Abstract
Objectives
After portosystemic anastomoses for biliopathy, some patients continue to suffer biliary obstruction. The effects of splenectomy and devascularization of the abdominal oesophagus and upper stomach are unclear. The aim of the current study was to determine the features of portal biliopathy (PB) in patients with non-cirrhotic portal hypertension, and to investigate outcomes in these patients after surgical procedures.
Methods
A retrospective study of 56 patients who underwent surgery for PB during 1996–2010 was conducted. Data on presenting features, treatment received and outcomes were analysed.
Results
In total, 41 of these patients had extrahepatic portal venous obstruction and 15 had non-cirrhotic portal fibrosis. Forty patients underwent shunt surgery and 16 underwent splenectomy and devascularization. Median bilirubin levels fell from 1.8 mg/dl (range: 0.4–5.9 mg/dl) to 1.0 mg/dl (range: 0.3–5.4 mg/dl) after shunt surgery and from 1.9 mg/dl (range: 0.6–4.0 mg/dl) to 1.2 mg/dl (range: 0.6–5.2 mg/dl) after splenectomy–devascularization. On follow-up, five of 33 patients had persistent jaundice after successful shunt surgery. These patients had a history of multiple endoscopic stentings and three patients had demonstrated a dominant common bile duct stricture preoperatively.
Conclusions
Portal biliopathy was reversed in 38 of 43 patients by either portosystemic shunting or splenectomy–devascularization. In five patients, direct biliary decompressive procedures were required because of shunt blockage or a non-reversible biliary stricture.
Keywords: pseudosclerosing cholangitis, extrahepatic portal venous obstruction, non-cirrhotic portal fibrosis, proximal lienorenal shunt, splenectomy–devascularization, biliary obstruction
Introduction
Although changes in the biliary tract in patients with portal hypertension were first described as early as 1944,1 the term ‘portal biliopathy’ was not coined until the early 1990s, when it was used to define structural changes in the extra- or intrahepatic bile ducts secondary to portal hypertension.2 Portal biliopathy (PB) is most commonly seen in extrahepatic portal venous obstruction (EHPVO), in which it has been reported to occur in 80–100% of patients, although it is asymptomatic in the majority.3,4 The condition has also been described in non-cirrhotic portal fibrosis (NCPF) and cirrhosis, albeit in smaller proportions of patients.5 Symptomatic PB is often managed by endoscopic stent insertion into the common bile duct (CBD). Choudhuri et al.6 first described a patient in whom a portosystemic shunt procedure was effective in reversing the radiological and biochemical biliary changes in symptomatic PB. Later, Chaudhary et al.7 described seven patients with PB who underwent portal decompression. However, two of these patients, despite having patent anastomoses, continued to require postoperative endoscopic stenting, indicating that effective portal decompression does not always reverse PB.
Several questions regarding the management of PB remain unanswered, including those concerning how to identify patients who will benefit from surgical intervention, the optimal operation (shunt surgery, devascularization procedures, splenectomy), and the percentage of patients who will require subsequent decompression of the biliary tree.
The aim of the current investigation was to evaluate the presenting features of PB in patients with non-cirrhotic portal hypertension (EHPVO and NCPF) who underwent surgical interventions (both shunt and non-shunt procedures) and to study short- and longterm outcomes in these patients.
Materials and methods
A retrospective review of data from a prospectively maintained database was performed. All patients who underwent surgical interventions for non-cirrhotic hypertension (EHPVO and NCPF) between 1996 and 2010 and who had symptomatic, biochemical or radiological findings suggestive of PB were identified and selected for analysis. Patients with cirrhosis of the liver were excluded from the study.
