Abstract
Background
Following potentially curative resection at this centre, patients with pancreatic adenocarcinoma (PAC) are routinely enrolled in a programme of clinical and radiographic surveillance. This study sought to evaluate its diagnostic yield.
Methods
All patients who underwent pancreaticoduodenectomy for PAC at this institution during 1998–2008 were identified. Patients with asymptomatic recurrence were compared with those with symptomatic recurrence. Factors associated with survival following the detection of recurrence were compared.
Results
A total of 216 of 327 (66.1%) resected patients developed recurrence. Asymptomatic recurrence was detected in 118 (54.6%) patients. Symptomatic recurrence was associated with multifocal disease or carcinomatosis, poor performance status and less frequent subsequent therapy. Median time to recurrence did not differ between groups, but survival after detection was shorter in symptomatic patients (5.1 months vs. 13.0 months; P < 0.001). Treatment was administered more frequently to asymptomatic patients (91.2% vs. 61.4%; P < 0.001). At recurrence, a preserved performance status score of ≤1, further therapy, low CA 19-9, and an isolated site of recurrence were independently associated with longer post-recurrence survival (P < 0.001).
Conclusions
Overall, 54.6% of cases of recurrent PAC were detected prior to the onset of symptoms using a standardized clinical and radiographic surveillance strategy. Although this retrospective analysis limits definitive conclusions associating this strategy with survival, these results suggest the need for further studies of postoperative surveillance.
Keywords: surveillance, pancreatic adenocarcinoma, recurrence, survival, pancreaticoduodenectomy, symptoms
Introduction
Patients with many solid cancers who develop recurrence following potentially curative resection can often be treated with chemotherapy, radiation or reoperation when their disease burden is limited. There is, thus, a sound rationale for the use of routine surveillance programmes designed to identify low-volume, recurrent disease prior to the onset of symptoms. Advantages associated with the surveillance of patients with high-risk colorectal cancer have been described.1–3 Routine surveillance has also been advocated for patients with breast cancer,4 gastric cancer5 and melanoma.6
Series of longterm survivors published within the last decade suggest that up to 27% of patients with localized pancreatic adenocarcinoma (PAC) who complete multimodal therapy survive for >5 years.7 Nonetheless, most patients who receive potentially curative therapy for PAC develop recurrence. For such patients, systemic chemotherapy is not curative. Similarly, reoperation following pancreatic resection is typically futile.8–10 Probably in response to a resulting therapeutic nihilism, few data have been published on the incidence, pattern and treatment of recurrent PAC,11 and no analysis has been performed of outcomes associated with surveillance or treatment of recurrence following multimodal therapy. Essentially, no data exist to help guide postoperative follow-up.
The National Comprehensive Cancer Network (NCCN) recommends the routine surveillance of patients who have undergone resection for PAC with a physical examination and clinical assessment for the presence of symptoms every 6 months for 2 years, but does not advocate radiographic imaging because data demonstrating its efficacy are lacking. The NCCN further recommends that recurrence be treated with chemotherapy and/or chemoradiation, preferably as part of a clinical trial.12 However, the frequency with which recurrent PAC is detected when patients are eligible for treatment using this strategy is unknown.
It has long been hypothesized that a comprehensive surveillance strategy that includes both clinical assessment and radiographic evaluation might identify recurrent PAC when it is most amenable to further treatment. At this institution, patients are enrolled into an intensive surveillance programme following resection, and are liberally treated for recurrence upon detection. Through an analysis of patients curatively treated for localized PAC, this retrospective study aims to describe patterns and timing of recurrent PAC, to determine the rates of asymptomatic and symptomatic recurrence detected with this surveillance strategy, to characterize patients' performance status and clinical characteristics at the time of recurrence, and to identify factors associated with survival following recurrence.
