Abstract
Background. Delay in pulmonary tuberculosis (PTB) diagnosis is one of the major factors that affect outcome and threatens continued spread of tuberculosis. This study aimed at determining factors associated with delayed PTB diagnosis among human immunodeficiency virus (HIV) infected individuals. Methods. A retrospective observational study was done using clinic records of HIV-infected PTB suspects attending an HIV/AIDS clinic at Tintswalo rural hospital in South Africa (SA) between January 2006 and December 2007. Using routine clinic registers, 480 records were identified. Results. PTB diagnosis delay was found among 77/176 (43.8%) of the patients diagnosed with PTB. The mean delay of PTB diagnosis was 170.6 days; diagnosis delay ranged 1–30 days in 27 (35.1%) patients, 31–180 days in 24 (33.8%) patients; 24 (31.2%) patients remained undiagnosed for ≥180 days. Independent factors associated with delayed diagnosis were: older age >40 years (Odds Ratio (OR) 3.43, 95% CI 1.45–8.08) and virological failure (OR 2.72, 95% CI 1.09–6.74). Conclusion. There is a considerable delayed PTB diagnosis among HIV-infected patients in rural SA. Older patients as well as patients with high viral load are at a higher risk of PTB diagnosis delay. Therefore efforts to reduce PTB diagnosis delay need to emphasised.
1. Background
An estimated one-third of the world's population is infected with tuberculosis (TB) [1]. The human immunodeficiency virus (HIV) pandemic has resulted in dramatic increases in TB case notification rates, particularly in resource-limited settings [2]. This is because people living with HIV have a much greater risk of developing active TB than HIV-uninfected individuals [3]. South Africa is one of the countries most heavily affected by the dual HIV and TB epidemics [4], with an estimated 31% of all global TB cases occurring among HIV-positive individuals [5]. For example, in Gugulethu, a township in Cape Town, over half of antiretroviral therapy (ART) clinic attendees had previously been treated for TB, one-quarter were diagnosed with active TB, and a further 10% developed TB during the first year following initiation onto lifelong ART [6].
In resource-limited settings, where the majority of HIV-TB cases occur, TB diagnostics are frequently limited to sputum smear microscopy, despite the increased likelihood of smear negative disease among immunosuppressed patients [7]. This scenario complicates TB diagnosis and frequently results in delay in TB diagnosis in HIV-infected patients [8]. This delayed detection often leads to increased mortality and treatment complications for patients [9]. In addition, undiagnosed TB in patients starting ART may result in symptomatic immune reconstitution inflammatory syndrome during early ART phase because of unmasking of TB disease by ART [10]. We have previously reported on high mortality due to TB among ART initiators in this community [11]. Reducing delays in PTB diagnosis could improve patients' health outcomes and minimise public risk of exposure to TB in the community. The purpose of this study was to investigate factors associated with the delay in TB diagnosis among HIV patients following entry into public sector HIV services in rural South Africa.
2. Methods
2.1. Study Setting
This study was conducted at Rixile Clinic, a dedicated nongovernmental-organisation-(NGO-) supported, rural HIV clinic situated at Tintswalo hospital in Bushbuckridge, Mpumalanga, South Africa. Three HIV-trained doctors and six primary health care nurses provide comprehensive care for HIV patients at the clinic, including provision of ART, and screening for and management of TB, opportunistic infections, and sexually transmitted infections. The clinic was accredited for provision of ART in October 2005. Since then more than 2,000 individuals have been initiated on ART. Bushbuckridge is a densely populated rural area with a population of approximately 600,000 people living in 133 villages. The region is one of 13 rural nodal areas of extreme poverty in South Africa.
2.2. Study Design
This was a retrospective cohort study of PTB suspects at Rixile Clinic. Data were extracted from the ART clinic records of patients identified as PTB suspects from the PTB suspects register over a two-year period from January 2006 to December 2007.
2.3. Study Population
The study population was comprised of adults (18 years and older) at the time of identification as a PTB suspect and with confirmed HIV infection. All patients had attended the Rixile clinic at least once. PTB suspects were defined as patients who were provided with sputum jars for Acid-Fast Bacilli screening for PTB.