Preoperative data collected included the demographic profile of the patients, their presenting complaints, indications for surgery, preoperative endoscopic treatment details, and preoperative laboratory investigations. Perioperative details included the type of surgery, intraoperative findings and immediate, early and delayed laboratory values. Postoperative follow-up details included the incidence of recurrent bleeding, recurrence or appearance of symptoms of biliary obstruction, endoscopic interventions, the presence or absence of signs of progressive end-stage liver disease, development of cholelithiasis or choledocholithiasis, requirement for bilioenteric bypass or cholecystectomy, as well as data on quality of life as indicated by return to work, presence or absence of symptoms of biliary obstruction, relief from frequent hospital visits and the level of general well-being of the patient. Follow-up was conducted by studying outpatient visits or by telephone and written communication.
Extrahepatic portal venous obstruction was defined as the obstruction of the extrahepatic portal vein with or without involvement of the intrahepatic portal veins. It did not include isolated thrombosis of the splenic vein or superior mesenteric vein (SMV). This form of hypertension is characterized by features of portal hypertension with a portal cavernoma.8
Non-cirrhotic portal fibrosis was defined as a disease of uncertain aetiology characterized by a normally functioning liver, a large spleen and a dilated unobstructed portal venous system. The liver biopsy often shows periportal fibrosis and thromboses in the small and medium branches of the portal vein.9
Portal biliopathy was defined in the current study as indicated by an elevation of total bilirubin to >1.5 mg/dl with a predominant increase in direct bilirubin and/or elevated serum alkaline phosphatase (SAP) (>125 IU/ml), or according to evidence on computed tomography (CT) or magnetic resonance imaging (MRI) of dilatation of extra- or intrahepatic biliary radicals, single or multiple strictures, CBD stones or a beaded appearance of the CBD.
Portal biliopathy was categorized as symptomatic when the patient's presenting complaints included jaundice, pruritus, pain, fever or a history of single or multiple endoscopic retrograde cholangiopancreatography (ERCP) with or without stenting. It was considered to be asymptomatic if the biochemical and/or imaging findings were suggestive of the bile duct abnormalities described earlier and the patient had no symptoms of biliary obstruction. Endoscopic retrograde cholangiopancreatography was performed only in symptomatic patients with features of cholangitis or bile duct stones.
Resolution of PB was defined in symptomatic patients as relief from symptoms of biliary obstruction and in asymptomatic patients as a significant decrease in bilirubin and SAP levels or a reversal of radiological signs of biliary obstruction. Failure of a surgical intervention was defined as an inability to achieve resolution of PB.
The preferred surgical intervention was a portosystemic shunt procedure. The choice of shunt surgery was based on the surgeon's preference, the presence or absence of massive splenomegaly with hypersplenism, a previous history of splenectomy or shunt surgery, and the presence of a suitable shuntable vein. A splenectomy–devascularization procedure was performed if there was no suitable shuntable vein or if there was a massive bleed. A cholecystectomy was performed along with a shunt or splenectomy–devascularization procedure if gallstones were seen on preoperative imaging and in the absence of significant gallbladder varices. A cholecystojejunostomy or a choledochojejunostomy was performed in patients in whom ERCP was not feasible or had failed to relieve biliary obstruction.
Statistical analysis was performed using Wilcoxon's signed rank test. A P-value of <0.05 was considered to indicate statistical significance. Analysis was conducted using spss Version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
During the study period of 14 years, a total of 113 patients with EHPVO and 42 patients with NCPF underwent surgery in the department. The median ages of patients without PB but with EHPVO or NCPF were 18 years (range: 4–57 years) and 33 years (range: 20–61 years), respectively. Recurrent upper gastrointestinal (GI) bleed was the most common indication for surgery and was seen in 61 of 113 (54.0%) and 17 of 42 (40.5%) patients with EHPVO and NCPF, respectively.
Clinical profile of PB
A total of 41 patients with EHPVO and 15 patients with NCPF were found to have PB; these represent the current study group of 56 patients (36.1%). The group included 39 male and 17 female patients with a median age of 26 years (range: 3–68 years). The median ages of patients in the EHPVO-PB and NCPF-PB groups were 23 years (range: 3–50 years) and 34 years (range: 20–68 years), respectively.
