Abstract
Background
Spontaneous liver bleeding (SLB) is a rare but potentially fatal complication. In contrast to the East, various benign pathologies are the source of SLB in the West. An accurate diagnosis and a timely implementation of appropriate treatment are crucial in the management of these patients. The present study presents a large Western experience of SLB from a specialist liver centre.
Methods
A retrospective analysis of patients presented with SLB between January 1995 and January 2011.
Results
Sixty-seven patients had SLB, 44 (66%) were female and the median age at presentation was 47 years. Abrupt onset upper abdominal pain was the presenting symptom in 65 (97%) patients. The aetiology for SLB was hepatic adenoma in 27 (40%), hepatocellular carcinoma (HCC) in 17 (25%) and various other liver pathologies in the rest. Emergency treatment included a conservative approach in 42 (64%), DSA and embolization in 6 (9%), a laparotomy and packing in 6 (9%) and a liver resection in 11 (16%) patients. Eleven (16%) patients had further planned treatments. Seven (10%) died during the same admission but the mortality was highest in patients with HELLP syndrome. At a median follow-up of 54 months all patients with benign disease are alive. The 1-, 3- and 5-year survival of patients with HCC was 59%, 35% and17%, respectively.
Conclusion
SLB is a life-threatening complication of various underlying conditions and may represent their first manifestation. The management should include initial haemostasis followed by appropriate staging investigations to provide a definitive treatment for each individual patient.
Keywords: hepatocellular carcinoma < liver, adenoma < liver, poly or simple cystic disease < liver, focal nodular hyperplasia < spontaneous liver bleeding
Introduction
Spontaneous liver bleeding (SLB) was first described in 1844 but remains a rare complication associated with various underlying predisposing conditions.1 The clinical presentation is often non-specific but potentially life threatening and therefore demands a prompt diagnosis and treatment. The emergency scenario could be further complicated by the presence of advanced liver disease rendering its appropriate management a great therapeutic dilemma and resulting in poor outcomes.2 In contrast to the East where an underlying hepatocellular carcinoma (HCC) is the predominant cause, a variety of benign pathologies dominated by hepatic adenoma is the aetiology for SLB in the West. Various treatment options have been proposed but it is the sound decision-making process involving a multidisciplinary team, taking into account all the patient- and disease-specific parameters, that play the most crucial role in the patient outcomes.3
The present study describes the aetiology, clinical presentation, management and outcome of patients who were diagnosed with SLB in a tertiary referral centre.
Patients and methods
The centre where this study was conducted provides specialist hepatobiliary pancreatic and liver transplant services. A retrospective search was performed using the prospectively managed liver unit database to identify all patients who presented with SLB between January 1995 and January 2011. Data regarding clinical presentation, diagnosis, any underlying predisposing condition and the treatment implemented were collected. In patients diagnosed with hepatocellular carcinoma, the status of their background liver, their liver function reserve according to the Child–Turcotte–Pugh (CTP) score and the potential underlying aetiology of their primary liver cancer were also recorded. Tumour markers including carcinoembryonic antigen (CEA), carbohydrate antigen CA19-9 and alpha-fetoprotein (AFP) were measured if there was any clinical suspicion of an underlying tumour. The primary outcome measure was patient survival. Complete clinical and follow-up data were available for all patients.
Results
During the study period a total of 4271 malignant and 2275 benign hepatobiliary patients were referred to the unit. A diagnosis of SLB was made in 67 patients and they were included in the present study. The median age at presentation was 47 years (range 21–76) and 44 (66%) were female. Sixty-five patients (97%) were referred after their initial evaluation and stabilization at local hospitals. Two (3%) were admitted directly after an incidental diagnosis of a subcapsular liver hematoma on imaging performed for evaluation of abnormal liver function tests. A detailed summary of clinical presentation is presented in Table 1.
Table 1.