Materials and methods
Patients and staging
The centre's prospectively maintained pancreatic database was retrospectively queried for patients diagnosed with potentially resectable or borderline resectable adenocarcinoma of the pancreatic head or uncinate process, who underwent pancreaticoduodenectomy during 1998–2008.13 Staging was established using multidetector, contrast-enhanced computed tomography (CT) and was confirmed when necessary by multidisciplinary review. The staging criteria have been previously reported.14
Primary treatment
Patients with localized PAC often received chemotherapy and/or chemoradiation, either on or off protocol, prior to resection.14–16 Most patients received external beam radiation (typically to 30.0 Gray or 50.4 Gray) with concurrent gemcitabine, 5-fluorouracil or capecitabine. Gemcitabine-based systemic chemotherapy was delivered prior to chemoradiation in selected patients. Patients who underwent surgery first routinely received postoperative therapy.17 Pancreaticoduodenectomy was performed in a standard fashion.18
Follow-up and surveillance
Following completion of all therapy, patients were scheduled for re-evaluation every 3–4 months with a physical examination, chest radiograph and abdominal CT scan. Serum carbohydrate antigen 19-9 (CA 19-9) was typically, but not always, assayed. Patients without evidence of disease after 2 years from diagnosis were evaluated every 6 months to year 5, when evaluations were reduced to annual intervals. Magnetic resonance imaging was only used when patients were unable to undergo CT, and positron emission tomography scans were rarely employed.
Recurrence was considered symptomatic when it was detected concurrently with a significant patient-initiated complaint that was new or had increased in either severity or frequency during the prior surveillance interval, or was detected during physical examination. Symptomatic recurrences were diagnosed either at scheduled visits or at accelerated, unscheduled visits. Asymptomatic recurrences were defined as those discovered by routine imaging at scheduled visits in the absence of new complaints or physical findings. Performance status at recurrence was reported using the Eastern Cooperative Oncology Group (ECOG) score.19
Recurrence was classified as local, regional or distant.20 The development of a new, low-density mass in the region of the resected pancreas or in the mesenteric root was considered local. Abdominal ascites and enlarging regional lymph nodes were considered regional. A new low-density mass in the liver, in the lung or outside the abdomen was considered distant. When radiographic findings were consistent with cancer, biopsy was rarely performed. Only the first site(s) of recurrence was recorded for this study. Following recurrence, treatment was initiated at the discretion of the multidisciplinary group. Gemcitabine-based systemic chemotherapy was typically employed; chemoradiation and re-resection were rarely used.
Statistical analysis
Chi-squared and Fisher's exact tests were used for categorical variables. Wilcoxon rank-sum test was used to compare continuous variables. To reveal any potential difference in actual patterns of surveillance between symptomatic and asymptomatic patients, the median interval between surveillance CT scans was calculated for each patient from postoperative day 30 (to exclude acute postoperative scans) to the date of recurrence by dividing this duration with the number of abdominal CT scans actually obtained in that period. Time to recurrence (TTR) in patients affected by recurrence was defined as the interval between tissue diagnosis and detection of recurrence. Disease-free survival (DFS) was measured from diagnosis to whichever occurred first of the date of recurrence or the date of the last follow-up. Overall survival (OS) was defined as the interval between diagnosis and the date of death from any cause or last follow-up, whichever occurred first. Post-recurrence overall survival (PROS) was defined as the interval between recurrence and the date of death from any cause or last follow-up, whichever occurred first. Overall survival and PROS were estimated using the Kaplan–Meier method. The log-rank test was used to compare OS and PROS among various subgroups. The variable ‘symptom at recurrence’ was treated as a time-varying covariate when assessing its effect on OS because symptoms were recorded at disease recurrence rather than disease diagnosis. It serves as a baseline covariate when assessing its effect on PROS. ‘Symptom at recurrence’ was considered as a time-dependent covariate when Cox proportional hazards models were used to analyse associations between PROS and clinical characteristics. Univariate factors with P < 0.10 were included in the multivariate model. Statistical analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA) and s-plus Version 8 (TIBCO Software, Inc., Somerville, MA, USA).