Symptomatic PB was seen in 24 of 56 (42.9%) patients. The median age of patients with symptomatic PB was 26 years (range: 13–68 years). Of these patients, 12 had undergone ERCP prior to operation. The median number of ERCPs per patient was 2.5 (range: 1–5); a total of 32 procedures had been performed. Two patients underwent ERCP with nasobiliary drainage and the rest underwent ERCP with stenting. Twelve patients had features of biliary obstruction with recurrent upper GI bleeding or hypersplenism and underwent surgery without prior endoscopic intervention. Jaundice, pruritus, cholangitis requiring ERCP and drainage were the chief indications for surgery in 13 of 24 patients, whereas recurrent upper GI bleed, lower GI bleed and hypersplenism were the chief indications for surgery in 11 of 24 patients with symptomatic PB.
A total of 32 (57.1%) patients had asymptomatic PB. The median age of this group of patients was 27 years (range: 3–61 years). Of these, six patients had only abnormalities on imaging (CT, MRI, magnetic resonance cholangiopancreatography) and 26 had altered liver function tests. Clinical features are given in detail in Table 1.
Table 1.
Clinical features of patients with portal biliopathy (PB)
| Finding | EHPVO-PB (n = 41), n | NCPF-PB (n = 15), n | Total (n = 56), n (%) |
|---|---|---|---|
| Upper GI bleeding | 26 | 8 | 34 (60.7) |
| Jaundice | 21 | 3 | 24 (42.8) |
| Cholangitis | 7 | 0 | 7 (12.5) |
| Abdominal pain | 6 | 6 | 12 (21.4) |
| Melaena | 5 | 2 | 7 (12.5) |
| Abdominal lump | 5 | 2 | 7 (12.5) |
| Hypersplenism | 4 | 4 | 8 (14.3) |
| Growth failure | 2 | 0 | 2 (3.5) |
| Pruritus | 2 | 0 | 2 (3.5) |
| Haemobilia | 1 | 0 | 1 (1.7) |
| Ascites | 0 | 3 | 3 (5.4) |
EHPVO, extrahepatic portal venous obstruction; NCPF, non-cirrhotic portal fibrosis; GI, gastrointestinal.
Imaging characteristics
Details of imaging characteristics are shown in Table 2. In patients with EHPVO, dilatation of intrahepatic biliary radicals was the most common finding (Figs 1 and 2). Three NCPF patients had evidence of nodular liver with ascites. However, cirrhosis was ruled out on liver biopsy.
Table 2.
Imaging characteristics of patients with portal biliopathy (PB)
| Imaging characteristic | EHPVO-PB (n = 27), n | NCPF-PB (n = 6), n | Total (n = 33), n |
|---|---|---|---|
| Intrahepatic bile duct dilatation | 13 | 1 | 14 |
| Bile duct stricture | 3 | 0 | 3 |
| Gallbladder stones | 9 | 2 | 11 |
| Common bile duct stones | 6 | 1 | 7 |
| Multiple strictures (pseudosclerosing cholangitis) | 5 | 0 | 5 |
| Extrahepatic biliary dilatation alone | 3 | 0 | 3 |
| Left hepatic duct dilatation alone | 1 | 0 | 1 |
| Nodular liver | 0 | 3 | 3 |
EHPVO, extrahepatic portal venous obstruction; NCPF, non-cirrhotic portal fibrosis.
Figure 1.
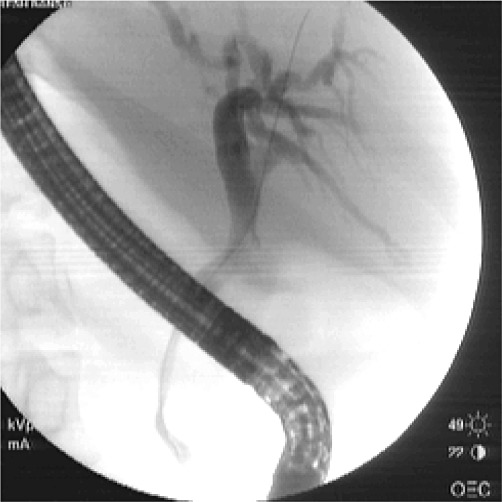
Endoscopic retrograde cholangiopancreatography showing dilatation of the intrahepatic biliary radicals with beaded appearance of the common bile duct
Figure 2.
