Abstract
Background
Chemotherapy has in some series been linked with increased morbidity after a hepatectomy. Hepatic injuries may result from the treatment with chemotherapy, but can also be secondary to co-morbid diseases. The aim of the present study was to draw correlations between clinical features, treatment with chemotherapy and injury phenotypes and assess the impact of each upon perioperative morbidity.
Patients and methods
Retrospective samples (n = 232) were scored grading steatosis, steatohepatitis and sinusoidal injury (SI). Clinical data were retrieved from medical records. Correlations were drawn between injury, clinical features and perioperative morbidity.
Results
Injury rates were 18%, 4% and 19% for steatosis, steatohepatitis and SI, respectively. High-grade steatosis was more common in patients with diabetes [odds ratio (OR) = 3.33, P = 0.01] and patients with a higher weight (OR/kg = 1.04, P = 0.02). Steatohepatitis was increased with metabolic syndrome (OR = 5.88, P = 0.02). Chemotherapy overall demonstrated a trend towards an approximately doubled risk of high-grade steatosis and steatohepatitis although not affecting SI. However, pre-operative chemotherapy was associated with an increased SI (OR = 2.18, P = 0.05). Operative morbidity was not increased with chemotherapy, but was increased with steatosis (OR = 2.38, P = 0.02).
Conclusions
Diabetes and higher weight significantly increased the risk of steatosis, whereas metabolic syndrome significantly increased risk of steatohepatitis. The presence of high-grade steatosis increases perioperative morbidity, not administration of chemotherapy per se.
Keywords: colorectal metastases < liver, chemotherapy < liver, colorectal carcinoma, chemotherapy, hepatectomy, perioperative morbidity
Introduction
Chemotherapy is being used with increasing frequency early in the treatment of patients with colorectal liver metastases (CRLM), even when these lesions are resectable.1 In addition to improved progression-free survival after a subsequent hepatectomy,2 this treatment increases the potential for perioperative morbidity and appears to be associated with a number of injury phenotypes now collectively referred to as chemotherapy-induced hepatic injuries (CIHI).3 CIHI is divided into two main groups: (i) chemotherapy-associated fatty liver diseases [the spectrum of which includes chemotherapy-associated simple steatosis (CASS) and chemotherapy-associated steatohepatitis (CASH)] and (ii) sinusoidal injuries [including sinusoidal dilation and congestion, sinusoidal obstruction syndrome, haemorrhagic centrilobular necrosis (HCN) and nodular regenerative hyperplasia (NRH)].
Controversy exists as to the effect of chemotherapy on peri-operative morbidity.4,5 Chemotherapy administration has not always been demonstrated as an independent risk factor for the development of post-operative complications, particularly in countries with higher background rates of obesity (and subsequent hepatic steatosis).4 On the other hand, CASH has been clearly associated with an increased perioperative mortality of 14.1% compared with 1.6% in those without steatohepatitis and is seen with increased frequency in association with irinotecan.6
Chemotherapy regimens for adjuvant and neoadjuvant treatment of colorectal carcinoma and CRLM consist of 5-flurouracil (5-FU) in association with oxaliplatin (FOLFOX or XELOX if capecitabine, the oral pro-drug of 5FU, is chosen) or 5-FU with irinotecan (FOLFIRI). 5-FU does not appear to be specifically associated with any injury phenotype other than CASS (with which oxaliplatin and irinotecan are also non-specifically associated).7
Oxaliplatin is associated with the development of sinusoidal injuries.8 This injury phenotype is associated with an increased rate of blood transfusion perioperatively,9 and increased morbidity in general.10
Non-alcoholic fatty liver diseases (NAFLD) in general are common, and phenotypically there is nothing differentiating CASH from non-alcoholic steatohepatitis (NASH), or differentiating CASS from simple steatosis secondary to obesity or diabetes, other than the association with administration of chemotherapeutics. This confounds attempts to ascribe risk to these chemotherapeutics specifically in terms of injury phenotype causation. It is known that factors such as diabetes and obesity increase rates of injuries such as steatosis.11 However, whether chemotherapy exerts an independent additive effect remains to be definitively demonstrated.
