Abstract
Objectives
Laparoscopic resection for benign liver disease has gained wide acceptance in recent years and hepatocellular adenoma (HA) seems to be an appropriate indication. This study aimed to discuss diagnosis and treatment strategies, and to assess the feasibility, safety and outcomes of pure laparoscopic liver resection (LLR) in a large series of patients with HA.
Methods
Of 88 patients who underwent pure LLR, 31 were identified as having HA. Diagnosis was based on radiological evaluation and resections were performed for lesions measuring >5.0 cm.
Results
The sample included 29 female and two male patients. Their mean age was 33.2 years. A total of 27 patients had a single lesion, one patient had two and one had four lesions. The two remaining patients had liver adenomatosis. Mean tumour size was 7.5 cm. Three right hepatectomies, 17 left lateral sectionectomies and 11 wedge resections or segmentectomies were performed. There was no need for blood transfusion or conversion to open surgery. Postoperative complications occurred in two patients. Mean hospital stay was 3.8 days.
Conclusions
Hepatocellular adenoma should be regarded as an excellent indication for pure LLR. Pure LLR is safe and feasible and should be considered the standard of care for the treatment of HA when performed by surgeons with experience in liver and laparoscopic surgery.
Keywords: liver surgery, hepatectomy, laparoscopy, hepatocellular adenoma, liver adenoma, laparoscopic liver resection
Introduction
Hepatocellular adenoma (HA) is a rare, benign, solid tumour of the liver,1,2 the incidence of which is increasing, especially in young women, as a result of the widespread use of oral contraceptives.3,4 The relationship between the use of oral contraceptives, particularly in high doses and for long periods, and the development of HA is well established.5,6 This tumour can also be associated with the use of anabolic steroids, type 1 glycogen storage disease, beta thalassaemia and haemochromatosis. Moreover, the disease is now diagnosed more frequently as a result of more accurate and commonly employed imaging studies.4 The annual estimated incidence of HA is three per 1 000 000 population.5
The natural history of patients with HA is not completely understood and treatment strategies are not completely defined. In patients with adenomas measuring <5.0 cm, most groups recommend the suspension of contraceptives and observation based on reports showing tumour regression or even disappearance.7,8 However, in HA sized >5.0 cm in diameter, surgical treatment is recommended because of the risk for malignant transformation, which can occur in 5–8% of patients, and the risk for rupture and haemorrhage, observed in 21–29% of cases.7,9,10 Treatment decisions are mostly based on the size of the lesion, although a recent paper has included gender as a relevant indicator for treatment, irrespective of lesion size, because of a 50% risk for malignant change.11
Laparoscopic approaches have been employed in the treatment of various benign and malignant diseases of the liver.12–15 Moreover, technical advances and patient outcomes that are comparable with or even superior to those of open surgery have resulted in their acceptance. It is recommended that any such laparoscopic procedure is performed by a surgeon who is experienced in liver surgery and trained in advanced laparoscopy. It has been suggested that the best candidates for this approach are patients with peripheral lesions located in the left lateral (segments II and III) and anterior (segments IVb, V and VI) segments, who require limited resections.16–18 Recently, however, major liver resections (more than three liver segments) have been reported as safe and feasible in specialized centres.19–22 The advantages of laparoscopic liver resection (LLR) include less postoperative pain, shorter length of hospital stay, shorter time to recovery and a lower incisional hernia rate.18,23,24 Given that the laparoscopic approach offers equivalent surgical treatment, including the advantages described above, in addition to improved aesthetic outcomes, it has gained wide acceptance for the treatment of benign liver disease, especially HA.13,18,23
Only a few series in the literature have focused on the laparoscopic treatment of benign liver tumours, and most of these are heterogeneous, involving patients with different benign disorders.13,18,22,23,25 Only one recent study has focused specifically on the results of laparoscopic treatment of HA.26 The aim of the present study is to debate treatment strategies and to assess the feasibility, safety and outcomes of pure LLR (PLLR) in the largest series yet reported of patients with HA.
