Abstract
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for 95% of primary liver cancer. For each of these malignancies the outcome is dismal; incidence is rapidly increasing and mechanistic understanding is limited. We observed abnormal proliferation of both biliary epithelium and hepatocytes in mice following genetic manipulation of Yes associated protein (YAP), a transcription co-activator. Here we comprehensively documented YAP protein expression in the human liver and primary liver cancers. We showed that nuclear YAP expression is significantly increased in human ICC and HCC. We found that increased YAP protein levels in HCC are due to multiple mechanisms including gene amplification, transcriptional and posttranscriptional regulation. Survivin, a member of the inhibitors-of-apoptosis protein (IAPs) family, has been reported as an independent prognostic factor for poor survival in both HCC and ICC. We found nuclear YAP expression correlates significantly with nuclear Survivin expression for both ICC and HCC. Furthermore, using mice engineered to conditionally overexpress YAP in the liver, we found Survivin mRNA expression depends upon YAP protein levels. Our findings suggested that YAP contributes to primary liver tumorigenesis and likely mediates its oncogenic effects through modulating Survivin expression.
Keywords: Yes associated protein, Survivin, Hepatocellular carcinoma, Cholangiocarcinoma
Introduction
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for the vast majority of primary liver malignancies and are among the most lethal and aggressive neoplasmas (1, 2). Primary liver cancer is the third most common cause of cancer mortality worldwide (2). For HCC patients, although advances have been achieved through development of multiple therapeutic approaches, the majority of patients still lack effective treatment and have a poor prognosis (1). Curative surgical resection offers the only hope for patients with ICC since it is not responsive to systemic chemotherapy and radiotherapy regimens. However, relatively few patients are suitable candidates for curative surgical resection and the recurrence rates remain high even after curative surgical resection (2). Therefore, there is urgent need to advance our understanding of the molecular mechanisms underlying these devastating cancers in order to devise novel strategies aimed at improving the prognosis.
Yes associated protein (YAP) is the nuclear effector of the Hippo signaling pathway (3). As a transcription co-activator, YAP can induce the expression of a class of genes which promote cell proliferation and inhibit cell death (4). The transcriptional co-activator activity of YAP can be inhibited by the Hippo signaling pathway through phosphorylating the conserved serine 127 residue (4). This phosphorylation leads to cytoplasmic retention of YAP (4), therefore, the activity of YAP can be reflected by its subcellular location: active YAP is located in the nucleus while inactive YAP can be found in the cytoplasm. Overexpression of YAP or ablation of upstream tumor suppressors in the Hippo pathway with genetically modified mouse models results in tissue over-growth which frequently leads to HCC and ICC (4–9). Yap deficiency in the mouse liver induces defects in bile duct development and proliferation (8), suggesting YAP plays an important role in biliary tract homeostasis. YAP has been identified as an independent prognostic marker for HCC, lung and ovarian cancer (10–12) and YAP is frequently over-expressed in lung, ovarian, pancreatic, colorectal, prostate carcinomas and brain malignancies (4, 13, 14). However, YAP expression in cholangiocarcinoma patients has not been well documented. Gene amplification of the Yap locus has been reported in a wide spectrum of human and murine malignancies including medulloblastomas, oral squamous-cell carcinomas, and carcinomas of the lung, pancreas, esophagus, liver, and mammary gland (15–21). No other mechanisms have been revealed to contribute to the elevated YAP expression in human tumors. Although a set of genes have been identified as transcriptional targets of YAP through genetically modified mouse models or cell lines, such as the IAP family member BIRC5/Survivin (4), the secreted Cystein-rich protein connective tissue growth factor (CTGF) (22), the EGF family member amphiregulin (AREG) (23) and the AXL receptor kinase (Axl) (24), none of them has been shown to correlate with YAP expression in human cancer patients. Thus, the purpose of this study is to investigate the expression of YAP in the normal and malignant human liver tissue; explore the mechanisms that contribute to the elevated YAP expression; and to find YAP regulated targets in cancer patients.
Materials and Methods
Human subjects
The use of human tissues in this study was approved by the Johns Hopkins Institution Review Board. All human liver samples are from patients undergoing surgical resection at the Johns Hopkins Hospital, Baltimore, MD. Tumor tissues and adjacent non-tumor tissues were collected at the time of surgery and stored at −80°C. Formalin-fixed, paraffin-embedded normal liver sections for interlobular bile duct, hilum and gallbladder each from 5 patients without liver disease were from pathology archives in the Johns Hopkins University School of Medicine.