It is likely that the increased perioperative morbidity that some have found in association with chemotherapy5 is actually secondary to a hepatic injury and not the chemotherapy as such. Additionally, the administration of chemotherapy that does not result in injury development may not be associated with higher rates of complications. These possibilities remain unproven.
In the present study, patient co-morbidity profile and chemotherapy administration are examined to separate out the component effects of each of these factors in the development of hepatic injury as seen histologically and to determine the effect of both chemotherapy in general and CIHI, specifically, on post-operative outcomes.
Patients and methods
Patients
Patients who had undergone a hepatectomy for any pathology between September 2001 and November 2009 were identified from the Victorian Cancer Biobank, Melbourne, Australia, a prospectively collected tissue bank. Additional patients who had undergone a hepatectomy for CRLM at The Alfred Hospital, Melbourne between May 1995 and September 2009 were identified from a database detailing these patient's clinical and operative outcomes. Clinical data relating to demographics, co-morbidities, details of primary colorectal disease, chemotherapy administration (adjuvant after colorectal primary, and any treatment for metastatic disease), as well as details of hepatic surgery including post-operative course, were collected where available from previously collated databases, or retrieved from review of the clinical history of identified patients.
Histological assessment
Haematoxylin and Eosin (H&E) slides of non-tumourous liver were available for all patients, and Masson's trichrome stains were generated from formalin-fixed paraffin-embedded tissue blocks containing non-tumourous liver from either the tissue bank specimens or pathology archive. Both H&E and Masson's slides were independently and blindly scored for degree of steatosis (high grade steatosis was defined as >33% fatty replacement), non-alcoholic fatty liver disease activity score (NAS),12 portal fibrosis score, degree of sinusoidal dilation (high grade defined as >1/3 of lobule involved)8 and presence or absence of four other features of sinusoidal injury (fibrotic venular occlusion, extravasated red blood cells, HCN and NRH). Steatohepatitis was diagnosed when steatosis was present at >5%, and the NAS was >2. Batches of 25 slides were independently scored by two pathologists, one from the Peter MacCallum Cancer Centre (W.M.), the other from the The Alfred Hospital (A.P.), with one observer present during all scoring (C.P.). A random sample of 25% of slides was independently and blindly scored twice by the same pathologist to confirm intra-observer consistency. Inter-observer consistency was also assessed and, where injury scores from each pathologist were two or more points discrepant, consensus was achieved by slide review with both pathologists and observer following collation of all data. American Association for the Study of Liver Diseases grade and stage13 were determined for all slides designated as NASH.
Statistical analysis
Associations between injury types were assessed using Fisher's exact tests. Hierarchical logistic regression models were used to assess the association of collected characteristics of samples with the development of high-grade injury and perioperative morbidity among all operations. These models tested the fixed effects of the characteristics and accounted for effects of the patient unit by adding patient as a random effect. Univariate and multiple variable models were estimated using the PROC GLIMMIX command in SAS (v9.2; SAS Institute Inc., Cary, NC, USA) using the method ‘between/within’ for computing the denominator degrees of freedom for tests of fixed effects. Spearman's correlation coefficient was used to quantify the agreement in injury grade between the reviewers.
Results
There were 221 patients, providing 232 samples (12 patients having had 2 separate hepatic operations for recurrent CRLM in that time period). More than half the patients (123/221) came from the Alfred Hospital (with 50 patients contributed in the last 5 years of the study period), with the remainder from the Victorian Cancer Biobank.
The median age of the cohort was 62 years (range 22–86) and 36% were female. From 232 samples, high-grade steatosis was found in 18%, high-grade sinusoidal dilation in 19% and high-grade steatohepatitis in 4% (Table 1). There was no association between high-grade steatosis and steatohepatitis (Fisher's P-value = 0.07).
Table 1.
