Abstract
Objectives
Portal vein embolization (PVE) can facilitate the resection of previously unresectable colorectal cancer (CRC) liver metastases. Bevacizumab is being used increasingly in the treatment of metastatic CRC, although data regarding its effect on post-embolization liver regeneration and tumour growth are conflicting. The objective of this observational study was to assess the impact of pre-embolization bevacizumab on liver hypertrophy and tumour growth.
Methods
Computed tomography scans before and 4 weeks after PVE were evaluated in patients who received perioperative chemotherapy with or without bevacizumab. Scans were compared with scans obtained in a control group in which no PVE was administered. Future liver remnant (FLR), total liver volume (TLV) and total tumour volume (TTV) were measured. Bevacizumab was discontinued ≥ 4 weeks before PVE.
Results
A total of 109 patients and 11 control patients were included. Portal vein embolization induced a significant increase in TTV: the right lobe increased by 33.4% in PVE subjects but decreased by 34.8% in control subjects (P < 0.001), and the left lobe increased by 49.9% in PVE subjects and decreased by 33.2% in controls (P = 0.022). A total of 52.8% of the study group received bevacizumab and 47.2% did not. There was no statistical difference between the two chemotherapy groups in terms of tumour growth. Median FLR after PVE was similar in both groups (28.8% vs. 28.7%; P = 0.825).
Conclusions
Adequate liver regeneration was achieved in patients who underwent PVE. However, significant tumour progression was also observed post-embolization.
Keywords: colorectal cancer liver metastases, tumour growth, portal vein embolization, bevacizumab, liver regeneration, degree of hypertrophy
Introduction
Colorectal cancer is diagnosed in approximately 142 570 Americans annually.1 Of these, 51 370 will die from the disease.1 Metastasis is the most common cause of death and occurs in the majority of patients.2,3 In recent decades, outcomes in patients with colorectal liver metastases (CRLM) have improved as a result of enhancements in chemotherapy and hepatic resection.4 In selected patients, the combination of chemotherapy and resection has increased 5-year survival to up to 50%, compared with only 10% in patients treated with chemotherapy alone.5,6 Perioperative chemotherapy regimens for patients with CRLM are based on either oxaliplatin or irinotecan. Bevacizumab, a human monoclonal antibody and an inhibitor of vascular endothelial growth factor (VEGF), has become part of first-line chemotherapy.7 VEGF plays a major role in tumour angiogenesis and is required for both tumour proliferation and healing of injured tissue. Randomized controlled trials have demonstrated that the addition of bevacizumab to standard chemotherapy increases tumour response, resectability rate and progression-free survival compared with chemotherapy alone.8–11
Unfortunately, despite the downsizing effect of preoperative chemotherapy, the majority of patients still have unresectable disease. The size of the future liver remnant (FLR) plays a major role in determining resectability. Therefore, strategies aimed at increasing the FLR if it is estimated to represent < 20–30% of organ size (in the absence of chronic liver disease) must be developed. Preoperative portal vein embolization (PVE) has been shown to be a safe and effective method of stimulating liver hypertrophy, increasing FLR and reducing post-hepatectomy complications.12–15
The regenerative process following PVE mirrors the regeneration stimulated after partial hepatectomy. Recent literature supports the safety of using preoperative chemotherapy in liver regeneration following PVE.16–18 Despite its increasing clinical usage, however, there are currently very few data regarding the effect of bevacizumab on liver regeneration after PVE.17 Moreover, the potential effect of PVE on tumour growth has been a subject of concern. In fact, some studies have suggested that, as well as causing hypertrophy of normal liver parenchyma, PVE also stimulates the growth of any tumour that is still present within the regenerating liver, including embolized and non-embolized sides.19–25
It is evident that the progression of tumours secondary to PVE could potentially affect resectability and overall survival in patients with CRLM. Any effect of pre-embolization chemotherapy on this potential tumour growth would therefore be an important clinical consideration. Therefore, the objectives of this observational study were to assess the effect of PVE on the volume of existing CRLM and to evaluate the effect of pre-embolization therapy, particularly the use of bevacizumab, on the volumes of metastases and FLR.