2.4. Data Collection and Management
Trained research assistants extracted data from the PTB suspects register and from patients' ART clinic medical records using a structured data collection tool. Data were abstracted on the following variables: sociodemographic factors, presenting symptoms, timing, and results of TB investigations, diagnosis category, date and regimen of TB treatment initiated, and details of HIV treatment, including ART initiation. Data were double-entered into Epi info version 3.5.1 (CDC, Atlanta, USA). Statistical analyses were conducted using STATA version 11 (Statacorp, College Station, USA).
2.5. Definition of TB Diagnosis Delay
TB diagnostic delay was defined as any diagnosis taking longer than 56 days from the time of requesting the first sputum examination. Although this definition is unconventional, it allows for the time required to complete all diagnostic examinations, including sputum culture, which is routinely requested among HIV-positive TB suspects with smear-negative results attending services at Rixile HIV clinic [12].
2.6. Statistical Analysis
Frequency distributions were used to describe categorical variables, and medians and interquartile ranges were used for continuous variables. The dependent variable diagnosis delay was categorized as a dichotomous variable (delay or nodelay). Bivariate associations were described using Chi-square tests. Variables that demonstrated significant association (P ≤ 0.1) with TB diagnosis delay were entered into a multivariate Cox proportional hazards regression model.
2.7. Ethical Consideration
Ethical approval for the study was obtained from the University of Witwatersrand Human Research Ethics Committee. Individual informed consent was not undertaken as the study used routinely collected clinic data.
3. Results
In the two-year study period, 630 individuals were identified as PTB suspects and recorded in the PTB suspect register. Sixty-two were excluded for the following reasons: 4 were younger than 18 years old, 42 did not return their sputum jars and did not attend any subsequent review, 1 had extra pulmonary TB, and there was no information on the outcome of TB diagnosis for 15 patients. Eighty-five patients' medical records were missing and 31 were duplicates. Thus, a total of 452 PTB suspects were included in the study. Figure 1 summarizes the patients' inclusion cascade.
Figure 1.
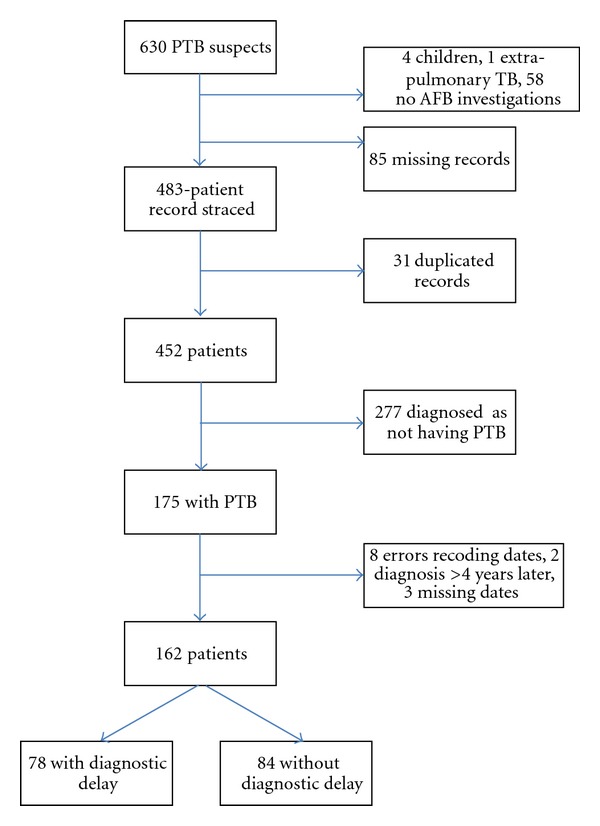
Cohort Flowchart **4 younger than 18 years, 1 with extrapulmonary TB, 58 with no AFB investigation requested. ***31 observations were duplicates ****8 with negative time interval to TB diagnosis, 2 with time interval to TB diagnosis >4 years, 3 with missing TB diagnosis date.
The demographic information of the 452 patients included in the preliminary analysis is summarized in Table 1. The ages of the patients ranged from 23 to 96 years old with a median age of 41 years (interquartile range (IQR) = 35–49). Seventy percent (316/452) of the participants were women; 175/446 (39.2%) were not married, 191/447 (42.7%) had attended primary school only, and more than half 403/449 (89.7%) were unemployed.