Tissue microarray
HCC and biliary cancer tissue microarrays were constructed with the preexisting paraffin-embedded tissues as described (13). A total of 11 HCC tissue microarrays including 87 HCC patient tumors and adjacent non-tumor tissues (each tumor and adjacent non-tumor tissue has 4 cores on the same TMA) and 2 biliary cancer tissue microarrays including 10 ICC patient tumors (each tumor has 2 cores on both TMA) were investigated in this study.
Animal procedures
The animal protocols were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. The inducible YAP transgenic mice (ApoE/rtTA-YAP) has been generated and described previously (4). Six-week-old transgenic mice were fed 0.2 mg/ml doxycycline (Sigma) in drinking water and their livers were harvested at indicated times. For each time point, at least three mice were used to calculate the mean and standard deviation.
DNA extraction and determination of genomic copy number
Genomic DNA in the tissue specimens was extracted with the DNeasy Tissue kit (Qiagen, Cat No. 69504). Quantitative real-time PCR was performed on a 7300 real-time PCR system (Applied Biosystems) with SYBR green PCR master mix (Applied Biosystems, Cat No. 4309155). The Cox8A gene, present on the same chromosome as Yap, was used as an internal control. Genomic DNA levels for Yap and Cox8A were determined based on standard curves derived from serial dilutions of human genomic DNA from normal liver tissues. The Yap gene amplifications were determined by the ratio of Yap and Cox8A genomic DNA levels. A ratio of more than 2 was considered as a Yap gene amplification. The primer sequences used are available upon request.
RNA isolation, reverse transcription, and real-time quantitative PCR
Total cellular RNA in the tissue specimens was extracted using the MasterPureTM RNA purification kit (Epicentre Biotechnologies, Cat No. MCR85102). cDNA was synthesized with random primer using AffinityScript™ Multi Temperature cDNA synthesis kit (Stratagene, Cat No. 200436). Real-time quantitative PCR (Q-PCR) was carried out on a 7300 real-time PCR system (Applied Biosystems) with SYBR green PCR master mix (Applied Biosystems, Cat No. 4309155). Beta-glucuronidase and Histone H2A.Z were used as housekeeping control for human and mouse, respectively. Relative differences in the expression of the candidate genes in different liver samples were determined using the 2-ΔΔCt method. The primer sequences used are available on request.
Immunostaining
Paraffin embedded tissue specimens were cut into 4-µm sections, dewaxed and hydrated. Antigen retrieval was performed by heating at 95°C−100°C in 10mM sodium citrate buffer (pH 6.0) for 20 minutes. Sections were blocked in 5% BSA for 10 minutes and then incubated with primary antibodies according to antibody manufacture’s protocol. After washing off the primary antibody with TBST, the sections were incubated with secondary antibodies according to antibody manufacture’s datasheet. For immunohistochemistry, DAB solution (DAKO) was applied and 50% hematoxylin was used for counterstain. For immunofluorescence, coverslips were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen, P36935). The primary antibodies used in the study were YAP (Epitomics, #2060), CK7 (DAKO, M7018), Survivin (Cell signaling, #2808), Glypican-3 (BioMosaics, #s-B0025R). The secondary antibodies used in this study were Envision anti-rabbit (DAKO; K4002), Alexa Fluor® 488 goat anti-mouse (Invitrogen, #11001) and Alexa Fluor® 568 goat anti-rabbit (Invitrogen, #11011).
Protein lysate and western blot analysis
Liver tissues were lysed in RIPA buffer (150 mM NaCl, 50mM Tris-HCl [pH7.4], 1% NP-40, 0.5% sodium-deoxycholate, 0.1% SDS, and 1mM PMSF) with protease inhibitors (Roche). The proteins were separated on SDS-polyacrylamide gels and transferred onto PVDF membranes (GE healthcare). The blots were probed with antibodies against human YAP (Epitomics, #2060) and normalized by GAPDH (Sigma, G9545). Signals were detected and quantified by Molecular Imager Gel Dox XR system from BioRad.