Materials and methods
Guidelines for meeting STROBE (strengthening the reporting of observational studies in epidemiology) criteria were used in the preparation of this manuscript.
Patients
This study was authorized by the Director of Professional Services at the McGill University Health Center as per institutional protocol. All patients who underwent PVE in preparation for liver resection (trisegmentectomy or staged resection, according to tumour board recommendations) were identified. The criteria for PVE were an FLR of < 30% or staged resection. Between January 2003 and May 2011, 168 patients underwent PVE; 127 of these had a diagnosis of CRLM and 41 had alternative diagnoses. Of the 127 CRLM patients, 18 were excluded because computed tomography (CT) scans were missing; therefore comparative volumes could be calculated in 109 patients. Only 89 of the 109 patients could be assigned to the bevacizumab and non-bevacizumab groups with certainty because some patients had received chemotherapy in other institutions (Fig. 1). Patients were also excluded if they had not received preoperative chemotherapy or were known to have biliary obstruction or cirrhosis. Basic demographic data, disease characteristics, surgery and chemotherapy data were reviewed retrospectively. To assess the effects of pre-embolization chemotherapy, the study group was subdivided into those who had received bevacizumab prior to embolization (n = 47) and those who had not (n = 42). A control group of patients with CRLM who had not undergone PVE was identified (n = 11). Control patients were selected if they had received neoadjuvant chemotherapy, had two CT scans both performed off-chemotherapy and before surgical resection, and if the time between scans was comparable with the corresponding interval in the PVE population.
Figure 1.
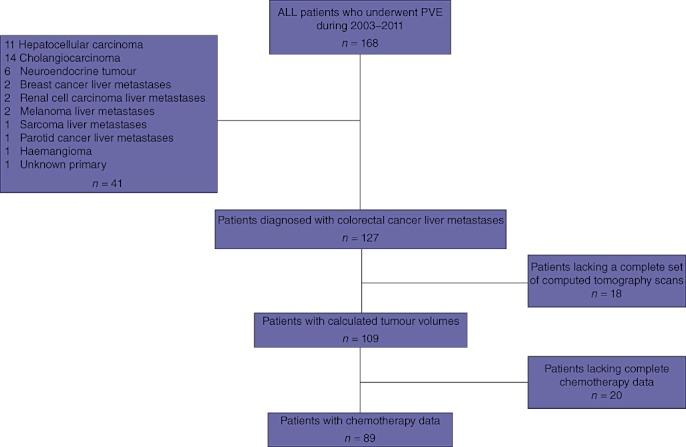
Distribution of patients who underwent portal vein embolization (PVE) during 2003–2011
Portal vein embolization
Portal vein embolization was administered prior to a planned trisegmentectomy or as part of a staged liver resection. The procedure was performed via an ipsilateral approach using 90–180-µm polyvinyl alcohol (PVA) particles and coils to occlude segmental branch origins. In patients undergoing right-sided embolization, the first embolization included both the anterior and posterior branches of the right portal vein. Patients who failed to achieve the recommended FLR underwent a subsequent embolization of any remaining segments in the right liver with or without embolization of segment IV branches. In general, standard chemotherapy alone was discontinued approximately 4 weeks prior to embolization, and regimens including bevacizumab were discontinued 6 weeks prior to embolization.
Volumetry
To obtain volumetric data, pre- and post-PVE CT scans were analysed using GE Medical Systems Advantage Windows 4.3 workstations (GE Healthcare, Chalfont St Giles, UK) with dedicated three-dimensional volume calculation software. Two radiologists were blinded to the patients' chemotherapy treatment. The volume of the FLR and total liver volume (TLV) were measured on the portal phase of thin-slice helical CT scans. Routine scans were performed prior to PVE and 3–4 weeks after PVE. The ratio between the FLR and TLV was determined before and after PVE and the absolute difference between these two ratios was defined as the degree of hypertrophy. Total tumour volumes (TTVs) and tumour volumes (TVs) in both embolized and non-embolized lobes were measured in all patients pre- and post-embolization.
