival (100%, 100% and 100%, respectively) than patients who did not achieve complete necrosis among the treated group of 93 patients (P = 0.07 and P = 0.09, respectively). However, no patient or tumour characteristics were significantly associated with a higher likelihood of achieving complete tumour necrosis following LRT (Table 3).
Table 3.
Analysis of patients by pathological response following loco-regional therapy (LRT)
| Complete response (n = 16, 17.2%) | Others (n = 77, 82.8%) | P-value | |
|---|---|---|---|
| Diagnosis of hepatitis C, n (%) | 11 (68.8) | 48 (62.3) | 0.65 |
| Number of lesions, mean ± SD | 1 ± 0.8 | 1.7 ± 0.9 | 0.01 |
| Largest lesion size, cm, mean ± SD | 2.7 ± 1.5 | 2.8 ± 1.3 | 0.80 |
| Total tumour diameter, cm, mean ± SD | 3.6 ± 1.1 | 3.9 ± 2.1 | 0.77 |
| Waiting time from LRT, days, mean ± SD | 38 ± 42.4 | 61.4 ± 27 | 0.43 |
| Waiting time from LRT, days, median (25–75% CI) | 38 (8–68) | 59 (42–83) | 0.43 |
| Number of neoadjuvant therapies, mean ± SD | 1.2 ± 0.4 | 1.1 ± 0.7 | 0.34 |
| Waiting time, days, mean ± SD | 118 ± 130 | 79.8 ± 119 | 0.06 |
| Waiting time, days, median (25–75% CI) | 72.5 (47–133.5) | 50 (27.3–86) | 0.06 |
| Differentiation: well, n (%) | 2 (12.5) | 9 (11.7) | <0.001 |
| Overall survival, months, mean ± SD | 41.4 ± 43 | 27.6 ± 31 | 0.27 |
| Disease-free survival, months, mean ± SD | 38.1 ± 38 | 26.2 ± 31.5 | 0.24 |
SD, standard deviation; CI, confidence interval.
There was no significant difference between patients in whom explanted tumours were found to have been radiologically understaged and those in whom explanted tumours were found to have been accurately staged in overall survival (P = 0.68) or disease-free survival (P = 0.74). Among patients who were radiologically understaged, there were no differences in overall 1-, 3- and 5-year survival between the LRT group (100%, 80.8% and 41.0%, respectively) and the non-LRT group (88.8%, 75.5% and 62.9%, respectively) (P = 0.75). Similarly, no statistical difference in 1-, 3- and 5-year disease-free survival emerged between the LRT group (89.6%, 69.3% and 31.7%, respectively) and the non-LRT group (81.0%, 70.5% and 47.0%, respectively) (P = 0.99).
To elucidate whether LRT would benefit any particular group of patients with HCC within the MC, outcomes were analysed for three subgroups of patients according to whether they demonstrated a single lesion measuring <3 cm (n = 129), a single lesion measuring 3–5 cm (n = 28) or multiple lesions (n = 68). There was no difference (P = 0.61) in 1-year (95.4% vs. 96.1%), 3-year (90.2% vs. 85.0%) or 5-year (77.3% vs. 80.0%) overall survival between patients receiving or not receiving LRT in the group with single lesions measuring <3 cm. Similarly, there was no difference (P = 0.48) in 1-year (92.3% vs. 92.3%), 3-year (84.6% vs. 92.3%) or 5-year (67.3% vs. 92.3%) overall survival between patients receiving or not receiving LRT in the group with single HCC lesions measuring 3–5 cm. There was no difference (P = 0.51) in 1-year (90.3% vs. 82.9%), 3-year (78.1% vs. 69.6%) or 5-year (55.9% vs. 62.6%) overall survival between patients receiving or not receiving LRT in the group with multifocal lesions. Comparisons of disease-free survival in patients treated and not treated with LRT showed no difference (P = 0.41) among patients with single HCC lesions measuring <3 cm in 1-year (95.4% vs. 94.7%), 3-year (77.7% vs. 79%) or 5-year (62.8% vs. 76%) survival, no differences (P = 0.28) among patients with single lesions measuring 3–5 cm in 1-year (84.6% vs. 92.3%), 3-year (76.2% vs. 92.3%) or 5-year (53.8% vs. 82.3) survival (Fig. 3), and no differences (P = 0.77) among patients with multifocal lesions in 1-year (80.0% vs. 80.1%), 3-year (69.9% vs. 68.5%) or 5-year (63.0% vs. 60.0%) survival (Fig. 4).