Table 1.
Socioeconomic characteristics of study participants.
| Characteristic | N | (%) |
|---|---|---|
| Marital status | ||
| Divorced | 81 | 18.2 |
| Married | 131 | 29.4 |
| Never married | 175 | 39.2 |
| Widowed | 59 | 13.2 |
| Total | 446 | 100.0 |
| Education level | ||
| No education | 90 | 20.1 |
| Primary | 191 | 42.8 |
| Secondary | 152 | 34.0 |
| Tertiary | 14 | 3.1 |
| Total | 447 | 100.0 |
| Occupation status | ||
| Salaried worker | 46 | 10.3 |
| Unemployed and willing to work | 142 | 31.6 |
| Unemployed and not willing to work | 261 | 58.1 |
| Total | 449 | 100.0 |
| Cigarette smoker | ||
| No | 359 | 80.7 |
| Yes | 86 | 19.3 |
| Total | 445 | 100.0 |
| Alcohol drinker | ||
| No | 355 | 79.9 |
| Yes | 89 | 20.1 |
| Total | 444 | 100.0 |
| Main material walls of house | ||
| Block cement | 229 | 51.0 |
| Brick | 115 | 25.6 |
| Mud | 105 | 23.4 |
| Total | 449 | 100.0 |
| People in households | ||
| 1 to 5 | 239 | 53.1 |
| 6 to 10 | 187 | 41.6 |
| >10 | 24 | 5.3 |
| Total | 450 | 100.0 |
N: number of study participants in each variable.
3.1. PTB Cases Diagnosed
Of the 452 PTB suspects, 162 (35.8%) PTB cases were diagnosed. Of those diagnosed with PTB, 40 (24.7%) were diagnosed on the basis of sputum smear positivity, 84 (51.9%) through positive sputum culture, and 38 (23.5%) through chest radiograph.
3.2. Outcomes of PTB Treatment
Out of the 162 patients diagnosed with PTB and started on TB treatment, 86 (53.1%) completed treatment, 14 (8.6%) died, 34 (21.0%) were lost to follow up, and 28 (17.3%) were still on treatment at time of data extraction.
3.3. Delay in Diagnosis of PTB
The median delay from presentation with PTB symptoms to PTB diagnosis was 55 days (IQR = 20–302). Delay in diagnosis of PTB of more than 56 days was observed among 78 (48.2%) of the 162 patients diagnosed with PTB. The overall delay was 57 to 86 days in 27 (34.6%), 87 to 223 days in 26 (33.3%), and >236 days in 25 (32.1%) of the patients diagnosed with PTB. The diagnosis was made by clinical history plus chest X-ray for 19 of the 78 (24.7%) and by sputum smear microscopy for 12 (15.4%) The majority of patients with diagnostic delay had their diagnosis made by sputum culture 47/78 (60.3%) (Table 2).
Table 2.
Diagnosis delay duration by method of diagnosis.
| Method of TB diagnosis | 57 to 86 days delay ∗∗N (%) | 87 to 236 days delay ∗∗N (%) | >236 days delay ∗∗N (%) | Total Number (%) | P value |
|---|---|---|---|---|---|
| Clinical history and chest X-ray | 4 (15.3) | 5 (19.2) | 10 (40) | 19 (24.7) | 0.016 |
| Sputum smear positive | 3 (11.5) | 2 (7.7) | 7 (28) | 12 (15.6) | |
| Sputum culture | 20 (74.1) | 19 (73.1) | 8 (32) | 47 (60.3) | |
| Total | 27 (100) | 26 (100) | 25 (100) | 78 (100) |
∗∗N: number of TB patients diagnosed within each time duration.
3.4. Factors Associated with TB Diagnosis Delay
Univariate analysis showed patient's age (P = 0.006), method of TB diagnosis (P = 0.025), detectable HIV viral load (P = 0.025), and ART use at the time of PTB diagnosis (P ≤ 0.001), to be significantly associated with TB diagnosis delay (Table 3). Age, viral load and ART use at the time of PTB diagnosis remained statistically significant in the multivariate analysis. Patients who were older than 40 years had a 1.57 times greater risk of TB diagnostic delay than those aged 18–40 years. The risk of TB diagnostic delay was 1.89 times greater in those with HIV viral load ≥400 copies/mL than patients with HIV viral load ≤400 copies/mL; however the observed association was not statistically significant P = 0.06. Those who were on ART at the time of PTB diagnosis were 51% less likely to have experienced diagnostic delay than those who were not on ART (Table 4).