Prevalence of hepatic injury in a cohort of 232 hepatic resection samples
| Injury | Definition | Per cent (n) |
|---|---|---|
| Steatosis | ||
| Grade 0 | 0% | 24% (56) |
| 1 | >0–5% | 31% (73) |
| 2 | >5–33% | 27% (63) |
| 3 | >33–66% | 12% (27) |
| 4 | >66% | 6% (14) |
| Sinusoidal injury | ||
| Grade 0 | Nil | 70% (163) |
| 1 | up to 1/3 lobular involvement | 11% (26) |
| 2 | >1/3–2/3 lobular involvement | 10% (23) |
| 3 | >2/3 to complete lobular involvement | 9% (21) |
| Steatohepatitis | ||
| Presence | steatosis >5%, NAFLD activity scorea >2 | 4% (10) |
NAFLD activity score (NAS) = sum of score for Mallory bodies, lobular inflammation, hepatocyte ballooning and perisinusoidal fibrosis (each factor scored 0 = absent, 1 = focal involvement of some lobules, 2 = focal involvement of most lobules, 3 = focal involvement of most or all, with diffuse involvement of some or most).12
There was good inter- and intra-observer correlations on most of the elements scored for hepatic injury (Table 2). Spearman's correlation co-efficients for steatosis were r = 0.84 (P < 0.0001) and r = 0.92 (P < 0.0001) for inter- and intra-observer scores, respectively. In particular, there were only four cases (2%) of steatosis scoring where the two pathologists differed by more than one point in terms of degree of steatosis. There was 100% agreement within one point when the same pathologist scored steatosis at two separate time points, and in 60 cases (97%) exactly the same score was awarded on both occasions.
Table 2.
Inter- and intra-observer variation in scoring of hepatic injuries in a cohort of 232 hepatic resection samples
| Parameter | Pathologist A.P. vs. pathologist W.M. | Pathologist A.P. 1st score vs. 2nd score | ||
|---|---|---|---|---|
| n | Spearman's correlation co-efficient (P-value) | n | Spearman's correlation co-efficient (P-value) | |
| Steatosis | 232 | 0.84 (<0.0001) | 62 | 0.92 (<0.0001) |
| Steatohepatitis | ||||
| Portal fibrosis | 226 | 0.39 (<0.0001) | 62 | 0.55 (<0.0001) |
| Perisinusoidal fibrosis | 226 | 0.41 (<0.0001) | 62 | 0.74 (<0.0001) |
| Lobular inflammation | 232 | 0.39 (<0.0001) | 62 | 0.56 (<0.0001) |
| Mallory bodies | 232 | −0.02 (0.82) | 62 | 1.0 (<0.0001) |
| Hepatocyte ballooning | 232 | −0.10 (0.13) | 62 | 0.33 (0.009) |
| Sinusoidal injury (SI) | 232 | 0.53 (<0.0001) | 62 | 0.52 (<0.0001) |
| Other SI features | ||||
| NRH | 232 | 0.28 (<0.0001) | 62 | 0.46 (<0.0001) |
| Extravasated RBC | 232 | 0.3 (<0.0001) | 62 | (not estimated) |
| HCN | 232 | −0.02 (0.79) | 62 | 1.0 (<0.0001) |
SI, sinusoidal injury; NRH, nodular regenerative hyperplasia; RBC, red blood cells; HCN, haemorrhagic centrilobular necrosis.
Agreement upon diagnosis of steatohepatitis was more problematic. The absence of steatohepatitis was agreed upon in 95% of cases (212 of the 223 cases without steatohepatitis), but the presence of the disease was only initially independently agreed upon in 50% of cases (5 of the 10 cases).
Spearman's correlation co-efficient for portal fibrosis, perisinusoidal fibrosis and lobular inflammation showed agreement that was statistically significantly greater than would be expected by chance (r = 0.39, 0.41, 0.39, respectively); however, Mallory bodies and hepatocyte ballooning scoring only agreed at levels expected by chance (r=−0.02, −0.10, respectively) (Table 2).
Regarding sinusoidal injury, there was lack of inter-observer agreement in 14% of cases (32 cases where the score was more than 1 point different); however, Spearman's correlation co-efficient for both inter- and intra-observer variation was consistent with agreement more than expected by chance (Table 2).