Grading of immunohistochemistry staining and correlation studies for HCC tissue microarray
Cytoplasmic and nuclear immunohistochemistry staining of YAP in hepatocytes were scored separately based on distribution and intensity. The distribution was scored on a four-point system. A score of 0 was given for complete lack of staining, 1 for staining in less than 10% of cells, 2 for 10%−50%, and 3 for more than 50% of cells. The intensity was also scored on a four-point system. A score of 0 was given for complete lack of staining, 1 for weak staining, 2 for medium staining, and 3 for strong staining. The final score for a patient was calculated by averaging the scores of all TMA cores in the same category. For each patient, both intensity and distribution scores no lower than 1.5 was considered strong in YAP staining; both intensity and distribution scores equal to 0 was considered negative; all others were considered low in YAP staining.
The nuclear expression of Survivin and cytoplasmic expression of Glypican-3 were scored as distribution only because there are similar levels in intensity. The final score for a patient was calculated by averaging the scores of all the cores of same patient. Correlation was calculated as nuclear YAP distribution scores vs. nuclear Survivin distribution scores and nuclear YAP distribution scores vs. cytoplasmic Glypican-3 distribution scores.
Grading of immunofluorescencea and correlation studies for ICC tissue microarray
Only strong fluorescent signal was considered as specific staining. Nuclear staining of YAP and Survivin in cholangiocytes were scored based on distribution as described above. The final score for each patient was calculated by averaging the scores of all the cores. Correlation was calculated as nuclear YAP distribution scores vs. nuclear Survivin distribution scores.
Statistial methods
Statistical significance of YAP staining in non-tumor and tumor tissues was determined using the Fisher exact test. The P values were calculated for positivity (negative vs. low + strong) and distribution of intensity (negative + low vs. strong) for non-tumor tissues compared to tumor tissues. The correlation analysis using Pearson’s test was performed with Graphpad Prism5 software.
Results
Elevated nuclear YAP expression in intrahepatic cholangiocarcinoma
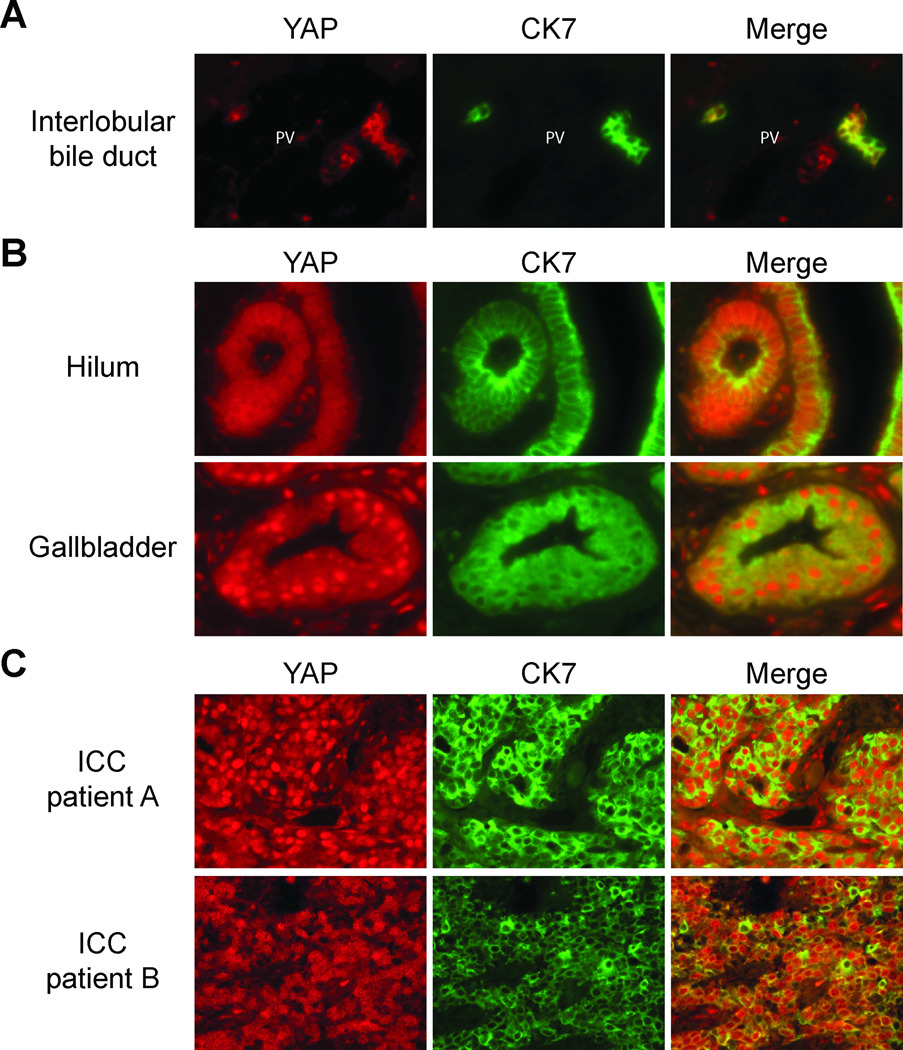