Presenting signs and symptoms of spontaneous liver bleeding in our study group (n = 67)
| Symptoms and signs | n (%) |
|---|---|
| Abdominal pain | 65 (97) |
| Malaise | 53 (79) |
| Vomiting | 42 (63) |
| Hematemesis | 1 (2) |
| Retrosternal pain | 4 (6) |
| Fever | 1 (2) |
| Jaundice | 5 (8) |
| Hypovolemic shock | 8 (12) |
Sixty-four of the 67 patients (95%) were submitted to a contrast-enhanced computed tomography scan (CE-CT) of the abdomen and pelvis at the referring hospital after initial resuscitation. One patient (2%) was evaluated only by an abdominal ultrasonography (US), and 2 (3%) underwent an emergency laparotomy and packing prior to imaging. After transfer from the referring hospitals, all patients were re-assessed using triple-phase CT. Eight patients (12%) underwent selective digital subtraction angiography (DSA) of the coeliac axis and superior mesenteric artery, with or without embolization. Magnetic resonance imaging (MRI) was performed in 54 patients (81%) for further characterization of lesions detected on a CT scan. The imaging revealed that 23 (34%) presented with haemoperitoneum, 36 (54%) with a subcapsular liver hematoma and 8 patients (12%) with a combination of a subcapsular hematoma and intra-peritoneal bleeding. Selected images are presented as Figs 1–4.
Figure 1.
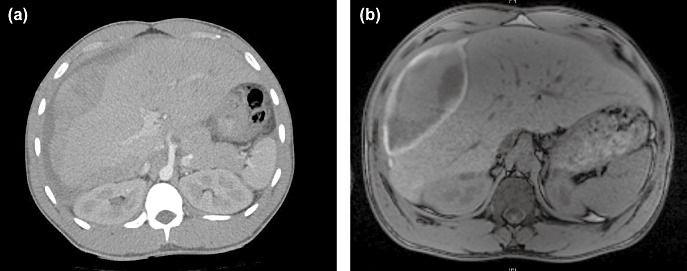
(a) Computed tomography and (b) magnetic resonance imaging images of a patient with spontaneous liver bleeding
Figure 4.
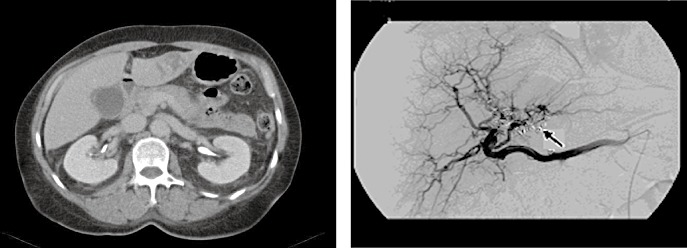
Spontaenous liver bleeding in a patient with underlying vasculitis
Figure 2.
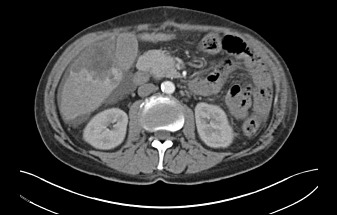
Computed tomography arterial phase-spontaneous liver bleeding with an underlying mass in the right lobe of the liver hepatocellular carcinoma
Figure 3.
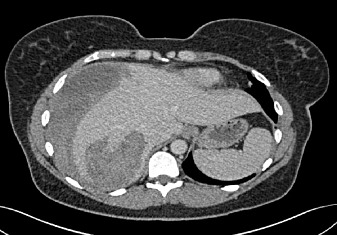
Computed tomography venous phase-right lobe (seg 6/7) intra-capsular haematoma owing to a ruptured hepatic adenoma
A detailed summary of the underlying aetiology for SLB in the present study along with the total number of patients diagnosed with that specific underlying pathology during the study period are presented in Table 2.
Table 2.
Underlying aetiology for spontaneous liver bleeding (SLB) (n= 67)
| Underlying pathology for SLB | n | Total number of patients diagnosed in study period |
|---|---|---|
| Benign Adenoma | 27 | 101 |
| Hepatocellular cancer (HCC) | 17 | 841 |
| Polycystic liver disease | 4 | 36 |
| Simple liver cyst | 1 | 130 |
| Polyarteritits nodosa (pseudoaneurysms) | 2 | 2 |
| HELLP syndrome | 4 | 6 |
| Nodular regenerative hyperplasia | 1 | 13 |
| Peliosis hepatis | 2 | 2 |
| Metastatic colorectal adenocarcinoma | 2 | 1884 |
| Focal nodular hyperplasia (FNH) | 3 | 116 |
| Haemangioma | 1 | 77 |
| No underlying pathology | 3 | – |
HCC, hepatocellular cancer; FNH, focal nodular hyperplasia.