Results
Overall survival in 327 patients
A total of 356 patients who underwent pancreaticoduodenectomy for PAC during 1998–2008 fulfilled the study inclusion criteria. Complete follow-up data were available for 327 (91.9%) patients. The median follow-up for all patients was 30.5 months (range: 3.3–147.8 months). In patients without recurrence, median follow-up was 46.1 months (range: 3.3–147.8 months). Neoadjuvant therapy was administered to 254 of 327 (77.7%) patients. Of the 73 patients who did not receive preoperative therapy, 50 (68.5%) patients were given postoperative therapy. The median OS and DFS in all 327 patients were 33.4 months [95% confidence interval (CI) 30.5–38.2] and 19.1 months (95% CI 16.8–22.7), respectively.
Timing and pattern of recurrence
Recurrent PAC was documented in 216 of 327 (66.1%) patients at last follow-up. The first site of recurrence was identified in the pancreas in 32 of 327 (9.8%) patients, as regional disease in 31 of 327 (9.5%) patients (peritoneum, n = 27; regional lymph nodes, n = 4), and at distant sites in 108 of 327 (33.0%) patients (liver, n = 58; lung, n = 35; other, n = 3; multiple distant sites, n = 12). In 45 of 327 (13.8%) patients, recurrence was documented simultaneously in at least two site groups.
The median TTR in patients with recurrence was 13.3 months (95% CI 11.8–14.7) from diagnosis. The first recurrence was documented within 2 years of diagnosis in 174 of 216 (80.6%) patients with recurrence (and 53.2% of all 327 patients) (Fig. 1). Recurrence was identified in 26.9% (88/327), 37.7% (86/228) and 17.4% (23/132) of patients at risk in each of the first 3 years following diagnosis. Thereafter, only 3.0–10.5% of patients at risk were diagnosed with recurrent disease annually to year 7.
Figure 1.
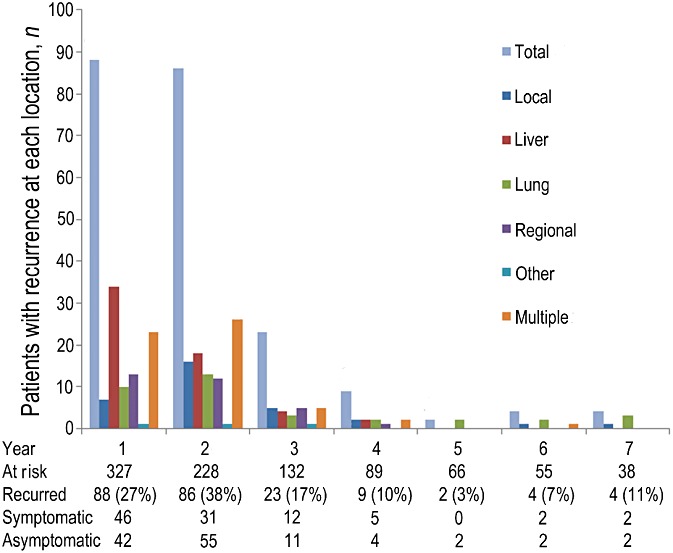
Recurrence was detected in the absence of symptoms in approximately half of all patients who experienced recurrence each year to year 7. Detection yield was highest in the first 2 years of surveillance and fell sharply thereafter
Detection and characteristics of recurrence
Ninety-eight of 216 (45.4%) recurrences were diagnosed in the presence of concurrent symptoms. Abdominal or back pain was reported by 76 of 98 (77.6%) symptomatic patients. Asymptomatic recurrence was diagnosed in 118 of 216 (54.6%) patients. Annual percentages of symptomatic recurrences ranged from 36.0% to 52.3%. There was no significant difference in the median frequency of surveillance CT scans between symptomatic and asymptomatic patients with recurrence (P > 0.325) (Table 1).
Table 1.