Normal human liver tissues for interlobular bile duct, hilum and gallbladder each from 5 patients were analyzed for YAP protein expression using immunofluorescence. A co-stain with biliary membrane marker CK7 was used to identify biliary epithelial cells (BECs). Because the nuclear presence of YAP reflects its transcription co-activator activity while YAP located in the cytoplasm indicates inactive YAP, we specifically examined the subcellular location of YAP along the intrahepatic biliary tree. For the BECs of interlobular bile ducts, YAP was located predominantly on the plasma membrane but little was present in the nucleus (Figure 1A). For the BECs of hilum and gallbladder, YAP was located both in cytoplasm and nucleus with strong nuclear YAP staining in gallbladder (Figure 1B). Having established YAP expression pattern in normal tissues, we next looked at malignant tissues. We evaluated nuclear YAP expression in 10 ICC patients and found elevated YAP expression compared to normal interlobular bile duct in 9 patients (90%) (YAP staining in representative patients shown in Figure 1C). This observation suggests that increased YAP activity could be a common feature in ICC patients.
Figure 1. Elevated nuclear YAP expression in intrahepatic cholangiocarcinoma.
(A)Interlobular bile ducts from human patients without liver disease co-stained with YAP and biliary membrane marker CK7. Note the membrane location of YAP in BECs of interlobular bile duct. (B) Bile ducts near hepatic hilum, and gallbladder from human patients without liver disease co-stained with YAP and biliary membrane marker CK7. Note the cytoplasmic and nuclear location of YAP in BECs of both hilum and gallbladder with gallbladder epithelial cells showed strong nuclear YAP staining. (C) Staining of YAP in two representative ICC patients. Note the strong nuclear YAP staining in ICC patients. PV, portal vein.
Nuclear YAP expression correlates with nuclear Survivin expression in intrahepatic cholangiocarcinoma
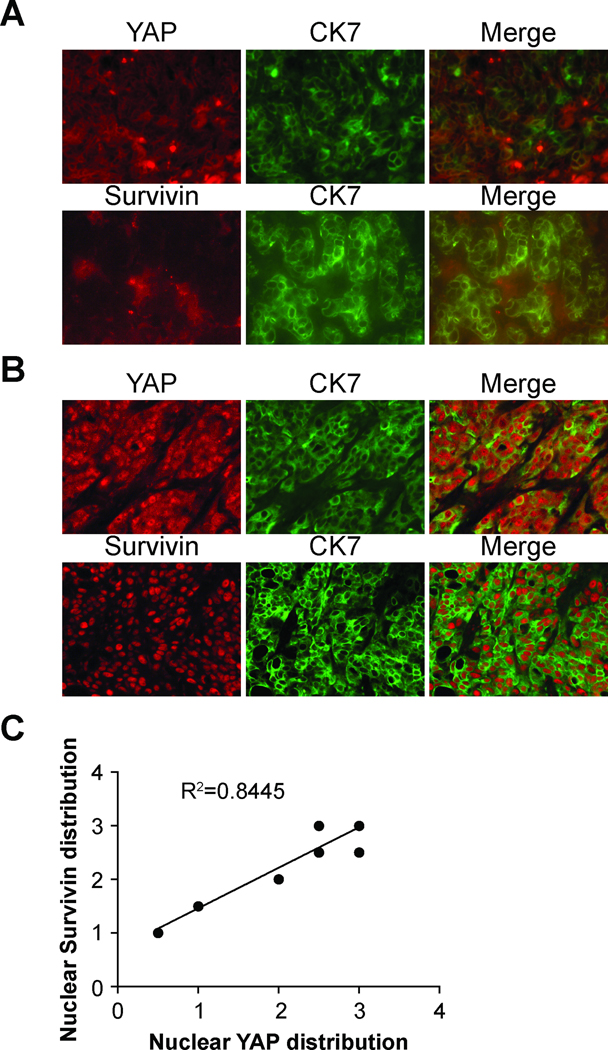
Transcription of Survivin has been shown to be regulated by YAP (4). Nuclear Survivin expression has been reported as a marker of poor prognosis in cholangiocarcinoma (25), and an un-biased genome-wide screen of commonly upregulated genes in ICC patients identified Survivin (26). Furthermore, from our unpublished data, Survivin mRNA levels was regulated by YAP during regeneration after biliary obstruction in mice (submitted). We decided to explore whether nuclear YAP expression associates with nuclear Survivin expression in ICC tumor samples. The distribution of positive YAP or Survivin nuclear expression was scored as described in the Methods section. Correlation analysis revealed significant positive association between nuclear YAP and nuclear Survivin expression (n=8, R2=0.8445, p=0.0012) (Figure 2). Therefore, YAP may regulate Survivin expression in ICC tumors.