Table 3.
Comparison of sociodemographic and clinical factors of participants with TB diagnosis delay.
| Characteristic | Diagnosis delay number (%) | No delay number (%) | P value |
|---|---|---|---|
| Patient age | |||
| 18 to 40 years | 33 (42.9) | 54 (64.3) | 0.006∗∗ |
| >40 years | 44 (57.1) | 30 (35.7) | |
| Sex | |||
| Female | 49 (62.9) | 58 (69.0) | 0.403 |
| Male | 29 (37.1) | 26 (31.0) | |
| Marital status | |||
| Divorced | 13 (16.9) | 13 (15.4) | 0.592 |
| Married | 25 (32.5) | 21 (25.0) | |
| Never married | 27 (35.1) | 38 (45.2) | |
| Widowed | 12 (15.5) | 12 (14.3) | |
| Occupation status | |||
| Salaried worker | 9 (11.7) | 7 (8.3) | 0.538 |
| Unemployed and willing to work | 21 (27.3) | 29 (34.5) | |
| Unemployed and not willing to work | 47 (61.0) | 48 (57.2) | |
| Smoke cigarette | |||
| No | 57 (74.0) | 66 (78.6) | 0.410 |
| Yes | 20 (26.0) | 18 (21.4) | |
| Alcohol drinking | |||
| No | 57 (74.0) | 65 (79.3) | 0.434 |
| Yes | 20 (26.0) | 17 (20.7) | |
| Education level | |||
| No education | 16 (20.8) | 14 (16.7) | 0.320 |
| Primary | 37 (48.0) | 33 (39.2) | |
| Secondary | 23 (29.9) | 33 (39.3) | |
| Tertiary | 1 (1.3) | 4 (4.8) | |
| Main material walls of house | |||
| Block cement | 38 (49.3) | 47 (56.0) | 0.377 |
| Brick | 19 (24.7) | 22 (26.1) | |
| Mud | 20 (26.0) | 15 (17.9) | |
| Method of TB diagnosis | |||
| Clinical history and CXR | 19 (24.7) | 19 (22.9) | 0.025∗∗ |
| Sputum AFB | 12 (15.6) | 28 (33.7) | |
| Sputum culture | 46 (59.7) | 36 (43.4) | |
| WHO-HIV clinical stage | |||
| 1 | 2 (3.6) | 2 (2.9) | 1.000 |
| 2 | 9 (16.4) | 16 (23.5) | |
| 3 | 42 (76.4) | 44 (64.8) | |
| 4 | 2 (3.6) | 6 (8.8) | |
| CD4 count (cells/mm3) | |||
| <50 | 4 (6.9) | 1 (1.8) | 0.193 |
| 50–200 | 15 (25.9) | 10 (17.9) | |
| >200 | 39 (67.2) | 45 (80.3) | |
| Viral load (copies/mL) | |||
| ≤400 | 31 (60.8) | 39 (81.3) | 0.025∗∗ |
| >400 | 20 (39.2) | 9 (18.7) | |
| BMI (Kg/m2) | |||
| Underweight | 16 (25.4) | 15 (22.4) | 0.828 |
| Normal weight | 34 (53.9) | 38 (56.7) | |
| Overweight | 12 (19.0) | 11 (16.4) | |
| Obese | 1 (1.5) | 3 (4.5) | |
| ART use at PTB diagnosis | |||
| No | 38 (50.0) | 62 (77.5) | ≤0.001∗∗ |
| Yes | 38 (50.0) | 18 (22.5) | |
| TB treatment outcome | |||
| Completed treatment | 41 (52.5) | 45 (53.6) | 0.640 |
| Died | 7 (9.1) | 7 (8.3) | |
| Lost to follow up | 14 (17.9) | 20 (23.8) | |
| Still on treatment | 16 (20.5) | 12 (14.3) |
∗∗ P value ≤ 0.05.