Tumour and patient characteristics in 216 patients who experienced recurrence after potentially curative resection
| Categories | All patients with recurrence | Patients without symptoms | Patients with symptoms | P-value |
|---|---|---|---|---|
| Recurrence, n (%) | 216 | 118 (54.6%) | 98 (45.4%) | |
| Primary stage, n (%) | ||||
| Potentially resectable | 191 | 99 (83.9%) | 92 (93.9%) | 0.030 |
| Borderline | 25 | 19 (16.1%) | 6 (6.1%) | |
| Time interval per CT, months, median (range) | ||||
| Recurrence at <2 years | 3.2 (1.3–10.1) | 3.4 (1.3–10.1) | 3.1 (1.5–6.4) | 0.330 |
| Recurrence at 2–5 years | 4.1 (2.9–17.1) | 4.1 (2.9–8.5) | 3.8 (3.3–17.1) | 0.810 |
| Time to recurrence, monthsa | ||||
| Median (range) | 13.3 (1.3–83.8) | 14.5 (1.3–83.5) | 12.3 (1.6–83.8) | 0.380 |
| Recurrence site, n (%) | ||||
| Local | 32 | 17 (14.4% | 15 (15.3%) | 0.010 |
| Regional | 31 | 11 (9.3%) | 20 (20.4%) | |
| Distant | 108 | 70 (59.3%) | 38 (38.8%) | |
| Multiple | 45 | 20 (16.9%) | 25 (25.5%) | |
| Performance status at recurrence (ECOG score), n (%) | ||||
| ≤1 | 166 | 116 (99.1%) | 50 (51.0%) | <0.001 |
| ≥2 | 49 | 1 (0.9%) | 48 (49.0%) | |
| CA 19-9 at recurrence, n (%) | ||||
| >40 U/ml | 149 | 80 (69.6%) | 69 (75.8%) | 0.320 |
| <40 U/ml | 57 | 35 (30.4%) | 22 (24.2%) | |
| Median (range), U/ml | 181 (1–21 096) | 135 (1–21 096) | 365 (1–20 934) | 0.050 |
| Treatment after recurrence, n (%) | ||||
| Yes | 157 | 103 (91.2%) | 54 (61.4%) | <0.001 |
| No | 44 | 10 (8.8%) | 34 (38.6%) | |
Time to recurrence was measured from the date of tissue diagnosis.
Evaluated and both clinically and statistically not significant: age at recurrence; gender; administration of neoadjuvant therapy prior to initial operation; estimated blood loss; use of vascular resection; resection margin; nodal status at initial operation; tumour size, and tumour differentiation.
CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9.
Initial demographics, treatment variables, perioperative factors and pathologic characteristics associated with the primary tumour did not differ between symptomatic and asymptomatic patients (Table 1). However, patterns of recurrence (P = 0.010) and distributions of ECOG scores (P = 0.001) differed between symptomatic and asymptomatic patients. The median CA 19-9 in symptomatic patients also trended higher than in asymptomatic patients (P = 0.050).
Median TTR did not differ between patients with symptomatic and asymptomatic recurrence [12.3 months (95% CI 11.6–13.0) vs. 14.5 months (95% CI 12.3–16.6); P = 0.380]. However, symptomatic patients had a shorter median PROS than patients without symptoms [5.1 months (95% CI 3.4–6.8) vs. 13.0 months (95% CI 10.8–15.1); P < 0.001]. Thus, the median OS of symptomatic patients [18.0 months (95% CI 15.9–24.7)] was shorter than in asymptomatic patients [29.6 months (95% CI 25.9–34.0)] (P = 0.003) (Fig. 2).
Figure 2.