Diabetes was associated with a higher rate of high-grade steatosis [odds ratio (OR) = 3.24, 95% confidence interval (CI) 1.36–7.71, P = 0.01] and a lower rate of high-grade sinusoidal injury (OR = 0.14, 95% CI 0.02–0.99, P = 0.02). Similarly, a body mass index (BMI) >30 kg/m2 was associated with high-grade steatosis (OR = 3.14, 95% CI 1.10–8.96, P = 0.05), and for each additional kilogram there was an increase of 4% in the likelihood of high-grade steatosis. Metabolic syndrome (a clinical syndrome characterized by at least three features from hypertension, hypertriglyceridaemia, low level high-density lipoprotein (HDL), hyperglycaemia or central adiposity14) was associated with steatohepatitis (OR = 5.88, 95% CI 1.36–25.00, P = 0.02). Male gender and hyper-cholesterolaemia were also associated with high-grade steatosis (OR for male gender = 2.77, 95% CI 1.16–6.62, P = 0.03; hypercholesterolaemia OR = 2.48, 95% CI 1.15–5.36, P = 0.03). There were no other associations between patient co-morbidity and injury phenotype.
Of those samples having exposure to chemotherapy (60.7% of cohort, n = 133), one-third of the patients had received treatment within 2 months of hepatic resection (n = 46) and 7.5% of the patients had been treated with chemotherapy twice, first for the colorectal primary, then again for CRLM (n = 10). Given the retrospective nature of the cohort, the data relating to duration of treatment with chemotherapy was poor. Just fewer than half the cohort had available data in this regard (49.6%). The average number of cycles of chemotherapy administered for treatment of the colorectal primary was 8.0 (median 6.0, range 1–26), and for hepatic metastases prior to hepatectomy, the average and median was 6.0 cycles (range 1–12). Analysis of injury type according to cycle number was not undertaken given the large gaps in these data.
The rates of steatosis and steatohepatitis were higher in those having any chemotherapy compared with those never exposed at 20% vs. 12% and 5% vs. 2%, respectively, although these differences did not reach statistical significance (Table 3). The rate of sinusoidal injury was not higher overall in patients treated with any chemotherapy, however, pre-operative treatment was associated with an increased risk of high-grade sinusoidal injury compared with patients who did not receive chemotherapy pre-operatively (OR = 2.18, 95% CI 1.01–4.74, P = 0.05).
Table 3.
Chemotherapy exposure and rate of injury among 218 patients undergoing hepatic resection
| Chemotherapy exposure | Number of samples | Steatosis (>33%) | Steatohepatitis | Sinusoidal injury (>1/3 lobule) |
|---|---|---|---|---|
| Never | 85 | 12% [6%–21%]a | 2% [0%–8%]b | 19% [11%–29%] |
| Any | 133 | 20% [14%–28%]a | 5% [2%–11%]b | 19% [13%–27%] |
| Preoperatively | 46 | 20% [9%–34%] | 4% [1%–15%] | 33% [20%–48%]c |
| Once only (but not preoperatively) | 56 | 20% [10%–33%] | 9% [3%–20%] | 15% [6%–27%]c |
Odds ratio (OR) = 1.90 [95% confidence interval (CI) 0.87–4.17, P = 0.14].
OR = 2.30 (95% CI 0.47–11.33, P = 0.49).
OR = 2.18 (95% CI 1.01–4.74, P = 0.05).
When controlling for diabetes, a BMI >30 kg/m2 or metabolic syndrome, the development of steatosis still has an OR of approximately 2.0 with administration of chemotherapy, although because of the small sample size, the CIs cross unity (Fig. 1). This suggests the effect of chemotherapy upon development of injury is independent of the effect these co-morbidities have in increasing the rate of injury. That is, chemotherapy is a factor increasing the rate of steatosis independent of co-morbidities which also increase the rate of steatosis.
Figure 1.