Materials and methods
Patients undergoing pure laparoscopic resection between 2007 and 2011 at one centre were identified from a prospective database. Of these 88 patients, 34 had a preoperative diagnosis of HA. Histological specimen examination confirmed the diagnosis of HA in 31 patients; in three patients histology showed focal nodular hyperplasia (Table 1). During the same period, three other patients with HA underwent conventional open surgery. In one of these, surgery was performed following rupture and intrahepatic haemorrhage; another had a centrally located lesion, and the third had two large nodules (sized >8.0 cm) on either side of the liver.
Table 1.
Indications for laparoscopic liver resection in 88 patients
| Indication | Patients, n |
| Liver adenoma | 31 |
| Colorectal metastases | 24 |
| Hepatocellular carcinoma | 16 |
| Biliary cystadenoma | 5 |
| Focal nodular hyperplasia | 3 |
| Breast cancer metastasis | 2 |
| Melanoma metastases | 2 |
| Fibrolamellar hepatocellular carcinoma | 1 |
| Peripheric cholangiocarcinoma | 1 |
| Haemangioendothelioma | 1 |
| Sarcoma metastases | 1 |
| Liver fibroma | 1 |
All patients were assessed according to their clinical history, physical examination and laboratory tests and were classified according to their American Society of Anesthesiologists (ASA) physical status score.27
Preoperative workup involved abdominal ultrasonography (US), computed tomography (CT) and/or magnetic resonance imaging (MRI). In cases of doubt, scintigraphies with Tc-99m hepatic iminodiacetic acid (HIDA) and sulphur colloid were performed. Preoperative US- or CT-guided biopsies were employed selectively only when diagnosis remained unclear after radiological investigation (Fig. 1).
Figure 1.
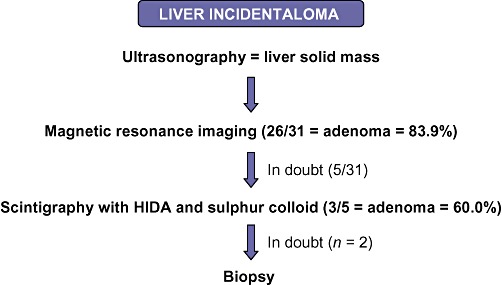
Diagnostic algorithm of hepatic incidentaloma in 31 patients with hepatocellular adenoma treated by laparoscopic liver resection. HIDA, Tc-99m hepatic iminodiacetic acid
All symptomatic patients and those with lesions of >5.0 cm were evaluated by a multidisciplinary team for resection. In patients with hepatic adenomatosis (10 or more HA lesions), only lesions of >5.0 cm were resected. Surgery was performed by the same surgical team in an elective setting in all cases.
Patients with peripheral nodules were submitted to non-anatomic resections as nodulectomies (sub-segmentectomies). In patients with large lesions, an anatomic resection with prior pedicle control was preferred. The Pringle manoeuvre was not routinely employed.
Data on the number, size and location of lesions, type of surgery performed, need for conversion to open surgery, length of intensive care unit (ICU) and hospital stay, blood transfusion requirements, perioperative complications and longterm outcomes were analysed.
Results
Of 34 patients with HA treated in the 4-year period 2007–2011, 31 (91.2%) underwent PLLR. Of these, 29 patients were female and two were male. The mean age of the sample was 33.2 years (range: 20–46 years). A history of oral contraceptive use was present in 25 cases.
The most common form of presentation was an incidental radiological finding (n = 21 patients), followed by abdominal pain (n = 9). Images produced by CT and MRI and analysed by expert radiologists predicted the diagnosis in 26 cases (83.9%). A diagnosis of HA was suggested in three of five patients, who underwent scintigraphy with HIDA and sulphur colloid. Two patients underwent CT-guided percutaneous biopsy to confirm the diagnosis (Fig. 1). Of the 31 patients, 30 were classified according to physical status as ASA grade I and one as ASA grade II.
Lesions were confined to the right hemiliver in nine patients and to the left hemiliver in 22 patients. A total of 27 patients had one lesion, one had two lesions and one had four lesions. Two patients had liver adenomatosis with more than 10 lesions (Table 2). In patients with adenomatosis, only tumours measuring >5.0 cm were resected. Mean tumour size was 7.5 cm (range: 3.0–14.0 cm).