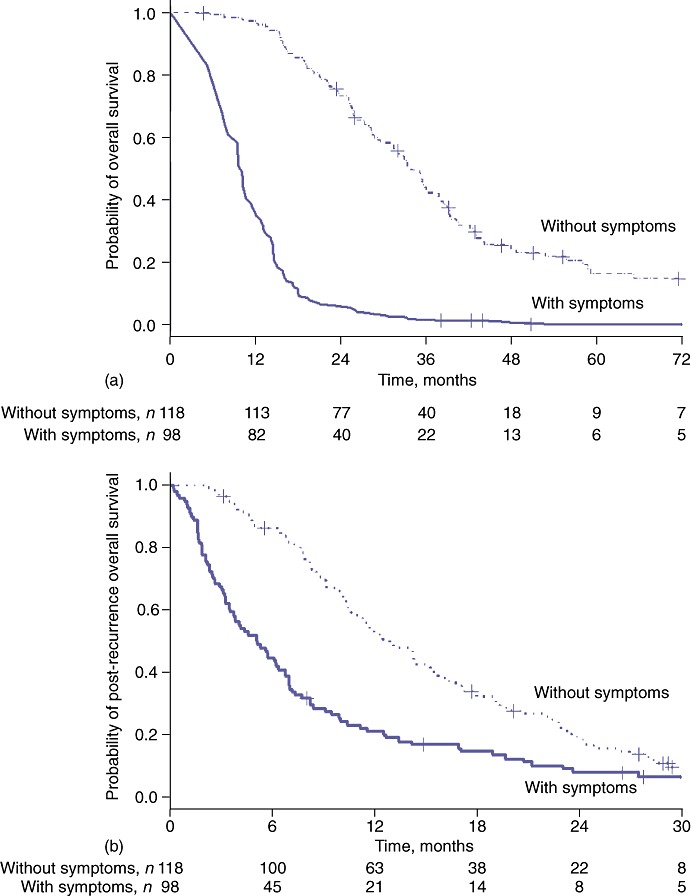
(a) Overall survival (P = 0.003) and (b) post-recurrence overall survival (P < 0.001) were both longer in asymptomatic patients compared with patients in whom recurrence was detected concurrently with symptoms
Treatment for recurrence
Further therapy was administered in 157 of 216 (72.7%) patients with recurrence. Treatment was administered more frequently in asymptomatic than in symptomatic patients (91.2% vs. 61.4%; P < 0.001). Median PROS was longer in treated patients than in untreated patients [11.8 months (95% CI 9.8–13.7) vs. 2.6 months (95% CI 2.6–3.2); P < 0.001].
Among 158 patients with preserved performance status (ECOG score ≤ 1) at recurrence, 136 (86.1%) received subsequent therapy. The reasons why the remaining 22 patients did not receive further treatment were patient choice (n = 11) and physician recommendation (n = 11). The median PROS of patients with ECOG scores of ≤ 1 treated following recurrence was longer than that of patients who were untreated [14.1 months (95% CI 11.5–16.6) vs. 4.2 months (95% CI 0.1–8.8); P < 0.001].
Factors associated with PROS
Four clinical factors independently associated with longer PROS were identified: isolated recurrence (local or distant recurrence was better than regional and multiple-site recurrence); preserved performance status (ECOG scores of ≤ 1); lower CA 19-9, and the administration of therapy for recurrence (Table 2). Further therapy was also independently associated with longer PROS in a separate multivariate model of the subgroup of patients with ECOG scores of ≤ 1 at recurrence. Among patients with ECOG scores of ≤ 1 at recurrence, younger age, lower estimated blood loss at pancreatectomy, lower CA 19-9 level at recurrence, longer TTR, and isolated (vs. multiple-site) recurrence were independently associated with longer PROS (Table 3).
Table 2.
Factors associated with post-recurrence overall survival (all patients, n= 216)
| Clinical characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Patient traits | ||||||
| Age | 1.02 | 1.01–1.04 | 0.002 | |||
| Perioperative factors | ||||||
| Length of stay | 1.56 | 1.07–2.27 | 0.020 | |||
| Estimated blood loss | 1.3 | 1.05–1.61 | 0.020 | |||
| Factors at recurrence | ||||||
| DFS > 1 year vs. ≤ 1 year | 1.64 | 1.22–2.17 | <0.001 | |||
| Symptomatic at recurrence | 2.02 | 1.52–2.68 | <0.001 | 1.16 | 0.81–1.67 | 0.410 |
| Recurrence group | ||||||
| Local vs. multiple | 0.41 | 0.25–0.66 | <0.001 | 0.38 | 0.22–0.64 | <0.001 |
| Regional vs. multiple | 0.57 | 0.35–0.92 | 0.020 | 0.60 | 0.36–1.00 | 0.040 |
| Distant vs. multiple | 0.45 | 0.31–0.64 | <0.001 | 0.46 | 0.31–0.69 | <0.001 |
| ECOG score at recurrence (ECOG 2–4 vs. 0–1) | 5.00 | 3.45–7.14 | <0.001 | 4.01 | 2.57–6.25 | <0.001 |
| CA 19-9 at recurrence | 1.22 | 1.13–1.31 | <0.001 | 1.19 | 1.10–1.28 | <0.001 |
| Treatment at recurrence | 0.24 | 0.17–0.35 | <0.001 | 0.31 | 0.21–0.47 | <0.001 |
Patient and tumour factors evaluated and insignificant on both univariate and multivariate analysis: gender; clinical stage at initial operation; neoadjuvant therapy; microscopic margin status; vascular resection; tumour size; tumour differentiation, and lymph node status.