Table 4.
Univariate and multivariate Cox proportional hazards regression analysis.
| Characteristic | Univariate HR (95% CI) | P-value | Multivariate HR (95% CI) | P value |
|---|---|---|---|---|
| Patient age | ||||
| 18 to 40 years | 1 | 1 | ||
| >40 years | 1.8 (0.53–1.32) | 0.05 | 1.57 (0.25–0.90) | 0.02 |
| Sex | ||||
| Female | 1 | |||
| Male | 1.26 (0.79–2.03) | 0.33 | — | — |
| Education level | ||||
| No education | 1 | |||
| Primary | 2.62 (1.28–5.34) | 0.25 | ||
| Secondary | 7.09 (0.87–7.56) | 0.17 | ||
| Tertiary | 1.54 (0.79–2.95) | 0.19 | ||
| Method of TB diagnosis | ||||
| History and CXR | 1 | |||
| Smear positive | 0.83 (0.48–2.14) | 0.95 | — | — |
| Sputum culture positive | 2.09 (1.19–3.65) | 0.09 | ||
| Viral load (copies/mL) | ||||
| <400 | 1 | 1 | ||
| >400 | 2.3 (0.87–1.56) | 0.07 | 1.89 (0.74–1.63) | 0.06 |
| CD4 Count (cells/mm3) | ||||
| <50 | 1 | |||
| 50–200 | 0.56 (0.18–1.72) | 0.31 | — | — |
| >200 | 0.64 (0.20–1.64) | 0.42 | ||
| ART use at PTB diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 0.82 (1.05–1.3) | 0.04 | 0.49 (0.36–0.97) | 0.04 |
| TB treatment outcome | ||||
| Completed treatment | 1 | |||
| Died | 2.42 (1.07–5.47) | 0.03 | — | — |
| Lost to follow-up | 0.92 (0.49–1.73) | 0.81 | ||
| Still on treatment | 0.79 (0.43–1.46) | 0.45 |
HR: hazard ratio; CI: confidence interval.
4. Discussion
A large proportion of PTB patients experienced diagnostic delays of greater than 56 days. These findings have important implications for informing current Stop-TB efforts aimed at reducing global TB incidence and mortality. TB diagnosis delay was associated with viral load ≥400 copies/mL. Delayed detection leads to patients presenting with advanced TB which promotes HIV/AIDS disease progression by accelerating viral multiplication [13].
TB diagnostic delay was significantly associated with patient's age. Older patients (>40 years) were at a much higher risk of TB diagnostic delay compared with those younger than 40 years. This is likely to be due to coexisting medical conditions in the elderly persons such as heart diseases and chronic chest problems, all of which may contribute to the difficulty of diagnosing TB and can thus result in delayed diagnosis and treatment [14, 15].
The majority of patients with diagnostic delay in this study (60.3%) were diagnosed with PTB by sputum culture. This may be because the study was conducted among HIV-infected patients, and studies have reported reduced diagnostic sensitivity of sputum smears and chest radiography in HIV infected patients [7]. Given the high percentage of patients diagnosed by TB culture, these findings support the practice of regular sputum culture tests to confirm sputum negative smears in ART clinics. Those who were not on ART at the time of PTB diagnosis were more likely to have delayed diagnosis than those who were on ART. ART unmasks TB in HIV-infected patients with subclinical disease making it easier to detect and diagnose [10].
There are several factors that have been reported from previous studies that influences delay in TB diagnosis. These could be patient, clinician, or health system related. Patient-related factors include choice of health care provider, stigma, alcoholism, substance abuse, general state of poor health, tobacco smoking, and chronic cough with blood-stained sputum [16–26]. Clinician-related factors include: poor training of clinical personnel in TB diagnosis, low level of TB awareness among private practitioners, and lack of knowledge of TB diagnosis among traditional healers [20, 22, 24–29]. Health system factors that play a role in delayed TB diagnosis include lack of diagnostic facilities and low access to health care services [26, 30].