Table 2.
Characteristics of patients with hepatic adenoma
| Patient | Age, years | Resected tumour characteristics | Surgical procedure |
|---|---|---|---|
| 1 | 40 | 3.0 cm, segment V | Non-anatomic resection |
| 2 | 37 | 3.0 cm, segment VI | Segmentectomy VI |
| 3 | 28 | 4.0 cm, segment IVb | Sub-segmentectomy IVb |
| 4 | 36 | 5.0 cm, segment II | Left lateral sectionectomy |
| 5 | 39 | 3.0 cm, segment II | Left lateral sectionectomy |
| 6 | 29 | 5.0 cm, segment III | Left lateral sectionectomy |
| 7 | 37 | 3.8 cm, segment III | Non-anatomic resection |
| 8 | 32 | 5.7 cm, segment III | Left lateral sectionectomy |
| 9 | 41 | 3.2 cm, segments II and III | Left lateral sectionectomy |
| 10 | 34 | 3.7 cm, segments II and III | Left lateral sectionectomy |
| 11 | 28 | 9.0 cm, segments VII and VIII | Right hepatectomy |
| 12 | 41 | 10.0 cm, segments VI and VII | Right hepatectomy |
| 13 | 45 | 10.5 cm, segment Va | Non-anatomic resection |
| 14 | 32 | One 5.0-cm lesion, three 1.5-cm lesions, all in right lobe | Right hepatectomy |
| 15 | 46 | 12.0 cm, segment IIa | Left lateral sectionectomy |
| 16 | 36 | 5.0 cm, segment VI | Non-anatomic resection |
| 17 | 36 | 8.0 cm, segment IVb | Sub-segmentectomy IVb |
| 18 | 35 | 8.0 cm, segments II and III | Left lateral sectionectomy |
| 19 | 42 | One 4.0-cm lesion (segment II), one 3.0-cm lesion (segment III) | Left lateral sectionectomy |
| 20 | 33 | 14.0 cm, segments II and III | Left lateral sectionectomy |
| 21 | 28 | 10.0 cm, segment III | Segmentectomy III |
| 22 | 29 | 5.0 cm, segments II and III | Left lateral sectionectomy |
| 23 | 32 | 10.0 cm, segments II and III | Left lateral sectionectomy |
| 24 | 29 | 9.0 cm, segment III | Non-anatomic resection |
| 25 | 20 | 8.0 cm, segment VI | Segmentectomy VI |
| 26 | 36 | 10.0 cm, segment VI | Non-anatomic resection |
| 27 | 31 | 10.0 cm, segments II and III | Left lateral sectionectomy |
Patient with liver adenomatosis (>10 lesions).
Operative procedures are shown in Table 2. There were neither intraoperative complications nor any need for conversion to open surgery. No patient had significant intraoperative bleeding. No patient required a Pringle manoeuvre or blood transfusion.
All patients with a diagnosis of HA achieved clear resection margins confirmed by histological examination of the surgical specimen.
Three patients, all of whom submitted to major resection, were transferred to the ICU for postoperative observation and were discharged on the first postoperative day. There were no instances of bile leak or abdominal collection postoperatively.
Postoperative complications occurred in two patients (6.5%). One patient, who underwent right hepatectomy, developed an empyema of the right hemi-thorax thought to have resulted from a small missed diaphragmatic injury, which was diagnosed on postoperative day 15 when the patient was readmitted to hospital with symptoms of fever and dyspnoea. After chest CT and thoracocentesis, the diagnosis of pleural empyema was established and a chest tube inserted. Drainage failed and the patient subsequently required thoracoscopic pleural decortication from which he recovered uneventfully and was discharged after 6 days. Another patient presented with an incisional hernia at a 12-mm trocar port site after 6 months of follow-up.
The mean postoperative hospital stay was 3.8 days (range: 1–15 days). All patients were alive and no recurrences were observed after a mean follow-up of 26 months (range: 2–50 months).