HR, hazard ratio; 95% CI, 95% confidence interval; DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9.
Table 3.
Factors associated with post-recurrence overall survival (patients with ECOG scores ≤ 1, n= 166)
| Clinical characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Patient traits | ||||||
| Age | 1.02 | 1.00–1.04 | 0.020 | 1.03 | 1.01–1.05 | 0.001 |
| Perioperative factors | ||||||
| Estimated blood loss | 1.28 | 1.00–1.65 | 0.050 | 1.44 | 1.09–1.92 | 0.010 |
| Factors at recurrence | ||||||
| DFS > 1 year vs. ≤ 1 year | 0.56 | 0.40–0.78 | <0.001 | 0.45 | 0.30–0.67 | <0.001 |
| Symptomatic | 1.37 | 0.97–1.95 | 0.080 | 1.05 | 0.71–1.55 | 0.820 |
| Recurrence group | ||||||
| Local vs. multiple | 0.39 | 0.22–0.67 | <0.001 | 0.42 | 0.22–0.79 | 0.007 |
| Regional vs. multiple | 0.40 | 0.22–0.71 | 0.002 | 0.48 | 0.26–0.90 | 0.020 |
| Distant vs. multiple | 0.39 | 0.25–0.60 | <0.001 | 0.40 | 0.26–0.66 | <0.001 |
| CA 19-9 at recurrence | 1.21 | 1.11–1.32 | <0.001 | 1.20 | 1.09–1.31 | <0.001 |
| Treatment at recurrence | 0.24 | 0.20–0.51 | <0.001 | 0.23 | 0.13–0.38 | <0.001 |
Patient and tumour factors evaluated and insignificant on both univariate and multivariate analysis: gender; clinical stage at initial operation; neoadjuvant therapy; length of stay at initial operation; microscopic margin status; vascular resection; tumour size; tumour differentiation, and lymph node status.
ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; 95% CI, 95% confidence interval; DFS, disease-free survival; CA 19-9, carbohydrate antigen 19-9.
Discussion
For the past two decades, the treatment strategy for localized adenocarcinoma of the pancreatic head at the study centre has emphasized objective clinical staging, liberal use of preoperative chemoradiation, and a uniform approach to the technical aspects of pancreaticoduodenectomy.7,21 This centre has also enrolled resected patients into a programme of clinical and radiographic cancer surveillance. This retrospective analysis of the diagnostic yield of the surveillance strategy found that approximately half of patients with new recurrence each year were detected in the absence of symptoms. The performance status of these asymptomatic patients was well preserved and 91.2% received further treatment. By contrast, recurrence detected concurrently with new symptoms or physical findings was often associated with carcinomatosis or multifocal disease, depressed performance status that often prohibited further therapy, and rapid clinical decline. These results suggest that radiographic surveillance programmes might identify patients with performance status and tumour biology that are most likely to benefit from subsequent therapy. However, the limitations and biases of retrospective analyses of surveillance programmes, like this one, highlight a need for further prospective study.