High viral load is a clinical indicator of declining immunological function of an HIV-infected individual which makes them to be at higher risk of developing active TB. In this era of ongoing HIV epidemic, diagnosis and clinical management of active TB poses a major challenge in patients with dual infection [31]. Due to immunosuppression, there is lack of pulmonary cavitations resulting in low bacillary concentration in sputum giving rise to high rate of negative sputum smear for AFB as well as negative chest radiographic findings. This has led to an over reliance of TB diagnosis through sputum culture which is an expensive and slow diagnostic method [32, 33]. This could be the reason why our study showed that a positive relationship exists between diagnostic delay and decline in immune function (viral load >400 copies/mL).
In considering the findings of this study it is important to bear in mind the following limitations: firstly, as this cohort was recruited from an ART clinic, they may have some differences in characteristics to those participants in hospitals, public health clinics, and the general community. Secondly, the study population did not include PTB suspects who were younger than 18 years, and 85 patients were excluded due to missing medical records. It is possible that some in both groups had active TB. Thirdly, accuracy of interpretation of routinely offered TB diagnostic tests (sputum smear and chest radiographs) depends on the skills and experience of the attending clinician. Interpretation of these tests is often subjective and prone to error; hence some of the results might have been incorrect. Fourthly, some patients in this cohort 42/630 (6.7%) did not return their sputum jars (lost to follow-up) and, therefore, were not investigated for AFB. It is possible that some of these patients had TB or had died of TB. Lastly we did not have data documenting the duration of TB symptoms prior to presenting at a health facility, therefore we would not know length of diagnosis delay attributed to TB patient's health seeking behaviour. The exclusion of patients who were lost-to-follow-up and the definition of delay from presentation at health system, rather than start of symptoms, make these findings conservative as the true proportion of patients experiencing diagnosis delays would be higher than what has been reported in this study.
5. Conclusion
The diagnostic delays for PTB demonstrated in this study, as well as the difficulty of diagnosing TB in HIV-infected patients, point to the need for improved screening of TB suspects in ART clinics. Novel TB diagnostic methods such as Gene-Xpert, which are rapid, highly sensitive, and specific, should be promoted in primary health care facilities in order to ensure the timely diagnosis of PTB.
Authors' Contribution
M. Moshabela, R. Zulliger, and P. Nyasulu conceived and designed the study. R. Boniface undertook the data collection and statistical analysis and wrote the first draft of the manuscript. All authors contributed in intellectual content and approved the final manuscript.
Conflict of Interests
The authors have no conflict of interest to declare.
Acknowledgments
The authors would like to thank all the staff of Rixile HIV clinic and the management of Tintswalo Hospital for their cooperation. The contribution of Ilona Sips in the draft of the paper is greatly appreciated. This study was supported by Rural AIDS and Development Action Research (RADAR), School of Public Health, University of Witwatersrand.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. Journal of the American Medical Association. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15(2):143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 3.allAfrica.com: South Africa: Explaining TB-HIV Integration. http://www.allafrica.com/Stories/201003240509.html.
- 4.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clinical Infectious Diseases. 2006;42(7):1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global tuberculosis control—epidemiology, strategy, financing. 2009, http://www.who.int.