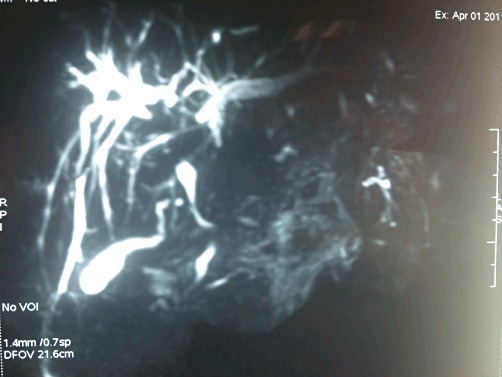
Magnetic resonance cholangiopancreatography showing dilatation of the intrahepatic biliary radicals and dominant common bile duct compression
Operations
Portosystemic shunt surgery was the most common procedure in the EHPVO-PB group, whereas shunt and non-shunt surgeries were performed almost equally in the NCPF-PB group. One patient in the NCPF group underwent a choledochojejunostomy along with the shunt procedure (Table 3).
Table 3.
Procedures performed in portal biliopathy (PB)
| Procedure | EHPVO-PB (n = 41), n | NCPF-PB (n = 15), n | Total (n = 56), n (%) |
|---|---|---|---|
| Proximal lienorenal shunt | 30 | 7 | 37 (66.1) |
| Distal lienorenal shunt | 1 | 0 | 1 (1.5) |
| Mesocaval shunt | 1 | 0 | 1 (1.5) |
| Lieno-adrenal shunt | 1 | 0 | 1 (1.5) |
| Splenectomy with devascularization | 8 | 8 | 16 (28.5) |
| Cholecystectomy with shunt or splenectomy | 6 | 2 | 8 (14.3) |
| Cholecystojejunostomy | 1 | 0 | 1 (1.5) |
| Choledochojejunostomy | 0 | 1 | 1 (1.5) |
EHPVO, extrahepatic portal venous obstruction; NCPF, non-cirrhotic portal fibrosis.
Laboratory investigations
Median preoperative bilirubin in the study group was 1.8 mg/dl (range: 0.4–5.9 mg/dl). Median SAP was 138 IU/ml (range: 23–765 IU/ml). After surgery (including shunt and non-shunt procedures), median bilirubin and SAP were 1.2 mg/dl (range: 0.3–5.4 mg/dl) (P < 0.001) and 109 IU/ml (range: 33–395 IU/ml) (P = 0.002), respectively. In patients with symptomatic biliopathy, median preoperative bilirubin and SAP were 2.0 mg/dl (range: 0.6–5.9 mg/dl) and 134 IU/ml (range: 50–765 IU/ml), respectively; these values decreased postoperatively to 1.3 mg/dl (range: 0.3–5.4 mg/dl) (P < 0.001) and 133 IU/ml (range: 60–395 IU/ml) (P = 0.290), respectively. In patients with asymptomatic biliopathy, median preoperative bilirubin and SAP levels were 1.7 mg/dl (range: 0.4–4.0 mg/dl) and 134 IU/ml (range: 23–483 IU/ml), respectively; these decreased postoperatively to 0.9 mg/dl (range: 0.3–5.2 mg/dl) (P = 0.002) and 92 IU/ml (range: 33–232 IU/ml) (P = 0.001), respectively. In patients who underwent shunt surgery for PB, median bilirubin decreased postoperatively from 1.8 mg/dl (range: 0.4–5.9 mg/dl) to 1.0 mg/dl (range: 0.3–5.4 mg/dl) (P < 0.001) and median SAP decreased from 150 IU/ml (range: 50–765 IU/ml) to 110 IU/ml (range: 33–395 IU/ml) (P = 0.003). In patients who underwent splenectomy–devascularization, median bilirubin decreased postoperatively from 1.9 mg/dl (range: 0.6–4.0 mg/dl) to 1.2 mg/dl (range: 0.6–5.2 mg/dl) (P = 0.023) and median SAP decreased from 105 IU/ml (range: 23–287 IU/ml) to 98 IU/ml (range: 60–218 IU/ml) (P = 0.27) (Table 4).