Statistics
Statistical analyses were performed using jmp Version 8.0 (SAS Institute, Inc., Cary, NC, USA). Normally distributed data were expressed as means and standard deviations; otherwise medians and ranges (interquartile ranges) were used. Nominal data were expressed as percentages. Differences in tumour growth against PVE and the use of bevacizumab were established using paired t-tests or Mann–Whitney U-tests as appropriate for continuous data. The chi-squared test was used for nominal data. Between-group differences were considered statistically significant at P < 0.05.
Results
Patients
A total of 127 CRLM patients who underwent PVE prior to liver resection were initially identified. Patients were excluded from the study group if they lacked two CT scans for volumetric calculations and thus 109 patients remained for tumour volume analysis (Fig. 1). Eleven control patients with two appropriately timed CT scans were also identified.
Patient demographics and preoperative variables are shown in Table 1. Among the 109 patients who received pre-embolization chemotherapy, receipt of bevacizumab was confirmed in 89 patients, 47 (52.8%) of whom were given pre-embolization bevacizumab. Complete details of the chemotherapy regimen were missing for some patients (Fig. 1) because they had been treated at a different institution. Chemotherapy was oxaliplatin-based in 22 and 17 patients in the bevacizumab and non-bevacizumab groups, respectively, and irinotecan-based in 13 and 12 patients in the bevacizumab and non-bevacizumab groups, respectively. One patient in the bevacizumab group and two in the non-bevacizumab group received chemotherapy using both oxaliplatin and irinotecan. Patients received a median of six (range: five to nine) chemotherapy cycles prior to embolization and the median time interval for all patients was 70 days (interquartile range: 51–100 days). Sixty patients (67.4%) underwent resection, including 30 patients (63.8%) in the bevacizumab group and 30 (71.4%) in the non-bevacizumab group (P = 0.167).
Table 1.
Baseline characteristics in the total study population
| PVE | No PVE | P-value | ||
|---|---|---|---|---|
| Bev (n = 47) | Non-bev (n = 42) | Bev (n = 11) | ||
| Male, n (%) | 26 (55.3) | 28 (66.7) | 9 (81.8) | 0.191 |
| Primary tumour, n (%) | ||||
| Colon | 33 (70.2) | 28 (66.7) | 6 (54.5) | 0.042a |
| Rectum | 6 (12.8) | 12 (28.6) | 5 (45.5) | |
| Missing data | 8 (17.0) | 2 (4.8) | 0 | |
| Lesions, n (%) | ||||
| Synchronous | 37 (70.2) | 37 (88.1) | 7 (63.6) | 0.051 |
| Metachronous | 3 (6.4) | 3 (7.1) | 3 (27.3) | |
| Missing data | 7 (14.9) | 2 (4.8) | 1 (9.1) | |
| Chemotherapy cycles, median (range) | 6.0 (5–9) | 7.5 (6–9) | 7.0 (6–16) | 0.266 |
| Chemotherapy regimen, n (%) | ||||
| Oxaliplatin-based | 22 (46.8) | 17 (40.4) | 5 (45.5) | 0.701 |
| Irinotecan-based | 13 (27.7) | 12 (28.6) | 2 (18.2) | 0.701 |
| Both | 1 (2.2) | 2 (4.8) | 0 | |
| Missing | 11 (23.3) | 11 (26.2) | 4 (36.3) | |
| Resected, n (%) | 30 (63.8) | 30 (71.4) | 10 (90.9) | 0.167 |
| Staged | 18 (60.0) | 15 (50.0) | 2 (20.0) | 0.316 |
| Trisegmentectomy | 12 (40.0) | 15 (50.0) | 8 (80.0) | |
| Right-sided embolization, n (%) | 46 (97.8) | 41 (97.6) | NA | |
| Segment IV embolizationb, n (%) | 4 (8.5) | 0 | NA | |
| Resected | 1 (25.0) | |||
| Unresectable | 2 (50.0) | |||
| Missing | 1 (25.0) | |||
| Days between CT scans, median (range) | 72 (52–116) | 65 (51–117) | 68 (47–92) | 0.581 |
| Days from chemotherapy to second CT scan, median (range) | 51 (30–107) | 44 (27–100) | 70 (47–116) | 0.220 |
There were more cases of rectal cancer in the control group; no difference was seen when comparing bev vs. non-bev in the PVE group (P= 0.179).