A primary objective of this study was to describe the timing and pattern of recurrent disease detected using an intensive surveillance strategy in patients with resected PAC. The present authors routinely recommend follow-up every 3–4 months during the first 2 years, every 6 months in years 3–5, and annually thereafter. This frequency of surveillance is similar to that recommended by the NCCN within the first 2 years after resection and is rational given the timing of recurrence in patients with resected PAC. Indeed, 80.6% of recurrences in this series occurred within the first 2 years. After 2 years, the detection of recurrence among survivors at risk decreased dramatically. Nonetheless, historically this centre has continued to follow patients beyond 2 years, at longer intervals, and has used these visits to evaluate patients not only for recurrence, but also for the nutritional, metabolic and psychosocial abnormalities that are unique to longterm post-pancreatectomy survivors. This policy is consistent with recent data demonstrating that regularly scheduled visits to a physician are welcomed by cancer patients who have undergone resection.22 However, radiographic surveillance itself may also be a source of considerable anxiety and fear of recurrence that translates into a negative effect on quality of life.23 The potential benefits of radiographic surveillance, particularly the longterm benefits, therefore require additional study.
A second objective of this study was to characterize the performance status of patients diagnosed with recurrent cancer using the present strategy. Poor performance status has been identified as an independent negative prognostic factor in patients with all stages of PAC treated with chemotherapy, chemoradiation or surgery.24–26 The reasons for this association are multifactorial, but include a significant disease burden or unfavourable tumour biology, which often occur in patients with poor performance status who are unable to undergo aggressive therapy. In this series, the presence of new symptoms at recurrence was associated with an ECOG score of ≥ 2 in 49.0% of patients. Symptoms were also more common in patients with carcinomatosis, multiple synchronous sites of recurrence and higher CA 19-9 levels. These data suggest that the onset of symptoms may indicate a rapid clinical decline that is already in progress and that precludes further therapy. This issue is particularly relevant given the potential role of FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, oxaliplatin), a newer regimen associated with both significant activity and toxicity, in the treatment of recurrent PAC.27
Although the results of this analysis suggest that the use of radiographic surveillance might identify patients with a performance status that indicates they are suitable for treatment and may even be associated with a survival benefit, this interpretation of the present data must be made cautiously and the influence of bias must be acknowledged. Lead time bias describes the possibility that the increase in survival duration following detection by a screening test is an artefact of earlier diagnosis and does not reflect a true prolongation of life. In the present series, the TTR of asymptomatic patients was equivalent to that of symptomatic patients, suggesting that lead time bias was not the primary reason for improved survival. Furthermore, the absence of an independent association between symptoms at recurrence and PROS on multivariate analysis can be explained by the close correlation between symptoms and performance status, of which the latter was retained in the final multivariate model. Length time bias may also have influenced these results. This bias reflects the possibility that a screening test may detect only patients with indolent disease and miss patients with more aggressive cancer. Whether the tumours in asymptomatic patients were more slow-growing than those in symptomatic patients, and whether the interval until the onset of symptoms and functional decline would have been longer in asymptomatic patients had they been followed until symptoms manifested, are unknown. Because this was not a prospective trial, it is possible that a disparity in the actual intensity of follow-up affected the results. However, little difference in the median time interval between surveillance scans emerged between patients with asymptomatic and symptomatic recurrences. Finally, this study is also limited by the absence of cost-effectiveness data. Given the current economic environment in which justification for health care expenditures is critical, this limitation is significant. A rigorous analysis is currently underway to address this issue.
Despite these limitations, this analysis has some important strengths. The foremost of these is the quality of the follow-up reported in this retrospective study. Indeed, to the authors' knowledge, this clinical series represents the most comprehensive follow-up of resected PAC patients with recurrences to be reported. Thorough clinical data, from diagnosis to death, for all patients with pancreatic tumours are prospectively logged in a multidisciplinary database maintained by dedicated personnel.13 Complete follow-up data were available for 91.9% of the 356 patients who fulfilled the present study's inclusion criteria. This analysis is therefore subject to minimal selection bias compared with prior analyses of PAC recurrence in which complete follow-up data referred to as few as 20% of patients.11,28
In summary, this study found that a regularly scheduled clinical and radiographic surveillance programme detected recurrence prior to the onset of symptoms in over half of patients who underwent curative resection for PAC. The detection yield was highest in the first 2 years of surveillance and fell sharply thereafter. If the primary purpose of postoperative surveillance is to detect recurrence when it is treatable, these data suggest that routine radiographic surveillance of patients with resected PAC may be worthwhile, particularly within the first 2 years. However, further prospective studies are necessary to determine the precise clinical efficacy of postoperative follow-up and treatment, as well as the optimal timing with regard to resource allocation and cost.