Table 4.
Effects of surgery in patients with portal biliopathy (PB) (n = 56)
| Investigations | Preoperative median (range) | Postoperative median (range) | P-value |
|---|---|---|---|
| Shunt surgery | |||
| Bilirubin, mg/dl | 1.8 (0.4–5.9) | 1.0 (0.3–5.4) | <0.001 |
| SAP, IU/ml | 150 (50–765) | 110 (33–395) | 0.003 |
| Non-shunt surgery | |||
| Bilirubin, mg/dl | 1.9 (0.6–4.0) | 1.2 (0.6–5.2) | 0.023 |
| SAP, IU/ml | 106 (23–287) | 98 (60–218) | 0.266 |
SAP, serum alkaline phosphatase.
Follow-up
There was no perioperative mortality. Overall, 43 of the 56 (76.8%) patients were followed for a median of 48 months (range: 14–120 months). Thirteen patients were lost to follow-up.
After operation, seven of 43 patients in the study group (including the EHPVO and NCPF subgroups) required ERCP with stenting for jaundice and fever. Of these, two patients had asymptomatic PB prior to shunt surgery and developed symptoms secondary to CBD stones at 3 years and 7 years, respectively, after shunt surgery and cholecystectomy; these patients were treated with ERCP, stone removal and sphincterotomy. These patients continue to be symptom-free and have not required any further endotherapy on follow-up. In the other five patients, the symptoms of biliopathy continued to require multiple ERCP and stent placements. One patient had a massive lower GI bleed from jejunal varices at 10 years after splenectomy–devascularization and died. Two patients continued to have dominant CBD stricture postoperatively, which required frequent stent exchange, and subsequently underwent successful Roux-en-Y hepaticojejunostomy at 16 months and 48 months after shunt surgery, respectively. The remaining two patients are currently maintained on endoscopic stents. All four of the patients with persistent jaundice underwent Doppler ultrasound (US) during follow-up, which revealed patent shunts in all but one patient, who experienced a rebleed after 6 months of lienorenal shunt. This shunt was shown to have thrombosed. The patient subsequently underwent a mesocaval shunt procedure. However, this patient continued to have persistent jaundice with evidence of patent shunt on longterm follow-up (at 36 months).
Eight of the 43 patients had rebleeding. Of these, two patients belonged to the group with persistent jaundice. As stated earlier, one of these patients died and one required a mesocaval shunt as a result of recurrent bleeding with a blocked shunt. The other six patients were successfully managed with endotherapy. A total of 38 of 43 patients were found to have returned to work after surgery, were free from symptoms of biliary obstruction, did not require frequent hospital visits or endoscopic treatments and were leading normal lives. Details of follow-up are given in Table 5.
Table 5.
Follow-up in patients with portal biliopathy (PB)
| Follow-up, months, median (range) | EHPVO-PB (n = 34) | NCPF-PB (n = 9) | Total (n = 43) | ||
|---|---|---|---|---|---|
| 42 (14–120) | 36 (14–72) | ||||
| Shunt (n = 29) | Non-shunt (n = 5) | Shunt (n = 4) | Non-shunt (n = 5) | ||
| Jaundice | 5 | 1 | 0 | 1 | 7 |
| ERCP required | 5 | 1 | 0 | 1 | 7 |
| Bilioenteric bypass | 2 | 0 | 0 | 0 | 2 |
| Bleed | 3 | 2 | 1 | 2 | 8 |
| Ascites | 0 | 0 | 1 | 2 | 3 |
| Mortality | 0 | 1 | 0 | 0 | 1 |
EHPVO, extrahepatic portal venous obstruction; NCPF, non-cirrhotic portal fibrosis; ERCP, endoscopic retrograde cholangiopancreatography.