Right-sided and segment VI embolization.
PVE, portal vein embolization; bev, bevacizumab; CT, computed tomography; NA, not available.
Portal vein embolization
Baseline characteristics and embolization data for patients who underwent PVE, by chemotherapy group (bevacizumab and non-bevacizumab), compared with those who did not undergo PVE, are shown in Table 1. In total, 105 patients (96.3%) underwent a right-sided embolization and four patients, all in the bevacizumab group, underwent segment IV and right portal vein embolization. One of these four patients had an extended right hepatectomy.
The median FLR in the 109 patients with CRLM who underwent PVE was 21.7% (range: 15.9–26.3%) before embolization and 28.7% (range: 23.2–35.4%) after embolization (P < 0.001). The median degree of hypertrophy was 6.0 (range: 1.6–10.2).
Tumour volumes
Overall, 77.1% of patients had an increase in TV. Statistically significant increases in TV were seen in both liver lobes (Tables 2 and 3); changes in TV in the PVE group differed markedly from those in the control group of patients who did not undergo PVE. Patients in the PVE group demonstrated a 33.4% increase in TV in the right lobe, whereas control subjects showed a 34.8% decrease in TV in the right lobe (P < 0.001). These percentages corresponded to a positive growth rate of 0.07 cm3/day (range: 0–0.27 cm3/day) in the PVE group and a negative rate of 0.06 cm3/day (range: 0.18–0.01 cm3/day) in the controls (P < 0.001). Patients in the PVE group showed an increase in TV of 49.9% in the left lobe, whereas control subjects demonstrated a decrease in TV of 33.2% in the left lobe (P = 0.022). Eight patients in the PVE group demonstrated unilateral disease on the first CT scan and developed new lesions on the second CT scan (i.e. after PVE), an event that was not observed in any patient in the control group. This difference did not reach statistical significance (P = 0.427).
Table 2.
Tumour volumetry in the right lobe in patients who did and did not undergo portal vein embolization (PVE)
| PVE (n = 109) | No PVE (n = 11) | P-value | |
|---|---|---|---|
| Tumour volume, cm3, median (range) | |||
| First CT scana | 21.2 (4.5–76.4) | 10.6 (3.2–14.8) | 0.080 |
| Second CT scan | 34.8 (11.5–112) | 6.6 (2.0–9.9) | < 0.001 |
| P-valueb | < 0.001 | 0.002 | |
| Change in tumour volume, % (range) | 33.4 (− 0.5 to 168.0) | − 34.8 (− 40.7 to − 26.1) | < 0.001a |
Tumour volumes calculated from first and second CT scans (with embolization during interval time for study group only).
Difference between pre- and post-embolization values.
CT, computed tomography.
Table 3.
Tumour volumetry in the left lobe in patients who did and did not undergo portal vein embolization (PVE)
| PVE (n = 109) | No PVE (n = 11) | P-value | |
|---|---|---|---|
| Tumour volume, cm3, median (range) | |||
| Pre-PVE | 0 (0–3.2) | 0 (0–7.4) | 0.694 |
| Post-PVE | 0 (0–6.0) | 0 (0–3.6) | 0.805 |
| P-value | < 0.001 | 0.625 | |
| Change in tumour volumea, % (range) | 49.9 (− 24.2 to 118.0) | − 33.2 (− 58.0 to 6.0) | 0.022 |
| New bilateral diseaseb, n (%) | 8 (7.3) | 0 | 0.595 |
Difference in tumour volumes in the left lobe between patients receiving PVE and control subjects, expressed as a percentage.
New lesions in the left lobe on the second computed tomography scan (i.e. after embolization in the PVE group).