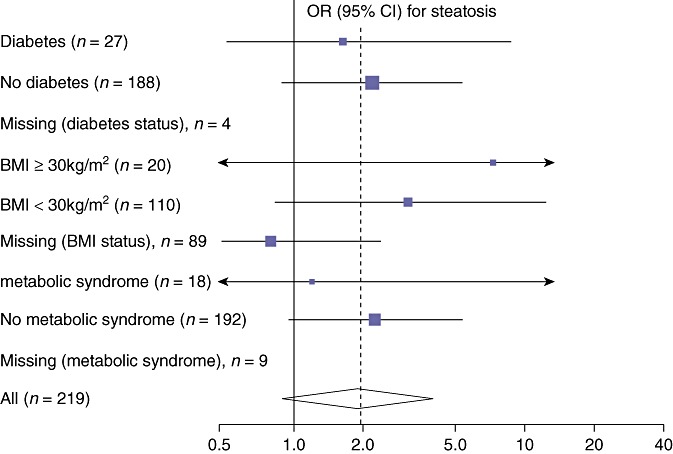
Odds ratio (OR) for developing steatosis when chemotherapy is administered in the presence or absence of diabetes, elevated body mass index (BMI) or metabolic syndrome. The effect of chemotherapy can be seen to be independent of the co-morbidity. For all patients, the OR for steatosis when administered chemotherapy is displayed as the diamond at the bottom of the diagram
There were 331 discrete operative procedures carried out during 230 operations for which data are available (some patients have multiple procedures such as resection of multiple separate segments of liver during one operation). This includes 21 repeat hepatectomies (of which 12 patients have tissue available for analysis). There was no surgical data for 11 patients for whom tissue was available. Portal vein embolization was employed pre-operatively in two patients.
Of these procedures, 139 (60.4%) were considered major hepatic resections (removing three or more hepatic segments) and the remaining 91 were minor hepatic resections (Table 4). The distribution of hepatic injuries between major and minor resections was equivalent. Perioperative morbidity was higher in patients undergoing a major resection (41% vs. 28%, Table 5), although this failed to reach statistical significance (Fig. 2).
Table 4.
Operative intervention in cohort
| Procedure | % (n) |
|---|---|
| Right hepatectomy | 23.3 (77) |
| Extended right hepatectomy | 7.6 (25) |
| Right posterior sectionectomy | 2.4 (8) |
| Central heptatectomy | 0.6 (2) |
| Left hepatectomy | 5.1 (17) |
| Extended left hepatectomy | 1.5 (5) |
| Left lateral sectionectomy | 9.7 (32) |
| Formal segmentectomy | 14.5 (48) |
| Wedge resection | 32.0 (106) |
| Intra-operative hepatic ablation | 3.3 (11) |
Table 5.
Rate of perioperative morbidity according to the presence or absence of hepatic injury and chemotherapy exposure
| Parameter | n | Rate of morbidity when present | Rate of morbidity when absent | OR for morbidity (95% CI) | P-value |
|---|---|---|---|---|---|
| High-grade steatosis | 223 | 51 % (20/39) | 30% (37/125) | 2.50 (1.20–5.23) | 0.02 |
| High-grade steatohepatitis | 223 | 50% | 35% | 1.85 (0.50–6.67) | NS |
| High-grade sinusoidal injury | 223 | 32% | 36% | 0.82 (0.39–1.85) | NS |
| Major vs. minor resection | 221 | 41% | 28% | 1.78 (0.99–3.19) | NS |
| Chemotherapy | 214 | 37% | 38% | 1.11 (0.62–2.00) | NS |
| Oxaliplatin | 169 | 41% | 35% | 1.32 (0.63–2.78) | NS |
| 5-Flurouracil | 173 | 40% | 35% | 1.28 (0.68–2.44) | NS |
OR, odds ratio; CI, confidence interval; NS, non-significant.
Figure 2.