Discussion
Portal biliopathy is most commonly associated with EHPVO. It is a late and progressive manifestation of the condition10 and may lead to hepatic dysfunction11 if not treated. Reported incidences range from 80% to 100%.3,4 However, only 20–30% of patients with PB have symptoms related to biliary obstruction.12,13 Portal biliopathy is also seen in patients with cirrhosis of the liver and NCPF, but its incidence in this context is lower (9–40%).5 In the current series, incidences of PB were 36.2% and 35.7% in patients with EHPVO and NCPF, respectively. Incidences of symptomatic biliopathy were 18.4% in EHPVO and 7% in NCPF, respectively. This low overall incidence may reflect the fact that biliary imaging or ERCP were performed in symptomatic patients only. The median age of patients with EHPVO without biliopathy was 18 years (range: 4–57 years), whereas the median age of patients with symptomatic biliopathy was 26 years (range: 13–50 years) (P = 0.005). Similarly, the difference in median age at presentation between patients with asymptomatic (19 years; range: 3–32 years) and symptomatic biliopathy was also significant (P = 0.034). This corroborates findings of previous studies indicating that symptomatic PB is a late and progressive manifestation of EHPVO, whereas asymptomatic changes may be present at initial presentation.
Most patients with PB were asymptomatic. Symptoms include jaundice, pruritus, fever and cholangitis. The incidences range from 5% to 38%.5,10 In the current study, 24 of 56 (42.9%) patients were symptomatic. However, even in this group, upper GI bleeding was the chief indication for surgery in 11 of 24 patients. Symptomatic biliopathy was the chief indication for surgery in 13 patients. Choudhuri et al.6 first described portosystemic shunting in a patient with CBD obstruction and portal cavernoma associated with bleeding. The authors found a reversal of the CBD obstruction after shunt surgery. Chaudhary et al.7 investigated the results of lienorenal shunt in seven patients with symptomatic biliopathy and reported a reversal of biliopathy in five patients; the remaining two patients required bilioenteric bypass at 6 months postoperatively.
In the current series, the most common surgery performed involved a proximal lienorenal shunt (66.1%); this was also the procedure of choice. Splenectomy with gastro-oesophageal devascularization was performed in 28.5% of patients.
Analysis of pre- and postoperative bilirubin and SAP levels in the study group showed a significant decrease in both in patients with biliopathy after surgical intervention (including both shunt and non-shunt surgeries) (P < 0.001 and P = 0.002, respectively). This supports the suggestion that any surgery that is designed to reduce portal pressure should also be effective in relieving biliary obstruction. These decreases in bilirubin and SAP levels were significant in both symptomatic and asymptomatic PB. When the study group was divided into patients who underwent shunt surgery and patients who underwent splenectomy–devascularization, the decrease in bilirubin remained significant in both groups (P < 0.001 and P = 0.023, respectively). However, the decrease in SAP after splenectomy–devascularization was not significant (P = 0.27). Khuroo et al.10 reported the results of four patients with PB and concluded that only shunt surgeries were effective in reversing biliopathy. Similarly, shunt surgery represented the preferred operative technique in the current study, especially in patients in whom PB was the chief indication for surgery, and splenectomy–devascularization was performed only in the absence of a viable shuntable vein. However, when patients who had undergone splenectomy–devascularization were reviewed, splenectomy–devascularization was found to be effective in relieving PB by significantly decreasing bilirubin levels. This probably reflects the reduction in portal flow caused by splenectomy. This concept has been proven in the context of living donor liver transplantation, in which splenic artery ligation and splenectomy have been shown to decrease portal flow by 10–60%.14,15
Compression of the bile duct by pericholedochal varices is postulated to represent the main cause of PB. The extrahepatic bile duct is surrounded by two venous plexuses: the epicholedochal (Saint) plexus,16 and the paracholedochal (Petren) plexus.17 Dilatation of these veins secondary to portal venous obstruction can cause compression of the bile duct. However, studies have also shown that in longterm cavernomatous dilatation, neogenesis and deposition of fibrous tissue over the bile duct do not revert with shunt surgery18 and can lead to stricture formation. Bile duct strictures have also been postulated to be secondary to ischaemic changes that occur following venous damage at the time of portal vein thrombosis, leading to vascular injury at the level of arterioles and capillaries.19 Dhiman et al.20 studied five patients with EHPVO and bile duct abnormalities and found complete reversal of bile duct changes on ERCP in only one patient post-shunt surgery suggestive of an ischaemic or fibrosing pathology in the others. However, in a study of 19 patients with symptomatic biliopathy, Vibert et al.21 noted reversibility of changes in all patients and concluded that ischaemia was unlikely to be the mechanism for this.