The effects of pre-embolization chemotherapy in the bevacizumab and non-bevacizumab groups on tumour growth after PVE are shown in Tables 4 and 5. The percentage increase in tumour growth was higher in the non-bevacizumab group than in the bevacizumab group, but the difference was not statistically significant. Rates of tumour growth in the non-bevacizumab and bevacizumab groups, respectively, were 56.2% vs. 34.5% (P = 0.764) in the right lobe, and 54.3% vs. 30.1% (P = 0.612) in the left lobe.
Table 4.
Tumour volumetry in the right lobe in patients who underwent portal vein embolization (PVE) with and without bevacizumab (bev)
| Bev (n = 47) | Non-bev (n = 42) | P-value | |
|---|---|---|---|
| Tumour volume, cm3, median (range) | |||
| Pre-PVE | 16.4 (3–75.5) | 22.4 (6.2–65.5) | 0.370 |
| Post-PVE | 21.2 (8.4–82.6) | 33.7 (11.7–117.0) | 0.255 |
| P-value | 0.003 | < 0.001 | |
| Change in tumour volumea, % (range) | 34.5 (− 2.5 to 212.2) | 56.2 (3.6–165.0) | 0.764 |
Change in tumour volumes in the right lobe, expressed as a percentage, in patients who did and did not receive bevacizumab (all received PVE).
Table 5.
Tumour volumetry in the left lobe in patients who underwent portal vein embolization (PVE) with and without bevacizumab (bev)
| Bev (n = 47) | Non-bev (n = 42) | P-value | |
|---|---|---|---|
| Tumour volume, cm3, median (range) | |||
| Pre-PVE | 0 (0–3.3) | 0 (0–2.6) | 0.435 |
| Post-PVE | 0 (0–5.2) | 0 (0–5.6) | 0.654 |
| P-value | 0.123 | 0.021 | |
| Change in tumour volumea, % (range) | 30.1 (− 62.4 to 190.6) | 54.3 (− 23.9 to 100.1) | 0.612 |
Change in tumour volumes in the left lobe, expressed as a percentage, in patients who did and did not receive bevacizumab (all received PVE).
Liver regeneration after PVE
A clinically significant increase in FLR volume was observed in both groups after PVE. Both groups had similar TLV prior to (P = 0.617) and after (P = 0.581) embolization.
The addition of bevacizumab to chemotherapy did not affect the pre- to post-embolization change in FLR volume. The proportion of the FLR increased from 20.8% to 28.8% in the bevacizumab group, and from 21.3% to 28.7% in the non-bevacizumab group (P = 0.825). Correspondingly, the mean degree of hypertrophy was comparable in the two groups (Table 6).
Table 6.
Liver volumetry by chemotherapy group in patients who underwent portal vein embolization (PVE) with and without bevacizumab (bev)
| Bev (n = 47) | Non-bev (n = 42) | P-value | |
|---|---|---|---|
| Total liver volume, cm3, median (range) | |||
| Pre-PVE | 1588 (1444–2000) | 1685 (1377–2073) | 0.617 |
| Post-PVE | 1635 (1415–2037) | 1766 (1385–2135) | 0.581 |
| Future liver remnant, %, median (range) | |||
| Pre-PVE | 20.8 (15.1–23.8) | 21.3 (14.9–26.4) | 0.373 |
| Post-PVE | 28.8 (21.9–34.5) | 28.7 (24.8–35.7) | 0.825 |
| Degree of hypertrophy | 7.5 (3.4–11.2) | 5.1 (1.0–12.5) | 0.127 |
Correlation of the percentage growth in the FLR with the percentage growth in TV revealed a statistically significant positive linear correlation between the growth of the remnant liver and the growth of tumours in the right lobe of the liver (P = 0.043) (Fig. 2).
Figure 2.