Figure 3.
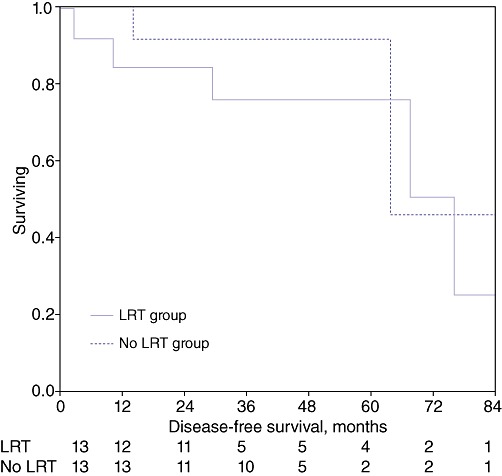
Kaplan–Meir survival curves: disease-free survival in patients with single hepatocellular carcinoma lesions measuring 3–5 cm in the treated and non-treated groups (P = 0.28). LRT, loco-regional therapy
Figure 4.

Kaplan–Meir survival curves: disease-free survival in multifocal hepatocellular carcinoma lesions in the treated and non-treated groups (P = 0.77). LRT, loco-regional therapy
Discussion
Although pre-transplant LRT is widely accepted as able to downstage HCC to meet MC requirements, or to treat MC-compliant HCC if the expected waitlist time is >6 months, its apparent lack of benefit does not indicate its application in MC HCC patients with short waitlist times. Although no large RCTs have compared outcomes in pre-transplantation treated vs. non-treated MC HCC patients, small single-centre studies indicate that there is no difference in post-transplant patient survival or tumour recurrence rate in the setting of short waitlist time.14
Although it would appear that treating small tumours with a high chance of inducing total tumour necrosis followed by rapid transplantation (within 3 months of LRT) may set a basis for cure, 8–12% of patients with MC HCC will develop post-transplant tumour recurrence independently of pre-transplant management.17 In the absence of accurate biomarkers indicating residual disease, this is considered likely to relate to the lack of acknowledgement of micro-metastasis at the time of treatment.
In this retrospective study, data for 225 HCC patients with MC-compliant disease (out of 275 HCC patients) who underwent LT were analysed. Patient outcomes and histopathologic tumour characteristics were compared in 93 patients who received neoadjuvant LRT and 132 patients who did not. The median waitlist time prior to transplantation in this population was short, at 48.0 days, and close to the median waitlist time reported across United Network for Organ Sharing (UNOS) regions during the post-MELD era until 2006.18 It is also of note that among all UNOS regions, Region 10, in which the centres in which the present study was conducted are located, reported the shortest median waiting time (2.7 weeks) for HCC patients who fulfilled MC requirements during the post-MELD era until 2009.19 The median waiting period in this region is also considerably shorter than the 6-month period recommended by the American Association for the Study of Liver Diseases (AASLD) guidelines1 for the application of neoadjuvant LRT in patients with MC HCC.
In the present study population, MELD scores at listing were lower in the LRT group, but the difference was less noticeable at transplantation. The better tolerance of LRT of patients with more preserved liver function explains this disparity, which has also been reported in other studies.14 Most of the 93 patients to whom LRT was delivered received a once-only treatment and only seven (7.5%) patients received more than one session of therapy (mean: 1.2 sessions). The most frequently used modalities were TACE (62.4%), RFA (30.1%) and DEB (9.7%).