- 6.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 7.Onyebujoh P, Rodriguez W, Mwaba P. Priorities in tuberculosis research. The Lancet. 2006;367(9514):940–942. doi: 10.1016/S0140-6736(06)68385-2. [DOI] [PubMed] [Google Scholar]
- 8.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease. 2000;4(2):97–107. [PubMed] [Google Scholar]
- 9.Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. International Journal of Tuberculosis and Lung Disease. 2006;10(1):24–30. [PubMed] [Google Scholar]
- 10.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet Infectious Diseases. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacPherson P, Moshabela M, Martinson N, Pronyk RM. Mortality and loss to follow-up among HAART initiators in rural South Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(6):588–593. doi: 10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Tuberculosis—A training Manualfor Health Workers. http://www.capegateway.gov.za/
- 13.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr. Effect if isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. The Lancet. 1993;342(8866):268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 14.Mostaza JL, García N, Fernández S, Bahamonde A, Fuentes MI, Palomo MJ. Analysis and predictors of delays in the suspicion and treatment among hospitalized patients with pulmonary tuberculosis. Anales de Medicina Interna. 2007;24(10):478–483. doi: 10.4321/s0212-71992007001000004. [DOI] [PubMed] [Google Scholar]
- 15.Karim F, Islam MA, Chowdhury AMR, Johansson E, Diwan VK. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy and Planning. 2007;22(5):329–334. doi: 10.1093/heapol/czm026. [DOI] [PubMed] [Google Scholar]
- 16.Pronyk RM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. International Journal of Tuberculosis and Lung Disease. 2001;5(7):619–627. [PubMed] [Google Scholar]
- 17.WHO. Diagnostic and Treatment Delay in Tuberculosis. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 18.Yamasaki-Nakagawa M, Ozasa K, Yamada N, et al. Gender difference in delays to diagnosis and health care seeking behavior in a rural area of Nepal. International Journal of Tuberculosis and Lung Disease. 2001;5(1):24–31. [PubMed] [Google Scholar]
- 19.Güneylioglu D, Yilmaz A, Bilgin S, Bayram U, Akkaya E. Factors affecting delays in diagnosis and treatment of pulmonary tuberculosis in a tertiary care hospital in Istanbul, Turkey. Medical Science Monitor. 2004;10(2):CR62–CR67. [PubMed] [Google Scholar]
- 20.Hooi LN. Case-finding for pulmonary tuberculosis in Penang. Medical Journal of Malaysia. 1994;49(3):223–230. [PubMed] [Google Scholar]
- 21.Kiwuwa MS, Charles K, Harriet MK. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: a cross-sectional study. BMC Public Health. 2005;5, article 122 doi: 10.1186/1471-2458-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajeswari R, Chandrasekaran V, Suhadev M, Sivasubramaniam S, Sudha G, Renu G. Factors associated with patient and health system delays in the diagnosis of tuberculosis in South India. International Journal of Tuberculosis and Lung Disease. 2002;6(9):789–795. [PubMed] [Google Scholar]
- 23.Steen TW, Mazonde GN. Pulmonary tuberculosis in Kweneng District, Botswana: delays in diagnosis in 212 smear-positive patients. International Journal of Tuberculosis and Lung Disease. 1998;2(8):627–634. [PubMed] [Google Scholar]
- 24.Bai LQ, Xiao SY. Factors associated with diagnostic delay for patients with smear-positive pulmonary tuberculosis in rural Hunan, China. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27(9):617–620. [PubMed] [Google Scholar]
- 25.Rojpibulstit M, Kanjanakiritamrong J, Chongsuvivatwong V. Patient and health system delays in the diagnosis of tuberculosis in Southern Thailand after health care reform. International Journal of Tuberculosis and Lung Disease. 2006;10(4):422–428. [PubMed] [Google Scholar]
- 26.Needham DM, Foster SD, Tomlinson G, Godfrey-Faussett P. Socio-economic, gender and health services factors affecting diagnostic delay for tuberculosis patients in urban Zambia. Tropical Medicine and International Health. 2001;6(4):256–259. doi: 10.1046/j.1365-3156.2001.00709.x. [DOI] [PubMed] [Google Scholar]
- 27.Lienhardt C, Rowley J, Manneh K, et al. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. International Journal of Tuberculosis and Lung Disease. 2001;5(3):233–239. [PubMed] [Google Scholar]
- 28.Liam CK, Tang BG. Delay in the diagnosis and treatment of pulmonary tuberculosis in patients attending a university teaching hospital. International Journal of Tuberculosis and Lung Disease. 1997;1(4):326–332. [PubMed] [Google Scholar]
- 29.Wandwalo ER, Mørkve O. Delay in tuberculosis case-finding and treatment in Mwanza, Tanzania. International Journal of Tuberculosis and Lung Disease. 2000;4(2):133–138. [PubMed] [Google Scholar]
- 30.Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: a cross sectional study. BMC Infectious Diseases. 2005;5, article 112 doi: 10.1186/1471-2334-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthew T. HIV and TB: dual immunosuppressive diseases. HIV Clinician/Delta Region AIDS Education & Training Center. 2010;22(2):1–5. [PubMed] [Google Scholar]
- 32.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. The Lancet Infectious Diseases. 2012;12(3):201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. Journal of Infectious Diseases. 2011;204(supplement 4):S1159–S1167. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]