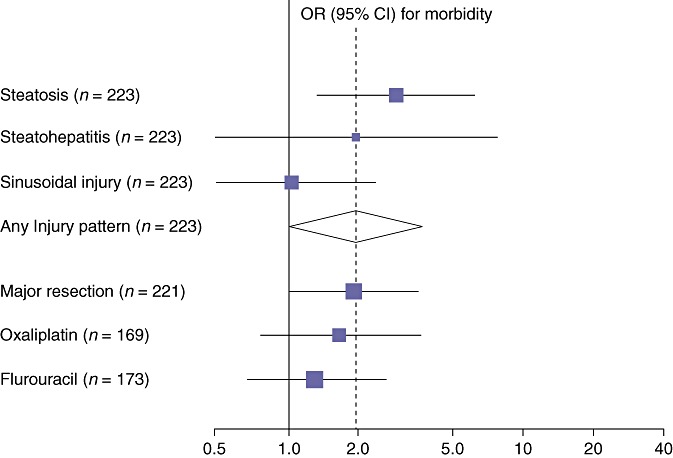
Odds ratio (OR) for perioperative morbidity amongst patients with or without hepatic injury, and in those administered chemotherapy. Only those patients with steatosis had a statistically significantly increased risk of perioperative morbidity [OR = 2.50, 95% confidence interval (CI) 1.20–5.23, P = 0.02]. Note that major vs. minor resection was associated with a higher rate of perioperative morbidity, with an OR = 1.78 that almost reached statistical significance (95% CI 0.99–3.19, P = 0.06)
The rate of operative morbidity in the cohort overall was 36% (95% CI 29%–42%), not varying with or without the administration of chemotherapy (37% vs. 38%) (Table 5 and Fig. 2). Major morbidity included post-operative bile leak [8% (5%–12%)], hepatic failure [1% (0%–3%)] and three patients returning to theatre for bleeding [1% (0%–4%)]. There were two deaths [mortality = 1% (0%–3%)].
There were 150 separate complications which either required treatment, or prolonged inpatient stay, documented for 83 patients from the 230 episodes where perioperative morbidity data were available. Although the Clavien–Dindo system15 was not retrospectively applied to the complications that were retrieved from the medical notes, by definition there were no Grade I complications recorded. The median number of complications per patient was one (range 1–9). There were 43 patients who suffered one complication, 25 who suffered two complications and 15 who suffered three or more complications. There were also 14 patients who were readmitted within 30 days, although one of these patients readmissions was for a planned lung resection, and another was readmitted to have a further disc of diaphragm excised after a pathology report documented an incomplete margin on the diaphragm that had been excised at the first operation, and so therefore neither of these strictly represent peri-operative complications.
Respiratory complications account for 30.7% of all the complications (46 separate complications), while 28% of the overall complications are hepatobiliary specific (42 episodes). The remainder were accounted for by general complications (62 episodes, 41.3%). There were 29, 31 and 47 patients affected by respiratory, hepatobiliary and general complications, and as such the rate of these complications in the cohort was 13%, 14% and 21%, respectively. Overall, 36% of patients suffered at least one complication during their post-operative course.
Pneumonia and pleural effusion account for over three-quarters of all respiratory complications (20 and 16 cases, respectively, Fig. 3a) and for 13.3% and 10.7% of complications overall. The majority of hepatobiliary complications were bile leak and subphrenic collection requiring intervention (18 and 14 cases respectively), again accounting for more than three-quarters of the complications in that subgroup (Fig. 3b). Bile leak and subphrenic collection represented 12.0% and 9.3% of complications overall. The commonest general complications were arrhythmia (13 cases, 8.7% of overall complications) and the requirement for a blood transfusion (11 cases, 7.3% of overall complications) (Fig. 3c).
Figure 3.