Six patients (9%) were taking aspirin at the time of presentation (2 with HCC, 2 with adenoma and 2 with hematoma and no underlying lesion). One patient (2%) with polycystic liver disease was on warfarin and 2 (3%) (1 with peliosis hepatis and 1 with a haematoma and no underlying pathology) were on treatment-dose low-molecular-weight heparin (enoxaparin). In addition, the oral contraceptive pill and hormone replacement therapy were among the prescriptions of 6 (9%) and 4 patients (6%) with a ruptured adenoma, respectively. Six patients (9%) (1 without any lesion, 3 with adenoma and 2 with peliosis hepatis) were receiving anabolic steroids.
Among the 17 HCC patients, 15 were cirrhotic and the underlying liver disease was of viral aetiology in 10. Of the 15 cirrhotic patients, 3 were CTP class A, 4 were class B and 8 were class C. The levels of AFP were elevated in 10 HCC patients. A diagnosis of HCC was established in 9 patients prior to presentation with SLB. Of the 27 patients diagnosed with adenoma 3 had a known history of multiple adenomatosis. The median follow-up period was 54 months (range 0–193 months).
Management
Adenoma (n = 27)
Twenty-two patients were successfully managed without any intervention. Two patients required selective hepatic artery embolization, and three patients underwent an emergency liver resection (two right hepatectomy and one left hepatectomy). There were no peri-operative deaths. All patients with ruptured adenoma were subsequently followed-up with abdominal US examination every 6 months for the first year and annually thereafter.
During follow-up, two patients had a left lateral segmentectomy (7 months after the bleeding episode), one underwent a right extended hepatectomy (12 months post discharge) and one had a right lobectomy (2 years after rupture) for a sudden increase in tumour size. At a median follow-up of 57 (range 17–193) months all patients were alive.
HCC (n-17)
Twelve patients were managed conservatively and of these only one was non-cirrhotic. Three patients who received palliative treatment owing to advanced liver disease died during the same admission. A selective DSA and embolization was performed in two patients. Three patients underwent a hepatic resection during the same admission after control of haemorrhage surgery; one patient without cirrhosis had a left hepatectomy but died 20 months later because of distant metastases. The second patient (CTP class A cirrhosis) underwent a non-anatomical resection of the lesion and is still alive 5 years after surgery. The third patient who underwent a laparotomy for haemostasis at the local referring hospital had peritoneal disease and died 2 months later. The in-hospital mortality for the HCC group of patients was 3 out of 17 patients.
After initial haemostasis, patients who received conservative treatment had definitive treatment for the underlying tumour as follows: transarterial chemoembolisation (TACE) in 3, radiofrequency ablation (RFA) +/− TACE in 2, a left hemihepatectomy 2 months after discharge in 1 patient, an extended left hepatectomy 4 months after discharge in 1 and palliative treatment for 4 patients. The 1-, 3- and 5-year survival was 59%, 35% and 17%, respectively.
Metastases from colorectal primary (n = 2)
One of the two patients with metastatic adenocarcinoma of colorectal origin was managed conservatively and the other with packing. Both died within the first year after their discharge.
Other benign pathologies
Four patients with polycystic liver disease were initially managed conservatively and subsequently evaluated for potential treatment options. Two patients underwent laparoscopic de-roofing of cysts 3 and 12 months after SLB for symptom relief. All patients are alive at a median follow-up of 65 months (range 48–96).
One patient with nodular regenerative hyperplasia underwent an emergency right hepatectomy after failed radiological control of a haemorrhage. The patient is alive at 4 years follow-up.
One of the two patients with peliosis hepatis underwent an emergency laparotomy and packing at a local hospital but unfortunately died 24 h later. The second patient underwent DSA embolization and is alive 56 months after discharge.
Of the four patients with HELLP syndrome, two underwent a right hemihepatectomy for a right liver lobe infarction and necrosis and the others had an emergency laparotomy for haemostasis. Three of them died during the immediate post-operative period. The fourth patient is alive 13 years after the right hepatectomy.