In the current series, 38 patients were found to be symptom-free on longterm follow-up and did not require multiple interventions for biliary obstruction. Therefore, in this group of patients, biliary obstruction probably reflected CBD compression alone, which was relieved after surgery.
Overall, seven patients in the study group developed jaundice postoperatively and required ERCP. Of these patients, five had symptomatic biliopathy (five of 24 patients) and four had undergone ERCP with multiple stent insertions preoperatively (with a median of three and a range of two to five attempts per patient). One patient died after 10 years of follow-up of a massive lower GI bleed suggestive of a progression of portal hypertension. One patient had recurrent upper GI bleed with blockade of the lienorenal shunt and underwent mesocaval shunt surgery, but he also had a preoperative history of recurrent episodes of cholangitis that required ERCP with stenting. Three of these patients had documented predominant CBD strictures on preoperative ERCP. All four patients had patent shunts on Doppler US on longterm follow-up. Two of these patients continued to have recurrent cholangitis postoperatively and required multiple stent changes and eventually underwent uneventful bilioenteric bypass at 18 months and 4 years after shunt surgery, respectively. All other patients remained free of any biliary symptoms. Overall, 38 of 43 patients resumed work after surgery and were found to be leading near-normal lives.
These findings indicate that shunt surgeries are effective in reversing the effects of CBD obstruction in the majority of patients. Patients who are more likely to respond to shunt surgery are those with: asymptomatic biliopathy; patent shunts; symptomatic biliopathy in the absence of multiple episodes of ERCP with stenting for preoperative cholangitis, and the absence of predominant CBD stricture on preoperative imaging. Symptomatic biliopathy is also a marker for advanced disease as these patients were significantly older than patients with asymptomatic biliopathy and had a higher incidence of persistent biliary obstruction than asymptomatic patients (five of 24 vs. two of 32 patients) in the current study. Thrombosis of shunts may contribute by causing the progression of portal hypertension, which, in turn, causes the compression of the CBD. Frequent ERCP and stent insertions are associated with recurrent cholangitis, which may act as a stimulus for neogenesis, fibrosis and stricture. In the current study, four of five patients with persistent biliary obstruction had a history of multiple ERCP and stent insertion in the preoperative period. Dominant CBD stricture may indicate an irreversible change in the CBD as all three patients with preoperative evidence of dominant stricture in the current study continued to require biliary decompression after shunt surgery.
Conclusions
Successful portosystemic shunt procedures relieve biliary obstruction in the majority of patients with PB. However, a small percentage of patients may continue to progress and eventually require a bilioenteric bypass. A history of shunt blockade, recurrent cholangitis requiring frequent stent changes preoperatively, and predominant bile duct stricture on preoperative imaging, may be associated with failure of resolution of PB. This study also shows that splenectomy with devascularization may alter the natural history of PB, probably by decreasing the portal flow, and thus can be considered a therapeutic procedure in patients without a shuntable vein.