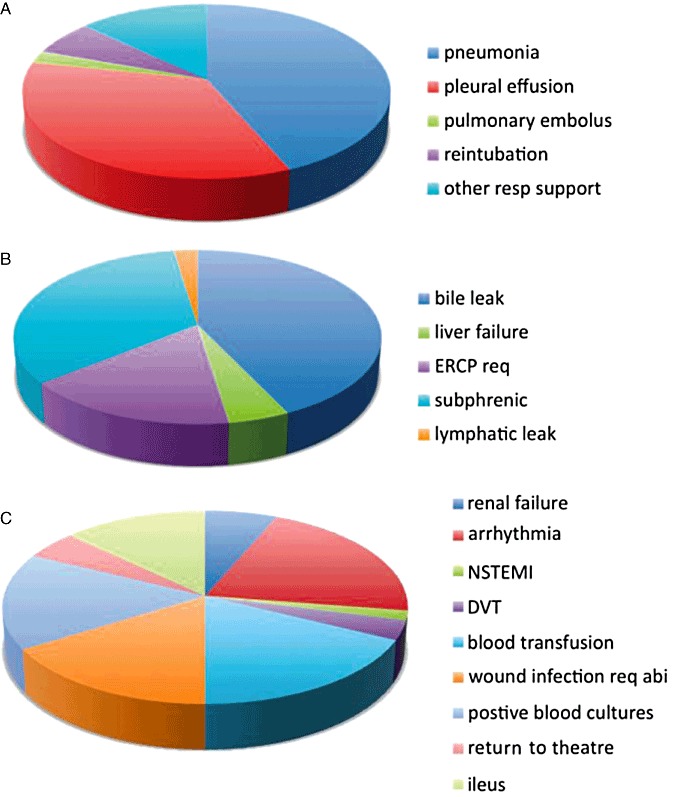
Perioperative morbidity. For each of the main groups of perioperative morbidity: (a) respiratory; (b) hepatobiliary specific; and (c) general complications, the proportion of each category that each individual disease process represents is displayed. ERCP req, endoscopic retrograde cholangiopancreatography required; Subphrenic, subphrenic collection requiring treatment intervention; NSTEMI, non-ST elevation myocardial infarction; DVT, deep venous thrombosis; Abi, antibiotics
Overall morbidity was higher among patients if their sample showed evidence of high-grade steatosis (OR = 2.50, 95% CI 1.20–5.23, P = 0.02; Table 5), with a larger effect seen in the subgroup of general medical perioperative morbidity (OR = 3.08, 95% CI 1.45–6.54, P = 0.004). There was a higher rate of respiratory complications with higher grades of steatosis (increasing from 7.7% in those with grade 0 steatosis to 21.4% in those with grade 4 steatosis), although the hepatobiliary-specific peri-operative morbidity subgroup did not demonstrate a statistically significant increase in the rate of morbidity with high-grade steatosis. The largest numerical increase was in the rate of wound infections in those with high-grade steatosis [5 wound infections in 39 patients with high-grade steatosis (13.0%) vs. 5 wound infections in 193 patients without high-grade steatosis (2.6%), P = 0.02]. The presence of sinusoidal injury or steatohepatitis and the administration of chemotherapy were not associated with increased morbidity.
Discussion
Injury patterns are common tissue-based responses
Phenotypically, the liver manifests injury in a limited number of ways.16 Steatosis is a common end result of many injurious agents, ranging from drugs to chronically elevated blood lipids.17 Steatohepatitis results from oxidative stress acting upon an already steatotic liver.18 These injuries can be induced by chemotherapy but are also associated with other risk factors such as obesity, diabetes and metabolic syndrome.19 This may confound analyses comparing patients administered chemotherapy with those not treated, as there will be a background rate of steatosis in the non-chemotherapy group, potentially obscuring the ability to detect increases in rates of steatosis secondary to chemotherapeutic treatment.
Diabetes, obesity and metabolic syndrome increase rates of simple steatosis and/or steatohepatitis
We have confirmed established associations between diabetes, weight and metabolic syndrome with steatosis and steatohepatitis in our cohort overall (OR = 3.24 and 1.04 with respect to steatosis for diabetes and higher weight per kg respectively, and OR = 5.88 with respect to steatohepatitis and metabolic syndrome). Clinically, these are highly significant increases in the risk of developing these injury patterns, and are independent of chemotherapy administration. When chemotherapy is administered, there is a suggested independent effect with an OR of approximately two for all of these injury patterns over and above that inferred from the co-morbidity itself [OR for steatosis 1.90 (0.87–4.17), for steatohepatitis 2.30 (0.47–11.33)]. That is, in both those with and without co-morbidity, chemotherapy appears to increase the rate of steatosis and steatohepatitis independent of comorbidity. The ability to demonstrate this with statistical significance was limited by the small sample size and the CIs therefore cross unity, this is an inherent limitation in fixed-number retrospective cohorts such as this. Our cohort size had approximately 80% power to detect an association with injury for a factor of prevalence of about 15% and with an OR of 2.85 with a significance level of α= 0.05. There appeared to be no difference in the rate of sinusoidal injury comparing patients administered chemotherapy overall compared with no chemotherapy, in spite of others having demonstrated an association.8 Nevertheless there was a statistically significantly higher rate of sinusoidal injury seen when chemotherapy was administered in the pre-operative setting (OR = 2.18).