Discussion
Since the first LLR, described by Gagner et al.28 in 1992, there have been considerable development and employment of this technique. Ferzli et al.29 were the first to report a non-anatomic LLR in a patient with a 9.0-cm HA located in segment IV. Furthermore, Azagra et al.30 performed the first anatomic resection in a patient with HA located in segments II and III.
Although there have been no randomized studies comparing open with laparoscopic surgical approaches in liver resection, several authors have demonstrated the safety of LLR for the treatment of benign and malignant lesions in retrospectives series.14,23,31–36 However, only one study has specifically addressed the feasibility, safety and outcomes of LLR in patients with HA.26
The experience described in the present study shows that there is only a limited role for open surgery for HA. It has been previously demonstrated that when patients with bleeding HA receive arterial embolization, their haemodynamics stabilize, the haematoma is reabsorbed and, in some cases, the tumour decreases in size, making resection safer and easier.7,37 In the present series, it was considered appropriate to undertake open surgery in one patient who presented with this type of complication and in two other patients with poorly accessible lesions.
Because LLR may allow for faster recovery, a shorter hospital stay, a lower risk for incisional hernia and good cosmetic results, there has been debate regarding the indications for minimally invasive treatment for benign liver tumours.18,23,24 Koffron et al.38 reported a large series of patients undergoing LLR, including a significant number of patients operated on for benign disease, and suggested a possible change in the management of young women with benign liver tumours whereby a more liberal indication for resection should be adopted. However, in a more recent report, the same authors returned to a more selective approach for resection.32 Buell et al.17 and Bryant et al.39 argued against widening the indications for resection of benign liver tumours, which they consider justified only by the fact that the surgical procedure involved is minimally invasive. These authors have emphasized that the indications for laparoscopic resection of benign liver tumours should follow the same criteria as those used for open surgery.17,39 It is important to emphasize that liver resection, even when performed laparoscopically, can lead to more complications than the benign tumour itself.
There seems to be no doubt that HA is an excellent indication for LLR; in the present experience, HA accounted for 35.2% of all instances of PLLR. Indications for HA resection include the presence of symptoms, diagnostic uncertainty and the risk for complications such as haemorrhage or malignant transformation. As well as symptoms, treatment decisions are mostly based on gender and the size of the lesion. A recent review has shown that 96% of HA complicated by malignant transformation measured >5.0 cm.40 Farges et al. recently showed that the prevalence of malignancy is 10 times greater in men than in women and recommended the routine resection of HA in men, irrespective of lesion size.11
Cho et al.9 recommended that in view of the lifelong risks for malignancy (5%) and bleeding (20–30%), most patients without comorbidities who have surgically favourable HA should undergo resection regardless of lesion size. Despite the low morbidity and mortality rates of minimally invasive liver resection, small lesions measuring 3.0–5.0 cm in asymptomatic patients should be subject to observation because data favouring resection are lacking. Other less invasive procedures, such as radiofrequency ablation and arterial embolization, employed in small series have not yet been proven effective.10,37,41
It is generally believed that asymptomatic patients with lesions of <3.0 cm should be observed and oral contraceptives discontinued. Indeed, some studies have reported occasional HA regression after the discontinuation of oral contraceptives,8,42 although in most cases lesions remained stable.7,43
Another contentious point refers to patients with liver adenomatosis (more than 10 liver adenomas). Dokmak et al.7 have recently shown that complications in these patients are related not to the number of nodules, but to the adenoma subtype and lesion size. These authors suggest that in patients with adenomatosis, only lesions of >5.0 cm should be resected.7
In the present series, 17 left lateral sectionectomies were performed. Left lateral and anterior segment resections have been reported as systematically safe and feasible and the present experience supports the views of others that left lateral resections should be routinely performed laparoscopically.30,33,34,44
It is not clear if large adenomas involving more than three segments should be treated with open or laparoscopic major liver resection. Nowadays, however, PLLR major resections can be performed safely as a result of technical advances and increased experience in surgeons. Indeed, reports from specialized centres have shown low morbidity and mortality rates following right hepatectomies or even extended liver resections.19–22,45,46 Although resections of segments II and III were performed in patients with large tumours, two of the three patients submitted to right hepatectomies had tumours measuring >9.0 cm and one had four lesions all located in the right hemiliver. Anatomic resection with pedicle control avoided bleeding and therefore no patient required a Pringle manoeuvre, transfusion or conversion to open surgery.