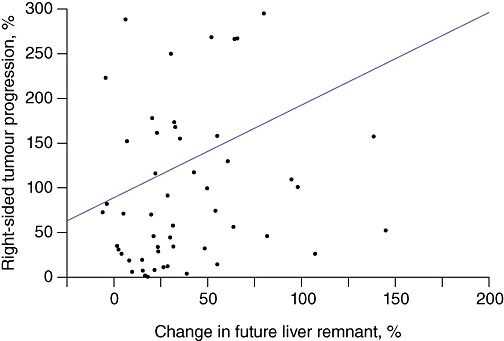
Correlation between percentage of future liver remnant growth and right-sided tumour progression
Discussion
Portal vein embolization is an important strategy in the optimization of resectability rates in CRLM and is reported to be safe and effective in stimulating contralateral liver growth, which can be a major limitation in the resectability of CRLM. There are concerns that PVE may simultaneously stimulate tumour growth and this may limit its use. This study has demonstrated that PVE stimulates tumour growth in both embolized and non-embolized lobes of the liver compared with control lobes in a group of patients who had not received PVE and had been off preoperative chemotherapy for a duration similar to that of the PVE patients. The addition of bevacizumab to chemotherapy administered before embolization trended towards a relative protective effect (although this did not reach statistical significance), reducing this enhanced tumour growth without affecting liver hypertrophy. To the authors' knowledge, this is the largest study to demonstrate the effects of PVE on liver hypertrophy and tumour growth in patients with CRLM.
Bevacizumab has been shown to improve pathologic response rates when combined with cytotoxic agents and has also been reported to exert a protective effect against sinusoidal injuries induced by oxaliplatin-based chemotherapy.26 Nevertheless, the inclusion of bevacizumab in treatment regimens for patients scheduled to undergo PVE and hepatic resection has been tempered by concerns regarding impaired wound healing and tissue regeneration, both of which are greatly dependent on angiogenesis and VEGF expression. Consistent with findings by Gruenberger and colleagues,27 the present study found no increased risk for morbidity post-resection in patients receiving perioperative chemotherapy with bevacizumab.28 However, existing data regarding the effects of bevacizumab on post-embolization hypertrophy remain scarce and inconsistent.29,30 In a retrospective study conducted at the MD Anderson Center, University of Texas, preoperative chemotherapy plus bevacizumab did not impair liver regeneration after PVE.29 Patients included in that study received oxaliplatin-based chemotherapy with (n = 26; median six cycles) or without (n = 17; median five cycles) bevacizumab, or received no chemotherapy before embolization (n = 22). After a median of 4 weeks post-PVE, no significant difference in the degree of hypertrophy was found among patients who had no chemotherapy, patients who received chemotherapy with bevacizumab and patients who received chemotherapy without bevacizumab (mean values 10.0%, 8.8% and 6.8%, respectively; P = 0.11). Conversely, Aussilhou and colleagues30 reported a significantly smaller increase in mean FLR volume in 13 patients receiving bevacizumab plus standard chemotherapy compared with 26 patients treated with chemotherapy only (561 cm3 vs. 667 cm3; P < 0.03).30 In that study, 30% of patients underwent portal vein ligation instead of embolization. Importantly, the mean number of bevacizumab cycles was 12, and the number of cycles above six was found to significantly reduce liver growth, as was age ≥ 60 years. It is noteworthy that prolonged chemotherapy has been identified previously as a factor contributing to impaired liver regeneration.31,32
In the present study, patients received a median of six chemotherapy cycles (five to nine in the bevacizumab group; six to nine in the non-bevacizumab group). This is consistent with the duration of treatment in the MD Anderson study.29 Volumetric CT assessments were completed within 3–4 weeks after PVE in the present study; this is in concordance with published data showing that the greatest increase in post-embolization liver volume (about 75%) occurs within 3 weeks after the procedure and is followed by a plateau phase of minimal regeneration.33
The progression of metastases after embolization was first described by Elias et al., who showed that four of five patients had tumour growth after PVE.20 However, that study included a small number of patients, lacked a control group and included patients with heterogeneous liver pathologies. In 2001, Kokudo et al. evaluated tumour proliferation after PVE using the Ki-67 labelling index, and showed that PVE induced a higher rate of proliferation compared with that in PVE-free controls.19 Although these authors included more patients (18 patients in the study group and 29 controls), there was no mention of peri-embolization chemotherapy. In 2007, Ribero et al. observed no changes in tumour size in 80 patients undergoing PVE.22 The authors did not, however, report the proportions of patients in whom tumour size increased or decreased and measured tumour diameters rather than volumes. It is the present authors' belief that tumour volume measurements are more accurate, especially when metastatic lesions are numerous, heterogeneous and uneven.