Figure 2. Nuclear YAP expression correlates with nuclear Survivin expression in intrahepatic cholangiocarcinoma.
(A) Representative ICC tumor with low nuclear YAP levels stained with YAP/CK7 and Survivin/CK7. Note the absence of nuclear staining for both YAP and Survivin. (B) Representative ICC tumor with high nuclear YAP levels stained with YAP/CK7 and Survivin/CK7. Note the strong nuclear staining for both YAP and Survivin. (C) Correlation curve between nuclear YAP distribution and nuclear Survivin distribution (n=8, Pearson’s test, R2=0.8445, p = 0.0012).
Elevated nuclear YAP expression in hepatocellular carcinoma
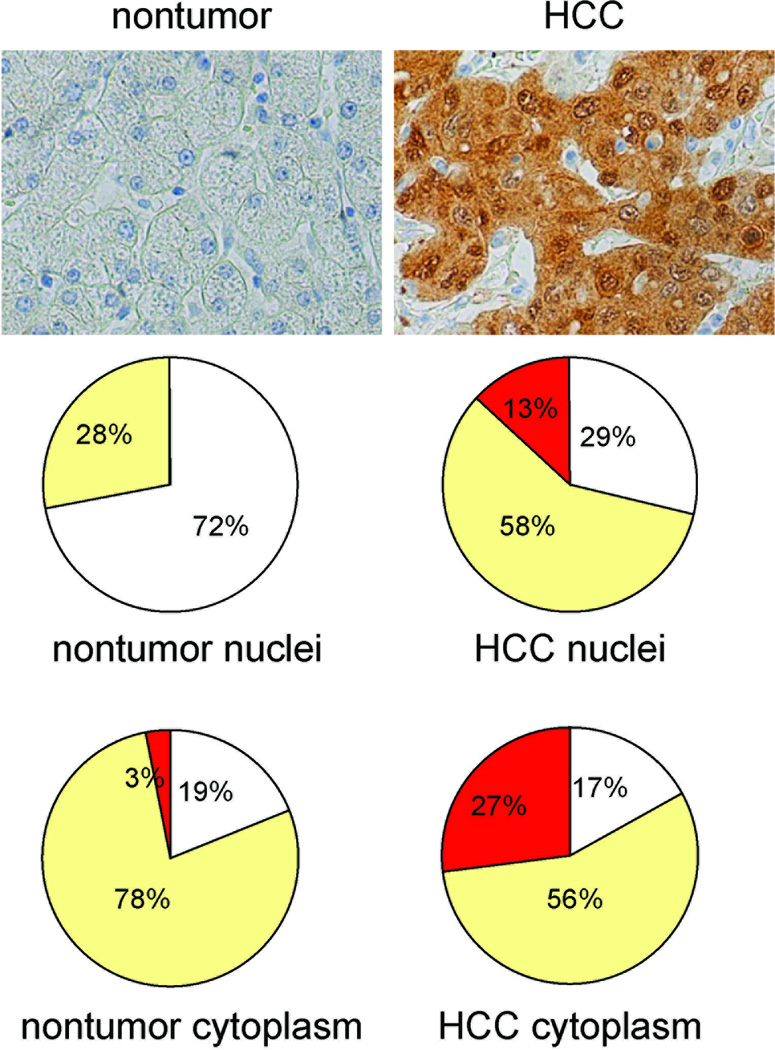
YAP expression in 87 HCC samples from 83 patients was evaluated by immunohistochemistry. The nuclear and cytoplasmic YAP staining in malignant and non-malignant hepatocytes was scored separately (Figure 3). Nuclear YAP staining was present in 62 of 87 (13%+58%=71%) of HCC specimens compared to 23 of 83 (28%) of non-tumor specimens (p < 0.001). The frequency of strong nuclear YAP expression was significantly higher in HCC specimens 11 of 87 (13%) than in non-tumor specimens 0 of 87 (0%) (p < 0.001). There is no difference in cytoplasmic YAP staining between tumor and nontumor specimens (83% vs. 81%, p=0.148). However, strong YAP cytoplasmic staining is significantly more frequent in tumor tissues (27% vs. 3%, p < 0.001).
