Abstract
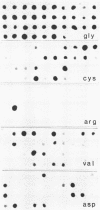
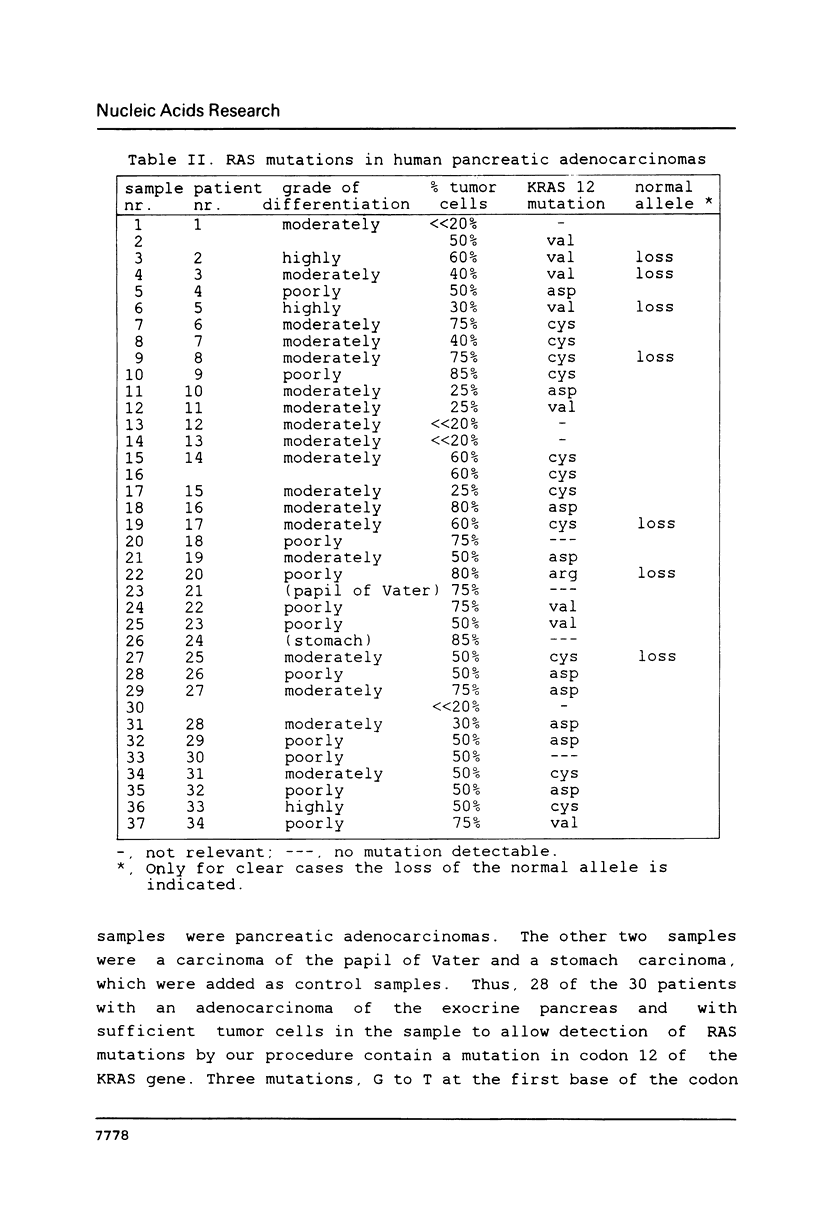
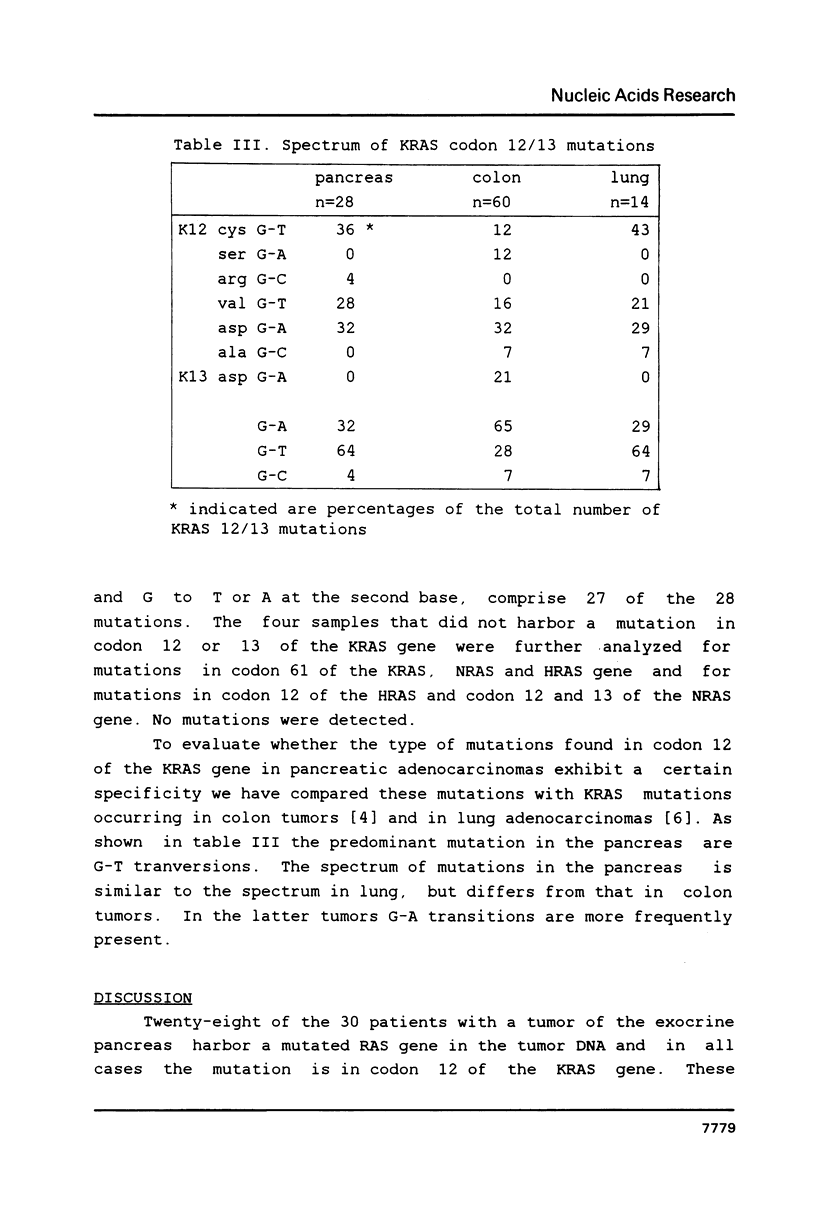
DNAs from human pancreatic adenocarcinomas were analyzed for the presence of mutations in codons 12, 13 and 61 of the NRAS, KRAS and HRAS gene. Formalin-fixed and paraffin-embedded tissue was used directly in an in vitro amplification reaction to expand the relevant RAS sequences. The mutations were detected by selective hybridization using mutation-specific synthetic oligonucleotides. In 28 of the 30 patients we found a mutation in codon 12 of the KRAS gene. This result confirms the findings of Almoguera et al. [Cell 53 (1988) 549-554] that KRAS mutations occur frequently in adenocarcinomas of the exocrine pancreas. The mutations are predominantly G-T transversions, in contrast to the KRAS mutations in colon tumors which are mainly G-A transitions. Furthermore, in a portion of the tumors the mutation appears to be homozygous.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., Jansen A. M., Veeneman G. H., van Boom J. H., van der Eb A. J. Three different mutations in codon 61 of the human N-ras gene detected by synthetic oligonucleotide hybridization. Nucleic Acids Res. 1984 Dec 11;12(23):9155–9163. doi: 10.1093/nar/12.23.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., van der Eb A. J., Janssen J. W., Delwel R., Löwenberg B., Colly L. P. Mutations in N-ras predominate in acute myeloid leukemia. Blood. 1987 Apr;69(4):1237–1241. [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Farr C. J., Saiki R. K., Erlich H. A., McCormick F., Marshall C. J. Analysis of RAS gene mutations in acute myeloid leukemia by polymerase chain reaction and oligonucleotide probes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1629–1633. doi: 10.1073/pnas.85.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Pellicer A. Mutational activation of oncogenes in animal model systems of carcinogenesis. Mutat Res. 1987 May;185(3):293–308. doi: 10.1016/0165-1110(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S., Roebuck B. D., Kuhlmann E. T., Curphey T. J. Induction of pancreatic carcinomas in rats with N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine: histopathology. J Natl Cancer Inst. 1985 Jan;74(1):209–217. [PubMed] [Google Scholar]