In the current study, significant increases in median TV were observed in both liver lobes (33.4% and 49.9% in the right and left lobes, respectively) in the PVE population, compared with the control group (decreases of 34.8% and 33.2% in the right and left lobes, respectively). Growth within the left lobe would potentially have more impact on resectability and therefore patient survival. The current study included eight patients (7.3%) who developed new lesions within the remnant liver lobe after embolization, seven of whom were rendered unresectable. None of the patients in the control group developed new lesions within the left lobe during the time interval between the scans. This difference is of major clinical significance as it may have an impact on patient survival. These results suggest that metastases that respond to chemotherapy continue to do so for some time after chemotherapy is stopped and that the regenerative milieu stimulated by the PVE is of a magnitude that reverses this effect. The fact that new lesions appear in some patients may indicate that micrometastases are being recruited in this regenerative environment.
Additionally, the current study demonstrated that patients who underwent PVE and who received pre-embolization bevacizumab had less pronounced overall tumour progression than patients who received chemotherapy only. However, this difference did not reach statistical significance. The addition of bevacizumab has been shown to improve pathologic response, as evidenced by a significant reduction in viable tumour cells and an increase in tumour fibrosis, resulting in a protective effect against sinusoidal injuries induced by oxaliplatin-based chemotherapy.34–36 The increased response rate and/or fibrosis may explain the less pronounced growth observed in the bevacizumab group after PVE. A prospective examination of pathology specimens is needed to confirm this theory.
The limitations of the current study are those inherent to retrospective analyses and make it impossible to make specific recommendations regarding treatment regimens. Prospective studies with homogeneous populations would be required to assess the optimal type and timing of treatment regimens in relation to PVE in patients with CRLM. Prospective studies are also required to assess the impact of PVE-induced tumour growth on outcomes such as resectability and longterm survival. In general, a single CT scan is performed between the end of chemotherapy and liver resection at this institution, which explains the relatively small number of control patients who underwent two CT scans between the cessation of chemotherapy and surgery.
In conclusion, the current study reports on the effects of pre-embolization bevacizumab on liver regeneration and tumour growth observed after PVE in the largest cohort of patients studied to date. These findings provide evidence that PVE induces significant tumour growth in patients with CRLM. Although PVE is an essential tool in the management of CRLM, its effectiveness can be enhanced by developing strategies that limit tumour growth without suppressing liver regeneration.
Acknowledgments
The authors thank the Deanship of Scientific Research, College of Medicine Research Centre, King Saud University for funding this work through research group project no. RGP-VPP-082, and the McGill University Health Center Hepatopancreatobiliary and Transplant Center Research Unit.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer. Cancer Staging Manual. 7th. New York, NY: Springer; 2010. p. 143. [Google Scholar]
- 4.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom PF National Comprehensive Cancer Network. Systemic therapy for advanced or metastatic colorectal cancer: National Comprehensive Cancer Network guidelines for combining anti-vascular endothelial growth factor and anti-epidermal growth factor receptor monoclonal antibodies with chemotherapy. Pharmacotherapy. 2008;28(11 Pt 2):18–22. doi: 10.1592/phco.28.11-supp.18S. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 10.Loupakis F, Bria E, Vaccaro V, Cuppone F, Milella M, Carlini P, et al. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: meta-analysis of randomized trials. J Exp Clin Cancer Res. 2010;29:58–66. doi: 10.1186/1756-9966-29-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch S, Spithoff K, Rumble RB, Maroun J Gastrointestinal Cancer Disease Site Group. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152–1162. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 12.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. discussion 680–681. [DOI] [PubMed] [Google Scholar]