To date, surgical indications for HA have been based on morphological and clinical criteria. In recent years, a new system of classification of HA based on genetic alterations has been proposed. Adenomas are classified into three groups according to, respectively, the expression of hepatocyte nuclear factor 1-α (HNF1α) and mutation of β-catenin; the third group shows no known mutation.1,2 In this latter group without mutation, two subgroups have been identified, of which one demonstrates inflammatory tissue and the other does not. Patients with HNF1α mutations present more frequently with multiple lesions, pronounced liver steatosis and lower malignant transformation rates (<5%). Patients with β-catenin mutations are usually men and have less steatotic and inflammatory findings but higher rates of malignant transformation. Patients with inflammatory type HA may be more susceptible to bleeding because of sinusoidal dilatation.1,47 The clinical application of this new system of classification is under investigation and deserves further study.1 The genetic profile of the present group of patients is under investigation. In the near future, patient selection for surgery will probably take into account clinical, histological and molecular alterations. At present, each centre should define its own policy for indications for and type of resection.
In conclusion, HA seems to be the best indication for LLR. Pure LLR for hepatic adenoma is feasible and safe, and results in low morbidity and mortality rates, even when major resection is necessary. Laparoscopic resection should be considered as a new standard of care for the treatment of HA when performed by surgeons with experience in liver and laparoscopic surgery.
Conflicts of interest
None declared.
References
- 1.Bioulac-Sage P, Blanc JF, Rebouissou S, Balabaud C, Zucman-Rossi J. Genotype phenotype classification of hepatocellular adenoma. World J Gastroenterol. 2007;13:2649–2654. doi: 10.3748/wjg.v13.i19.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 3.Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316–321. doi: 10.1007/s12072-008-9075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman P, Pugliese V, Machado MA, Montagnini AL, Salem MZ, Bacchella T, et al. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg. 2000;24:372–376. doi: 10.1007/s002689910059. [DOI] [PubMed] [Google Scholar]
- 5.Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- 6.Rosenberg L. The risk of liver neoplasia in relation to combined oral contraceptive use. Contraception. 1991;43:643–652. doi: 10.1016/0010-7824(91)90007-3. [DOI] [PubMed] [Google Scholar]
- 7.Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-centre surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 8.Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, et al. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33:234–236. doi: 10.1097/00004836-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Cho SW, Marsh W, Steel J, Holloway SE, Heckman JT, Ochoa ER, et al. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol. 2008;15:2795–2803. doi: 10.1245/s10434-008-0090-0. [DOI] [PubMed] [Google Scholar]
- 10.Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicentre analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640–648. doi: 10.1245/s10434-008-0275-6. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]
- 12.Katkhouda N, Mavor E, Gugenheim J, Mouiel J. Laparoscopic management of benign cystic lesions of the liver. J Hepatobiliary Pancreat Surg. 2000;7:212–217. doi: 10.1007/s005340050178. [DOI] [PubMed] [Google Scholar]
- 13.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumours. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 14.Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, Belli A, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–1048. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]
- 15.Abu Hilal M, Underwood T, Zuccaro M, Primrose J, Pearce N. Short- and medium-term results of totally laparoscopic resection for colorectal liver metastases. Br J Surg. 2010;97:927–933. doi: 10.1002/bjs.7034. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection – 2804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 17.Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 18.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs. open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 19.Saint Marc O, Cogliandolo A, Piquard A, Famà F, Pidoto RR. Early experience with laparoscopic major liver resections: a case–comparison study. Surg Laparosc Endosc Percutan Tech. 2008;18:551–555. doi: 10.1097/SLE.0b013e318180c93b. [DOI] [PubMed] [Google Scholar]