Both patients with ruptured hepatic artery pseudoaneurysms owing to underlying PAN were successfully managed conservatively, and are alive 8 and 35 months after SLB.
The patient diagnosed with an underlying hemangioma was managed conservatively. Two of the 3 patients with focal nodular hyperplasia underwent a right hemihepatectomy and one had a left lateral segmentectomy. All patients diagnosed with either hemangioma or FNH remain alive at the latest follow-up.
Of the 3 patients with spontaneous liver rupture without identified underlying pathology, 1 patient was managed conservatively and is alive at follow-up. One underwent a right hepatectomy and remains alive 84 months after treatment. The last patient who underwent an emergency laparotomy and packing died on the second.
The emergency management of patients presenting with SLB is outlined as a flow sheet in Fig. 5.
Figure 5.
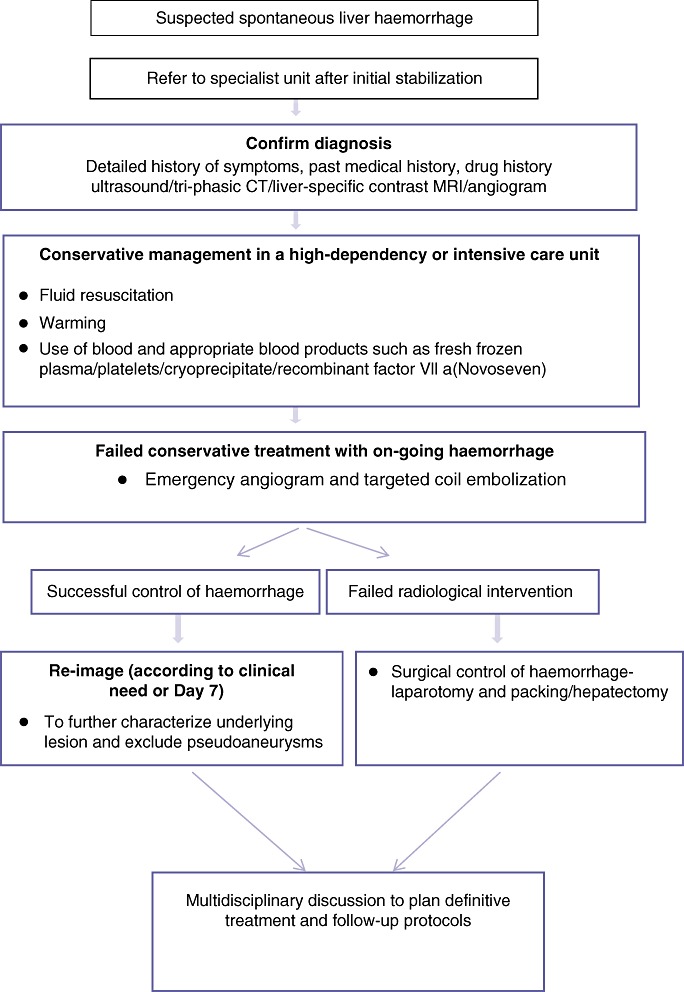
Algorithm for the management of spontaneous liver bleeding (SLB)
Discussion
Spontaneous liver bleeding is a rare life-threatening complication that warrants a timely diagnosis and effective control of haemorrhage. The presenting symptoms are often non-specific and SLB may also be the first manifestation of an unknown malignant or benign liver tumour. Fifty-nine (92%) patients in the present study presented with non-specific signs and symptoms. Some authors have reported an accurate pre-surgical diagnosis of SLB in only 60% of patients.4 Careful history and physical examination in conjunction with appropriate diagnostic work-up can help to establish the correct diagnosis. A triple phase CT scan of the abdomen and pelvis was the imaging modality of choice used for assessment of patients in the present study. A multi-detector CT (MDCT) scan has higher sensitivity and resolutional capacity than ultrasonography and it is faster and more easily accessible in emergency situations compared with MRI.5,6 A conventional angiography remains the gold standard for the diagnosis of an active hemorrhage8; however, the improvement in sensitivity of MDCT placed it at the frontline of the diagnostic armamentarium.7,9,10 The pathognomonic CT finding in the acute setting is active contrast extravasation and depiction of an area of blush with attenuation values similar to or greater than that of an adjacent enhancing vessel.10 In cases of demonstrated active bleeding, the implementation of selective visceral angiography with embolization of the bleeding arterial branches offers an opportunity of managing the patient in a minimal invasive manner and planning a further therapeutic strategy in an elective setting, as demonstrated in this series. Occasionally the only initial finding on US or a CT scan performed as an emergency is a subcapsular hematoma secondary to bleeding of a small underlying lesion in the periphery of the liver that is not detected by imaging.11,12 The stabilization of the patient with conservative measures, such as the embolization of the feeding arteries of the lesion, allow the attending multidisciplinary team to repeat the CT scan or employ MRI imaging for better characterization of the cause of the hemorrhage and definite management of the patient.