Acknowledgments
The authors acknowledge the contribution of Dr Bandar Ali Alqahtani, Gyan Burman Liver Fellow, Sir Ganga Ram Hospital in data collection.
Conflicts of interest
None declared.
References
- 1.Fraser J, Broun AK. A clinical syndrome associated with a rare anomaly of vena portal system. Surg Gynecol Obstet. 1944;78:520–524. [Google Scholar]
- 2.Sarin SK, Bhatia V, Makwane U. Portal biliopathy in extrahepatic portal vein obstruction. Ind J Gastroenterol. 1992;2:A82. [Google Scholar]
- 3.Dilawari JB, Chawla YK. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–276. doi: 10.1136/gut.33.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS, et al. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol. 2002;17:6–16. doi: 10.1046/j.1440-1746.2002.02596.x. [DOI] [PubMed] [Google Scholar]
- 5.Dilawari JB, Chawla YK. Extrahepatic portal venous obstruction. Gut. 1988;29:554–555. doi: 10.1136/gut.29.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhuri G, Tandon RK, Nundy S, Mishra NK. Common bile duct obstruction by portal cavernoma. Dig Dis Sci. 1988;33:1626–1628. doi: 10.1007/BF01535956. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary A, Dhar P, Sarin SK, Sachdev A, Agarwal AK, Vij JC, et al. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–329. doi: 10.1046/j.1365-2168.1998.00591.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, et al. Members of the APASL Working Party on Portal Hypertension. Consensus on extrahepatic portal vein obstruction. Liver Int. 2006;26:512–519. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, et al. Members of the APASL Working Party on Portal Hypertension. Non-cirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398–413. doi: 10.1007/s12072-007-9010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khuroo MS, Yattoo GN, Zargar SA, Javed G, Dar MY, Khan BA, et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 11.Dhiman RK, Behera A, Chawla YK, Dilawari JB, Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condat B, Vilgrain V, Asselah T, O'Toole D, Rufat P, Zappa M, et al. Portal cavernoma-associated cholangiopathy: clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–1308. doi: 10.1053/jhep.2003.50232. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal AK, Sharma D, Singh S, Agarwal S, Girish SP. Portal biliopathy: a study of 39 surgically treated patients. HPB. 2011;13:33–39. doi: 10.1111/j.1477-2574.2010.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary non-function in a small-for-size liver graft – successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626–628. doi: 10.1053/jlts.2003.50081. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Yamamoto S, Oya H, Nakatsuka H, Tsukahara A, Kobayashi T, et al. Splenectomy for reduction of excessive portal hypertension after adult living related donor liver transplantation. Hepatogastroenterology. 2002;49:1652–1655. [PubMed] [Google Scholar]
- 16.Saint JH. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operations on the extrahepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 17.Petren T. Die extrahepatischen Gallenwegsvenen und ihre pathologische anatomische Bedetung. Verh Anat Ges. 1932;41:139–143. [Google Scholar]
- 18.Bechtelsheimer H, Conrad A. Morphologisches Bild der Kavernosen Transformation der Pfortader. Leber Magen Darm. 1980;2:99–106. [PubMed] [Google Scholar]
- 19.Batts KP. Ischaemic cholangitis. Mayo Clin Proc. 1998;73:380–385. doi: 10.1016/S0025-6196(11)63706-3. [DOI] [PubMed] [Google Scholar]
- 20.Dhiman RK, Puri P, Chawla Y, Minz M, Bapurai JR, Gupta S, et al. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischaemic? Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 21.Vibert E, Azoulay D, Aloia T, Pascal G, Veilhan LA, Adam R, et al. Therapeutic strategies in symptomatic portal biliopathy. Ann Surg. 2007;246:97–104. doi: 10.1097/SLA.0b013e318070cada. [DOI] [PMC free article] [PubMed] [Google Scholar]