- 20.O'Rourke N, Fielding G. Laparoscopic right hepatectomy: surgical technique. J Gastrointest Surg. 2004;8:213–216. doi: 10.1016/j.gassur.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Gumbs AA, Bar-Zakai B, Gayet B. Totally laparoscopic extended left hepatectomy. J Gastrointest Surg. 2008;12:1152. doi: 10.1007/s11605-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 22.Dagher I, O'Rourke N, Geller D, Cherqui D, Belli G, Gamblin C, et al. Laparoscopic major hepatectomy. An evolution in standard of care. Ann Surg. 2009;250:856–860. doi: 10.1097/SLA.0b013e3181bcaf46. [DOI] [PubMed] [Google Scholar]
- 23.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumours. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 24.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–248. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 25.Gigot JF, Hubert C, Banice R, Kendrick ML. Laparoscopic management of benign liver diseases: where are we? HPB. 2004;6:197–212. doi: 10.1080/13651820410023950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Hilal M, Di Fabio F, Wiltshire RD, Hamdan M, Layfield DM, Pearce NW. Laparoscopic liver resection for hepatocellular adenoma. World J Gastrointest Surg. 2011;3:101–105. doi: 10.4240/wjgs.v3.i7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Society of Anesthesiologists. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 28.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumour. Surg Endosc. 1992;6:99. [Google Scholar]
- 29.Ferzli G, David A, Kiel T. Laparoscopic resection of a large hepatic tumour. Surg Endosc. 1995;9:733–735. doi: 10.1007/BF00187953. [DOI] [PubMed] [Google Scholar]
- 30.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy – technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 31.Abu Hilal M, Pearce NW. Laparoscopic left lateral liver sectionectomy: a safe, efficient, reproducible technique. Dig Surg. 2008;25:305–308. doi: 10.1159/000155222. [DOI] [PubMed] [Google Scholar]
- 32.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392. doi: 10.1097/SLA.0b013e318146996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–811. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Abu Hilal M, McPhail MJ, Zeidan B, Zeidan S, Hallam MJ, Armstrong T, et al. Laparoscopic versus open left lateral hepatic sectionectomy: a comparative study. Eur J Surg Oncol. 2008;34:1285–1288. doi: 10.1016/j.ejso.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case–control study. J Am Coll Surg. 2003;96:236–242. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 36.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoot JH, van der Linden E, Terpstra OT. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249–1253. doi: 10.1002/bjs.5779. [DOI] [PubMed] [Google Scholar]
- 38.Koffron A, Geller D, Gamblin TC, Abecassis M. Laparoscopic liver surgery: shifting the management of liver tumours. Hepatology. 2006;44:1694–1700. doi: 10.1002/hep.21485. [DOI] [PubMed] [Google Scholar]
- 39.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection – understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 40.Stoot JH, Coelen RJ, De Jong MC, De Jong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB. 2010;12:509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atwell TD, Brandhagen DJ, Charboneau JW, Nagorney DM, Callstrom MR, Farrell MA. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol. 2005;184:828–831. doi: 10.2214/ajr.184.3.01840828. [DOI] [PubMed] [Google Scholar]
- 42.Edmondson HA, Reynolds TB, Henderson B. Regression of liver cell adenomas associated with oral contraceptives. Ann Intern Med. 1977;86:180–182. doi: 10.7326/0003-4819-86-2-180. [DOI] [PubMed] [Google Scholar]
- 43.Marks WH, Thompson N, Appleman H. Failure of hepatic adenomas to regress after discontinuance of oral contraceptives. An association with focal nodular hyperplasia and uterine leiomyoma. Ann Surg. 1988;208:190–195. doi: 10.1097/00000658-198808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94:58–63. doi: 10.1002/bjs.5562. [DOI] [PubMed] [Google Scholar]
- 45.Dagher I, Di Giuro G, Dubrez J, Lainas P, Smadja C, Franco D. Laparoscopic versus open right hepatectomy: a comparative study. Am J Surg. 2009;198:173–177. doi: 10.1016/j.amjsurg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Koffron AJ, Kung R, Baker T, Fryer J, Clark L, Abecassis M. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant. 2006;6:2522–2525. doi: 10.1111/j.1600-6143.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 47.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]