The majority of the published literature describing SLB is from the East where the high incidence of HCC makes it the most common underlying cause for bleeding. The management and outcomes of a ruptured HCC very much depend on the severity of underlying liver disease. In this current series the most frequent cause of spontaneous liver bleeding was hepatic adenomas (27 patients, 40%). Hepatic adenomas usually occur in young women on long-term oestrogen therapy13,14 and the risk of a rupture may be as high as 30% to 50% in tumours >4 cm.15–17 The management of patients with bleeding hepatic adenomas depends on their hemodynamic status at the time of admission. In haemodynamically unstable patients after unsuccessful conservative management temporary haemostasis can be achieved with embolization or ligation of the feeding arterial branches or even the main hepatic arterial branch.18 Most experts advocate the delayed resection of the liver adenomas based on the fact that the natural history of such lesions is unpredictable and the cost benefit of long-term surveillance of these patients may outweigh the risks of surgical resection.19,20 In the present study, the patients who were managed conservatively and those who underwent successful coil embolization of the feeding vessels were submitted to a repeat CT scan to exclude pseudoaneurysms or re-bleed. There were no episodes of re-bleeding in the study cohort. The follow-up protocol included twice yearly US examination for the first year and annual US thereafter, along with measurement of AFP. Only patients with multiple adenoma, progressive advancement of their tumour size or suspicion of malignant transformation and those who opted for a surgical treatment subsequently underwent an operation in the form of some kind of hepatectomy. The incidence of malignancy in the entire group of patients with adenoma during the study period (101 patients) was 3% but none of them had SLB. Only in patients where haemostasis could not be attained with conservative measures, an immediate resection with enucleation or a partial hepatectomy was the preferred treatment. The treatment options should be individualized based on the clinical parameters and morphology of the underlying adenoma. All offending medication should be discontinued after a rupture.21,22
The incidence of a spontaneous rupture with HCC is between 3% and 15%.23,24 In the present study, patients with advanced liver disease or disseminated tumours were managed palliative. If signs of continued bleeding were clinically or radiologically evident, attempts at haemostasis with transarterial embolization were made. After the initial stabilization of the patient and precise staging of the disease with consideration of all patient- and tumour-related factors, further curative treatment in the form of a hepatectomy or palliative therapy in the form of TACE, RFA or chemotherapy was implemented. More recent studies have shown that early mortality and the long-term survival rate is not dependent on the modality of treatment but on the patient's disease state, CTP class in the presence of cirrhosis, total bilirubin level and the severity of shock.25–27 An urgent hepatectomy was performed in 2 of the 17 patients after an initial control of the haemorrhage. One of them was non-cirrhotic and the other was CTP class A, both of their tumours were small, single and easily accessible.