In the present study, the HCC recurrence rate over a mean follow-up of 32.2 months was 10.7%, and 1-, 3- and 5-year disease-free survival rates were 91.1%, 79.3% and 70.6%, respectively (Fig. 2). These findings are similar to historical data.3,20,21 No differences in patient survival or tumour recurrence rate emerged between the groups who did and did not receive LRT. This finding concords with results reported by the only other similar study to be published,14 which compared outcomes in 31 patients who received pre-transplant LRT with those in 33 patients undergoing transplantation without previous LRT. Among the 31 patients who received LRT, 23 were listed in the MELD era and their mean ± SD waiting time was 54 ± 61 days. This study found similar survival rates in the treated and untreated groups of 87.5% for overall survival and 71–75% for disease-free survival, over a follow-up of 36 months.
In the present study, tumours that had been staged as within the MC by imaging using triphasic CT or MRI were found to be understaged according to the analysis of explanted specimens in 23.1% of patients (Table 2). Similar rates (20.5–43.0%) of understaging by radiology have been documented by others.11–13 Analysis showed significant increases in the mean number of lesions (1.8 vs. 1.3; P = 0.03) and size of lesions (3.2 cm vs. 2.1 cm; P≤ 0.001) in this group of patients compared with those who were correctly staged.
Given that the MC cover a diverse range of HCC tumours, a subgroup analysis was performed in an effort to elucidate whether outcomes would differ among groups according to the number or size of tumours (single lesions of <3 cm, single lesions of 3–5 cm, multifocal disease). No disparity in patient survival or tumour recurrence was noticed in the groups with, respectively, single lesions of <3 cm (overall survival, P = 0.61; disease-free survival, P = 0.41), single lesions of 3–5 cm (overall survival, P = 0.48; disease-free survival, P = 0.28) (Fig. 3), or multifocal disease (overall survival, P = 0.51; disease-free survival, P = 0.77) (Fig. 4).
The occurrence of tumour necrosis of ≥60% has been associated with increased disease-free survival.22,23 In the present study, pathology analyses indicated that 16 (17.2%) patients achieved complete tumour necrosis induced by pre-transplant LRT and exhibited a 5-year recurrence-free survival rate of 100%. Given that the ideal goal of LRT is to achieve total tumour necrosis, it appears that current technologies are still evolving and that a minimal number of patients are treated completely.
Accordingly, and in view of the lack of accurate biomarkers that facilitate the recognition of patients who will develop post-transplant tumour recurrence, time has come to represent a diagnostic tool to unveil tumour behaviour. Yao et al.4 showed that when HCC patients in whom disease characteristics initially exceeded MC requirements were downstaged and remained within the MC without evidence of tumour progression over a period of time (≥3 months), they achieved post-transplant outcomes similar to those of patients whose disease initially fell within MC requirements.4 In an effort to reduce current recurrence rates in MC HCC patients, Roberts et al.15 proposed the application of an ‘ablating and waiting’ protocol in this patient population and raised concerns about rapid transplantation (within 3 months following LRT) as applied in some centres with short waitlist times. An RCT comparing post-transplant outcomes in patients who do not show disease progression after LRT during an observation period of 3–6 months with outcomes in patients who do develop disease progression would be worth considering. The rationale behind this initiative is solid and relates to the current scarcity of organs and the need to optimize organ utilization and patient selection.
The present study has some drawbacks. It is retrospective in nature and was performed at two different transplant centres using a population treated over a period of 7 years. The treating physicians involved have been multiple, and LRT modalities, imaging technologies and ways of interpreting tumour response to therapy have changed or varied. The majority of the study patients (57.3%) across both groups had small tumours, a characteristic that can be considered as a surrogate for favourable biologic behaviour, and hence the significance of LRT may have been minimized. This possibility is again supported by the fact that a relatively higher number of untreated than treated patients had well differentiated tumours. However, the study's significance is supported by the large number of patients involved, which makes it one of the largest retrospective studies of its nature.