Acknowledgments
This study was supported by the Khalifa Bin Zayed Al Nahyan Foundation and the Various Donor Pancreatic Research Fund at MD Anderson Cancer Center.
Conflicts of interest
None declared.
References
- 1.Baca B, Beart RW, Jr, Etzioni DA. Surveillance after colorectal cancer resection: a systematic review. Dis Colon Rectum. 2011;54:1036–1048. doi: 10.1007/DCR.0b013e31820db364. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicentre, randomized, controlled trial. J Clin Oncol. 2006;24:386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow-up after curative resection for colorectal cancer: systematic review and meta-analysis of randomized trials. BMJ. 2002;324:813–820. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas MP, Telaro E, Russo A, Moschetti I, Coe L, Fossati R, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005;(1):CD001768. doi: 10.1002/14651858.CD001768.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JJ, Gonen M, D'Angelica M, Jaques DP, Brennan MF, Coit DG. Is detection of asymptomatic recurrence after curative resection associated with improved survival in patients with gastric cancer? J Am Coll Surg. 2005;201:503–510. doi: 10.1016/j.jamcollsurg.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Salerni G, Lovatto L, Carrera C, Puig S, Malvehy J. Melanomas detected in a follow-up programme compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549–555. doi: 10.1001/archdermatol.2010.430. [DOI] [PubMed] [Google Scholar]
- 7.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Longterm survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Fleming JB, Lee JE, Pisters PW. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010;15:1205–1213. doi: 10.1634/theoncologist.2010-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seelig MH, Janot M, Chromik AM, Herzog T, Belyaev O, Weyhe D, et al. Redo-surgery following curative resection of pancreatic carcinoma: the difference between true and suspected recurrence. Dig Surg. 2009;26:222–228. doi: 10.1159/000219332. [DOI] [PubMed] [Google Scholar]
- 11.Meyers MO, Meszoely IM, Hoffman JP, Watson JC, Ross E, Eisenberg BL, et al. Is reporting of recurrence data important in pancreatic cancer? Ann Surg Oncol. 2004;11:304–309. doi: 10.1245/aso.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. Pancreatic adenocarcinoma V2. NCCN Clinical Practice Guidelines in Oncology, 2011. 2011.
- 13.Hwang RF, Wang H, Lara A, Gomez H, Chang T, Sieffert N, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008;15:1356–1366. doi: 10.1245/s10434-008-9833-1. [DOI] [PubMed] [Google Scholar]
- 14.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 16.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 17.Aloia TA, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–355. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Yen TW, Abdalla EK, Pisters PW, Evans DB. Pancreaticoduodenectomy. In: Von Hoff DD, Evans DB, Hruban RH, editors. Pancreatic Cancer. Sudbury, MA: Jones & Bartlett; 2005. pp. 265–286. [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 20.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz MH, Wang H, Balachandran A, Bhosale P, Crane CH, Wang X, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16:68–78. doi: 10.1007/s11605-011-1748-7. [DOI] [PubMed] [Google Scholar]
- 22.Kiebert GM, Welvaart K, Kievit J. Psychological effects of routine follow-up on cancer patients after surgery. Eur J Surg. 1993;159:601–607. [PubMed] [Google Scholar]
- 23.Thompson CA, Charlson ME, Schenkein E, Wells MT, Furman RR, Elstrom R, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in longterm lymphoma survivors. Ann Oncol. 2010;21:2262–2266. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, et al. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan S, Rana V, Janjan NA, Abbruzzese JL, Gould MS, Das P, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107:2589–2596. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 26.Ueno H, Okada S, Okusaka T, Ikeda M. Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology. 2000;59:296–301. doi: 10.1159/000012186. [DOI] [PubMed] [Google Scholar]
- 27.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 28.Asiyanbola B, Gleisner A, Herman JM, Choti MA, Wolfgang CL, Swartz M, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-fluorouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. 2009;13:752–759. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]