Polyarteritis nodosa (PAN) is a rare form of systemic vasculitis that has been associated with visceral artery aneurysm formation as a result of the underlying transmural inflammation. Gastrointestinal involvement is frequently seen in PAN, but isolated vasculitis of the liver with a ruptured hepatic artery aneurysm is an extremely rare initial clinical presentation. A clinical diagnosis is established if 3 of the 10 diagnostic criteria described by the American College of Rheumatologists (ACR) are present.28 In two patients who presented with a ruptured hepatic artery pseudoaneurysm, the diagnosis of PAN was established using ACR criteria. In addition to the administration of the appropriate immunosuppressive therapy for vasculitis, one of these patients underwent multiple percutaneous transarterial embolization of the ruptured pseudoaneurysms. Such cases emphasize the importance of suspecting the presence of some form of vasculitis as the underlying cause when multiple visceral pseudoaneurysms and other pertinent symptoms included in the patient's history are encountered. The aim of acute treatment is initial resuscitation followed by control of the haemorrhage at specialized centres with expert interventional radiologists. Immunosuppression and, if required, cytotoxic agents should be promptly commenced which could be life saving. Long-term follow-up of these patients with interval radiological imaging is essential.
HELLP syndrome is a multisystemic disorder that complicates pregnancy or appears soon after delivery. It is frequently associated with pre-eclampsia or eclampsia. Sudden onset abdominal pain with anaemia or signs of shock along with evidence of haemolysis, altered liver tests, and renal dysfunction on blood profile in the appropriate clinical setting should raise a suspicion of hepatic rupture. Death results largely from complications such as disseminated intravascular coagulation, pulmonary oedema or acute renal insufficiency.29 Aggressive correction of coagulopathy with blood products and organ supportive treatment in an intensive care setting is critical in the management of these patients. The grade of hepatic involvement varies from a minor subcapsular haematoma to an extensive parenchymal rupture. Transarterial embolization of selected vessels or even the right or left hepatic artery pedicles should be considered in patients with extensive parenchymal bleeding. Emergency surgical treatments are reserved for patients unresponsive to minimally invasive radiological interventions or in the event of massive hepatic parenchymal necrosis as encountered in two out of four patients in the present study. Liver transplantation is indicated in the context of fulminant hepatic failure.30–32 The outcomes in this group of patients are poor with a very high in-hospital mortality.
Peliosis hepatis (PH) is a rare condition characterized by the presence of cystic, blood-filled cavities within the hepatic parenchyma. The aetio-pathogenesis of this disease is unclear; however, injury to the sinusoidal endothelium has been proposed to be the most likely pathology. Peliosis hepatis may be associated with an underlying malignancy, acquired immune deficiency syndrome renal or cardiac transplantation or use of medications such as anabolic steroids and oral contraceptives.33 A combination of contrast CT and MRI is the investigation of choice, early globular vessel-like enhancement during arterial phase with centrifugal progression on portal venous and delayed phase without any mass effect on hepatic vessels is consistent with PH. The natural history of this disease has not been clearly defined; limited literature in the form of case reports suggests spontaneous regression or progression to chronic liver disease or a spontaneous liver rupture.34 In patients presenting with SLB after control of a haemorrhage, subsequent management should include stopping the suspected causative medications or treatment of the associated medical conditions.
Nodular regenerative hyperplasia (NRH) is a rare lesion that is associated with non-cirrhotic portal hypertension. The clinical diagnosis is usually incidental and SLB is an extremely rare initial presenting symptom. Although histological diagnosis is the gold standard, a non-invasive clinical diagnosis can be established with the use of a triphasic CT, liver-specific contrast (Gadolinium, Primovist) MRI and serum AFP measurement in combination. It is estimated that NRH is complicated by clinically overt portal hypertension in more than 50% of patients.35,36 The benign nature of the lesion and the high incidence of co-existent portal hypertension favour the avoidance of any unnecessary liver resection even in the presence of complications such as a spontaneous haemorrhage. The emergency treatment of choice is control of the haemorrhage by minimally invasive transarterial embolization followed by investigations to identify any associated diseases. Patients with NRH have a high incidence (74%) of an associated malignancy, prothombotic or rheumatological disease.37 The definitive treatment after control of the haemorrhage should include treatment for any concomitant diseases. In those with associated portal hypertension, the follow-up should aim at prevention and treatment of complications related to portal hypertension.
In the present study, two patients presented with SLB with an underlying metastatic adenocarcinoma of colorectal origin. It is preferable to achieve haemostasis using conservative means including embolization and planned surgical management after patient stabilization.
From the above experience, we suggest that the preferred management plan for SLB is initial haemostasis using conservative or radiological embolization methods followed by appropriate staging investigations to plan a definitive surgical treatment. Emergency surgery should be reserved for failed conservative and radiological intervention treatments. In patients with underlying cirrhosis the outcomes are poor.