Hepatic injury presence increases the rate of perioperative morbidity
The ability to demonstrate increased perioperative morbidity secondary to chemotherapy2,9,20 is similarly confounded in the literature. This may be because there are high rates of injuries such as steatosis in those not administered chemotherapy in some cohorts, obscuring the ability to detect the effect of chemotherapy. Equally, not all patients develop CIHI, and those that do not develop hepatic injury should not be at higher risk of perioperative morbidity. The EORTC 409832 trial did demonstrate increased perioperative morbidity in the group treated with pre-operative chemotherapy, although this was not stratified by the presence or absence of CIHI. We have observed no increase in perioperative morbidity in those administered chemotherapy as a whole, but have shown that those who display high-grade steatosis are at an increased risk of morbidity. Thus it may be concluded that it is the development of hepatic injury (be it from chemotherapy, obesity, diabetes or metabolic syndrome) that predisposes to perioperative morbidity, not the administration of chemotherapy as such.
Prediction of CIHI could lower perioperative morbidity
Concerns about inferior outcomes in patients subjected to a hepatectomy after chemotherapy should really be restated to acknowledge that inferior outcomes are more likely to be seen in those who develop or have liver injury. Efforts should therefore centre on establishing who is at risk of damage from chemotherapy and avoiding treatment in those patients, rather than abandoning chemotherapy pre-operatively altogether. Identifying patients at high risk of developing injury before treatment commences remains the goal. Clearly some patients, such as younger females without diabetes or hypercholesterolaemia, are less likely to a have pre-existing injury and may therefore be more likely to tolerate therapy well. Patients who do not develop CIHI can therefore expect to enjoy the improvements in progression-free survival that have been demonstrated2 without fear of increased peri-operative morbidity secondary to hepatic injury. Similarly, patients who do develop CIHI must be identified as quickly as possible so their treatment can be altered accordingly.
Conclusion
The presence of hepatic injury increases perioperative morbidity regardless of the cause of injury. Chemotherapy may increase the rate over and above the increase seen from comorbidities such as diabetes and obesity in some patients. The pre-treatment identification of which patients will develop injury in response to chemotherapy would be extremely useful in avoiding toxic therapies in those patients, while not denying the improvements pre-operative treatment may have in those that are not likely to develop CIHI as a result.
Acknowledgments
Charles Pilgrim is supported by the Royal Australasian College of Surgeons (RACS) Surgeon Scientist Scholarship, the RACS Foundation for Surgery Reg Worcester Research Fellowship and the Melbourne Research Scholarship from the University of Melbourne. Some of the data and tissues used in this project were provided by the Victorian Cancer Biobank with appropriate ethics approval. The Victorian Cancer Biobank is supported by the Victorian Government.
Conflicts of interest
None declared.
References
- 1.Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963–965. doi: 10.1016/S0140-6736(08)60429-8. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 4.Makowiec F, Mohrle S, Neeff H, Drognitz O, Illerhaus G, Opitz OG, et al. Chemotherapy, liver injury, and postoperative complications in colorectal liver metastases. J Gastrointest Surg. 2011;15:153–164. doi: 10.1007/s11605-010-1368-7. [DOI] [PubMed] [Google Scholar]
- 5.Morris-Stiff G, Tan YM, Vauthey JN. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol. 2008;34:609–614. doi: 10.1016/j.ejso.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 7.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2008;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 8.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 9.Aloia T, Sebagh M, Plasse M, Karam V, Levi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 10.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 11.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 12.Mendler MH, Kanel G, Govindarajan S. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver Int. 2005;25:294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 16.Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–684. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- 17.Salt WB., 2nd Nonalcoholic fatty liver disease (NAFLD): a comprehensive review. J Insur Med. 2004;36:27–41. [PubMed] [Google Scholar]
- 18.Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 19.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 20.Mehta NN, Ravikumar R, Coldham CA, Buckels JA, Hubscher SG, Bramhall SR, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]