- Longnecker D. S., Roebuck B. D., Yager J. D., Jr, Lilja H. S., Siegmund B. Pancreatic carcinoma in azaserine-treated rats: induction, classification and dietary modulation of incidence. Cancer. 1981 Mar 15;47(6 Suppl):1562–1572. doi: 10.1002/1097-0142(19810315)47:6+<1562::aid-cncr2820471419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mills P. K., Beeson W. L., Abbey D. E., Fraser G. E., Phillips R. L. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988 Jun 15;61(12):2578–2585. doi: 10.1002/1097-0142(19880615)61:12<2578::aid-cncr2820611232>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Paterson H., Reeves B., Brown R., Hall A., Furth M., Bos J., Jones P., Marshall C. Activated N-ras controls the transformed phenotype of HT1080 human fibrosarcoma cells. Cell. 1987 Dec 4;51(5):803–812. doi: 10.1016/0092-8674(87)90103-6. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Runge R. G., Birt D., Gingell R., Lawson T., Nagel D., Wallcave L., Salmasi S. Z. Current knowledge of pancreatic carcinogenesis in the hamster and its relevance to the human disease. Cancer. 1981 Mar 15;47(6 Suppl):1573–1589. doi: 10.1002/1097-0142(19810315)47:6+<1573::aid-cncr2820471420>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Pinkert C. A., Ornitz D. M., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987 Mar 27;48(6):1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Wynder E. L. An epidemiological evaluation of the causes of cancer of the pancreas. Cancer Res. 1975 Aug;35(8):2228–2233. [PubMed] [Google Scholar]
- Wynder E. L., Mabuchi K., Maruchi N., Fortner J. G. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973 Mar;50(3):645–667. doi: 10.1093/jnci/50.3.645. [DOI] [PubMed] [Google Scholar]