In conclusion, spontaneous liver bleeding is a life-threatening complication of various underlying conditions and may represent their first manifestation. Benign liver diseases are the predominant causes for SLB in the West. A high index of suspicion and good decision making are required for prompt diagnosis of the causative pathological entity and implementation of appropriate therapy for each individual patient.
Conflicts of interest
None declared.
References
- 1.Abercrombie J. Hemorrhage of the liver. Lond Med Gaz. 1844;34:792–794. [Google Scholar]
- 2.Recondare A, Bonariol L, Caratozzlo E, Callegari F, Bruno G, Dipaola F, et al. Management of spontaneous bleeding due to hepatocellular carcinoma. Minerva Chir. 2002;57:347–356. [PubMed] [Google Scholar]
- 3.Chen ZY, Qi QH, Dong ZL. Etiology and management of hemorrhage in spontaneous liver rupture: a report of 70 cases. World J Gastroenterol. 2002;8:1063–1066. doi: 10.3748/wjg.v8.i6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergara V, Muratone A, Bouzari H. Spontaneous rupture of hepatocellular carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770–772. doi: 10.1053/ejso.2000.1001. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AG, Jr, Duarte AC. Clinical presentation and management of liver adenoma hemorrhagic complications. Am Surg. 2010;76:654–655. [PubMed] [Google Scholar]
- 6.Choi BG, Park SH, Byun JY, Jung SE, Choi KH, Han JY. The findings of ruptured hepatocellular carcinoma on helical CT. Br J Radiol. 2001;74:142–146. doi: 10.1259/bjr.74.878.740142. [DOI] [PubMed] [Google Scholar]
- 7.Roy-Choudhury SH, Gallacher D, Pilmer J, Rankin S, Fowler G, Steers J, et al. Relative threshold of detection of active arterial bleeding: in vitro comparison of MDCT and Digital Subtraction Angiography. AJR. 2007;189:238–246. doi: 10.2214/AJR.07.2290. [DOI] [PubMed] [Google Scholar]
- 8.Baum S, Nusbaum M, Blakemore WS, Finklestein AK. The preoperative radiographic demonstration of intra-abdominal bleeding from undetermined sites by percutaneous selective celiac and superior mesenteric arteriography. Surgery. 1965;58:797–805. [PubMed] [Google Scholar]
- 9.Alani A, Ring EJ. Localization of gastrointestinal bleeding superiority of 99mTc sulfur colloid compared with angiography. AJR. 1981;137:741–748. doi: 10.2214/ajr.137.4.741. [DOI] [PubMed] [Google Scholar]
- 10.Sivit CJ, Paclet MH, Taylor GA. Life-threatening intraperitoneal bleeding demonstration with CT. Radiology. 1989;171:430. doi: 10.1148/radiology.171.2.2704807. [DOI] [PubMed] [Google Scholar]
- 11.Ulu EM, Arzu U, Ekici Y, Hunca C, Coskun M. Multidetector CT findings of spontaneous rupture of hepatic adenoma in a patient with hepatic adenomatosis. Diagn Interv Radiol. 2009;15:135–138. [PubMed] [Google Scholar]
- 12.Casillas VJ, Amenclola MA, Gascue A, Pinnar N, Levi JU, Perez JM. Imaging of nontraumatic hemorrhagic hepatic lesions. Radiographics. 2000;20:367–378. doi: 10.1148/radiographics.20.2.g00mc10367. [DOI] [PubMed] [Google Scholar]
- 13.Grazioli I, Federle MP, Ichikawa T, Balzano F, Nalesnik M, Maclariaga J. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology. 2000;216:395–402. doi: 10.1148/radiology.216.2.r00jl38395. [DOI] [PubMed] [Google Scholar]
- 14.Psalha PA, Semelka RC, Armao D, Woosley JT, Firal Z, Schneider G. Hepatocellular adenomas in men: MRI findings in four patients. J Magn Reson Imaging. 2005;22:258–264. doi: 10.1002/jmri.20375. [DOI] [PubMed] [Google Scholar]
- 15.Hugh TJ, Poston GJ. Benign liver tumor and masses. In: Blumgart LH, Fong Y, editors. Surgery of the Liver and Biliary Tract. Philadelphia, PA: WB Saunders Co. Ltd; 2000. pp. 1397–1422. [Google Scholar]