- 14.Abulkhir A, Limongelli P, Healey AJ, Curley SA, Vauthey JN. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 15.Wicherts DA, de Haas RJ, Andreani P, Sotirov D, Salloum C, Castaing D, et al. Impact of portal vein embolization on longterm survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240–250. doi: 10.1002/bjs.6756. [DOI] [PubMed] [Google Scholar]
- 16.Nafidi O, Désy D, Létourneau R, Côté J, Plasse M, Vandenbroucke F, et al. Hypertrophy of the non-embolized liver after chemotherapy. HPB. 2009;11:103–107. doi: 10.1111/j.1477-2574.2009.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Kumamoto T, Matsuyama R, Takeda K, Nagano Y, Endo I. Influence of chemotherapy on liver regeneration induced by portal vein embolization or first hepatectomy of a staged procedure for colorectal liver metastases. J Gastrointest Surg. 2010;14:359–368. doi: 10.1007/s11605-009-1073-6. [DOI] [PubMed] [Google Scholar]
- 19.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 20.Elias D, De Baere T, Roche A, Ducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 21.Barbaro B, Di Stasi C, Nuzzo G, Vellone M, Giuliante F, Marano P. Preoperative right portal vein embolization in patients with metastatic liver disease. Metastatic liver volumes after RPVE. Acta Radiol. 2003;44:98–102. [PubMed] [Google Scholar]
- 22.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effect on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 23.Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and regeneration in mice. J Hepatol. 2006;45:35–42. doi: 10.1016/j.jhep.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Maggiori L, Bretagnol F, Sibert A, Paradis V, Vilgrain V, Panis Y. Selective portal vein ligation and embolization induce different tumoral responses in the rat liver. Surgery. 2010;149:496–503. doi: 10.1016/j.surg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 25.van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436–444. doi: 10.1159/000184735. [DOI] [PubMed] [Google Scholar]
- 26.Zalinski S, Bigourdan JM, Vauthey JN. Does bevacizumab have a protective effect on hepatotoxicity induced by chemotherapy? J Chir (Paris) 2010;147(Suppl 1):18–24. doi: 10.1016/S0021-7697(10)70004-1. [DOI] [PubMed] [Google Scholar]
- 27.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhury P, Hassanain M, Bouganim N, Salman A, Kavan P, Metrakos P. Perioperative chemotherapy with bevacizumab and liver resection for colorectal cancer liver metastasis. HPB. 2010;12:37–42. doi: 10.1111/j.1477-2574.2009.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorzi D, Chun YS, Madoff DC, Abdalla EK, Vauthey JN. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765–2772. doi: 10.1245/s10434-008-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aussilhou B, Dokmak S, Faivre S, Paradis V, Vilgrain V, Belghiti J. Preoperative liver hypertrophy induced by portal flow occlusion before major hepatic resection for colorectal metastases can be impaired by bevacizumab. Ann Surg Oncol. 2009;16:1553–1559. doi: 10.1245/s10434-009-0447-z. [DOI] [PubMed] [Google Scholar]
- 31.Sturesson C, Keussen I, Tranberg KG. Prolonged chemotherapy impairs liver regeneration after portal vein occlusion – an audit of 26 patients. Eur J Surg Oncol. 2010;36:358–364. doi: 10.1016/j.ejso.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Vauthey JN, Zorzi D. In search of the black sheep: is it bevacizumab or extended chemotherapy? Ann Surg Oncol. 2009;16:1463–1464. doi: 10.1245/s10434-009-0450-4. [DOI] [PubMed] [Google Scholar]
- 33.Donadon M, Ribero D, Morris-Stiff G, Abdalla EK, Vauthey JN. New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res. 2007;1:20–27. [PMC free article] [PubMed] [Google Scholar]
- 34.Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 35.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumour response assessment in predicting the outcome in patients with colorectal liver metastases treated with neoadjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 36.Maru DM, Kopetz S, Boonsirikamchai P, Agarwal A, Chun YS, Wang H, et al. Tumour thickness at the tumour–normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]