In conclusion, based on this and other studies, LRT followed by rapid transplantation in MC HCC patients does not appear to impact on post-transplant outcome and its indication is without solid foundation. Indications for LRT in selected patients with single lesions of 3–5 cm or multifocal disease also remain questionable, but further studies are required to investigate outcomes in these subgroups. Further strategies to identify the 10% of patients who will develop tumour recurrence must be sought. The role of LRT in the setting discussed in this study may change in light of future evolutions in technology and the search for accurate biomarkers of disease behaviour.
Conflicts of interest
None declared.
References
- 1.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan Criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Yao FY, Hirose R, LaBerge JM, Davern TJ, 3rd, Bass NM, Kerlan RK, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Kerlan RK, Jr, Hirose R, Davern TJ, 3rd, Bass NM, Feng S, et al. Excellent outcome following downstaging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barakat O, Wood RP, Ozaki CF, Ankoma-Sey V, Galati J, Skolkin M, et al. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl. 2010;16:289–299. doi: 10.1002/lt.21994. [DOI] [PubMed] [Google Scholar]
- 7.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–509. doi: 10.1097/SLA.0b013e318148c704. discussion 509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitale A, Boccagni P, Brolese A, Neri D, Srsen N, Zanus G, et al. Progression of hepatocellular carcinoma before liver transplantation: dropout or liver transplantation? Transplant Proc. 2009;41:1264–1267. doi: 10.1016/j.transproceed.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 10.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R, et al. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 11.Chao SD, Roberts JP, Farr M, Yao FY. Short waitlist time does not adversely impact outcome following liver transplantation for hepatocellular carcinoma. Am J Transplant. 2007;7:1594–1600. doi: 10.1111/j.1600-6143.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 12.Mejia GA, Gomez MA, Serrano J, Garcia I, Tamayo MJ, Pareja F, et al. Correlation between the radiologic and histologic size of hepatocellular carcinoma in patients eligible for liver transplantation. Transplant Proc. 2006;38:1394–1395. doi: 10.1016/j.transproceed.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Shah SA, Tan JC, McGilvray ID, Cattral MS, Cleary SP, Levy GA, et al. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2006;81:1633–1639. doi: 10.1097/01.tp.0000226069.66819.7e. [DOI] [PubMed] [Google Scholar]
- 14.Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–673. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl. 2010;16:925–929. doi: 10.1002/lt.22103. [DOI] [PubMed] [Google Scholar]
- 16.Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol. 2010;105:610–612. doi: 10.1038/ajg.2009.663. [DOI] [PubMed] [Google Scholar]
- 17.Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- 19.Kadry Z, Schaefer EW, Uemura T, Shah AR, Schreibman I, Riley TR. Impact of geographic disparity on liver allocation for hepatocellular cancer in the United States. J Hepatol. 2012;56:618–625. doi: 10.1016/j.jhep.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Decaens T, Roudot-Thoraval F, Hadni-Bresson S, Meyer C, Gugenheim J, Durand F, et al. Impact of UCSF criteria according to pre- and post-OLT tumour features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006;12:1761–1769. doi: 10.1002/lt.20884. [DOI] [PubMed] [Google Scholar]
- 21.Lee FT., Jr Treatment of hepatocellular carcinoma in cirrhosis: locoregional therapies for bridging to liver transplant. Liver Transpl. 2007;13(Suppl. 2):24–26. doi: 10.1002/lt.21327. [DOI] [PubMed] [Google Scholar]
- 22.Chan KM, Yu MC, Chou HS, Wu TJ, Lee CF, Lee WC. Significance of tumour necrosis for outcome of patients with hepatocellular carcinoma receiving locoregional therapy prior to liver transplantation. Ann Surg Oncol. 2011;18:2638–2646. doi: 10.1245/s10434-011-1779-z. [DOI] [PubMed] [Google Scholar]
- 23.Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]