- 16.Gibbs J, Litwin A, Kahlenberg M. Contemporary management of benign liver tumors. Surg Clin N Am. 2004;84:463–480. doi: 10.1016/j.suc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Leese T, Farges O, Bismuth H. Lver cell adenoma. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558–564. doi: 10.1097/00000658-198811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zonder van PE, Tilanus HW, et al. Treatment of ruptured hepatocellular adenoma. Br J Surg. 2001;88:207–209. doi: 10.1046/j.1365-2168.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagorney DM. Are hepatic adenomata premalignant? HPB Surgery. 1996;10:59–61. doi: 10.1155/1996/59629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumors. Dig Surg. 2002;19:109–113. doi: 10.1159/000052022. [DOI] [PubMed] [Google Scholar]
- 21.Muller J, Keeffee EB, Esquivel CO. Liver transplantation for treatment of giant hepatocellular adenomas. Liver Transpl Surg. 1995;1:99. doi: 10.1002/lt.500010205. [DOI] [PubMed] [Google Scholar]
- 22.Weimann A, Ringe B, Kempnauer J, Lamesch P, Gratz KF, Prokop M, et al. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg. 1997;21:983–990. doi: 10.1007/s002689900337. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto M, Sudo T, Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. 1991;86:67–71. [PubMed] [Google Scholar]
- 24.Battula N, Madanur M, Priest O, Srinivasan P, O'Grady J, Heneghan M, et al. Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg. 2009;197:164–167. doi: 10.1016/j.amjsurg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma. A systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 26.Ong GB, Taw JL. Spontaneous rupture of hepatocellular carcinoma. BMJ. 1972;4:146–149. doi: 10.1136/bmj.4.5833.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CL, Fan ST, Lo CM. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725–3733. doi: 10.1200/JCO.2001.19.17.3725. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MD, Savage CO. Vasculitis in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005;19:215–233. doi: 10.1016/j.bpg.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981–991. doi: 10.1097/01.AOG.0000126245.35811.2a. [DOI] [PubMed] [Google Scholar]
- 30.Nunes JO, Turner MA, Fulcher AS. Abdominal imaging features of HELLP syndrome: a 10-year retrospective review. AJR. 2005;185:1205–1210. doi: 10.2214/AJR.04.0817. [DOI] [PubMed] [Google Scholar]
- 31.Araujo AC, Leao MD, Nobrega MH, Bezerra PF, Pereira FV, Dantas EM, et al. Characteristics and treatment of hepatic rupture caused by HELLP syndrome. Am J Obstet Gynecol. 2006;195:129–133. doi: 10.1016/j.ajog.2006.01.016. Epub 2006 Mar 30. [DOI] [PubMed] [Google Scholar]
- 32.Shames BD, Fernandez LA, Sollinger HW. Liver transplantation for HELLP syndrome. Liver Transpl. 2005;11:224–228. doi: 10.1002/lt.20285. [DOI] [PubMed] [Google Scholar]
- 33.Choi SK, Jin JS, Cho SG, Choi SJ, Kim CS, Choe YM, et al. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009;15:5493–5497. doi: 10.3748/wjg.15.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouya H, Vignaux O, Legmann P, de Pigneux G, Bonnin A. Peliosis hepatis: triphasic helical CT and dynamic MRI findings. Abdom Imaging. 2001;26:507–509. doi: 10.1007/s00261-001-0023-x. [DOI] [PubMed] [Google Scholar]
- 35.Ferlitsch A, Teml A, Reinisch W, Ulbrich G, Wrba F, Homoncik M, et al. 6-thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol. 2007;102:2495. doi: 10.1111/j.1572-0241.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 36.Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400–1409. doi: 10.3748/wjg.v17.i11.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, Stanley AJ, et al. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001–1005. doi: 10.1097/MEG.0b013e3283360021. [DOI] [PubMed] [Google Scholar]