Figure 3. Elevated nuclear YAP expression in hepatocellular carcinoma.
(A) Evaluation of YAP expression in human non-tumor liver and hepatocellular carcinoma. Top panel: representative photomicrographs of non-tumor and tumor tissues from same HCC patients stained for YAP. Middle and bottom panels: nuclear and cytoplasmic YAP expression depicted as a percentage of patients with no (white), low (yellow) and strong (red) staining.
Elevated YAP protein levels in hepatocelluar carcinoma are due to multiple mechanisms
To determine if the elevated YAP expression in HCC tumors is due to gene amplification (21), we analyzed levels of Yap genomic alleles and mRNA by quantitative-PCR and YAP protein levels by immunohistochemistry for paired HCC tumors and adjacent non-tumor tissues (Table 1). Among the 17 tumor tissues from 14 HCC patients, 4 tumors (24%) showed Yap gene amplification, 8 tumors (47%) showed Yap mRNA upregulation and 7 tumors (41%) showed YAP protein increase compared to adjacent non-tumor tissues. However, gene amplification and mRNA up regulation did not always correlate with an increase in YAP protein levels. Interestingly, 2 out of 4 tumors with Yap gene amplifications and 4 out of 8 tumors with increased Yap mRNA did not have increased YAP protein levels. Furthermore, 2 of 7 HCC tumors with elevated YAP protein were not associated with either gene amplification or mRNA upregulation (Table 1). This suggested that the elevated YAP protein levels in HCC tumors are due to multiple mechanisms including gene amplification, transcriptional and post-transcriptional mechanisms.
Table 1.
Characterization of YAP expression in hepatocellular carcinomas and adjacent cirrhotic tissue
| Patient No. |
YAP (malignant liver) | YAP (non-malignant liver) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gradea | gDNAb | mRNAc | Staind | Grade | gDNA | mRNA | Stain | |
| 1 | T3 | 0.67 | 4.07 ↑ | High ↑ | C4 | 0.73 | 0.52 | Low |
| 2 | T3 | 1.30 | 1.17 | Neg | C4 | 1.20 | 0.78 | Neg |
| T3 | 1.21 | 0.48 | Neg | |||||
| 3 | T3 | 1.57 | 0.50 | Neg | C4 | 0.55 | 0.35 | Neg |
| T4 | 16.58 ↑ | 1.64 ↑ | Low ↑ | |||||
| 4 | T4 | 1.05 | 3.76 ↑ | High | C4 | 2.40 ↑ | 0.73 | High |
| 5 | T3 | 0.75 | 2.26 | Neg | C4 | 3.03 ↑ | 1.57 | Low ↑ |
| 6 | T3 | 0.99 | 3.07 ↑ | Low | C4 | 1.54 | 1.42 | Low |
| T1&T2 | 1.43 | 3.27 ↑ | Low | |||||
| 7 | T4 | 1.56 | 5.14 ↑ | Low | C4 | 1.68 | 1.85 | Low |
| 8 | T1&T2 | 1.65 | 9.25 ↑ | High ↑ | C1 | 1.32 | 0.23 | Low |
| 9 | T1 | 1.82 | 2.90 | Low ↑ | C2 | 0.74 | 3.90 | Neg |
| 10 | T1&T2 | 1.41 | 4.98 | High ↑ | C4 | 1.54 | 2.51 | Low |
| 11 | T3 | 1.52 | 10.36 ↑ | High ↑ | C4 | 1.45 | 1.97 | Neg |
| 12 | T3 | 2.72 ↑ | 2.46 | Neg | C4 | 1.66 | 3.04 | Neg |
| 13 | T4 | 7.83 ↑ | 4.67 | Neg | C1 | 1.50 | 7.49 | Neg |
| 14 | T1&T2 | 4.57 ↑ | 1.34 | Low ↑ | C1 | 1.78 | 1.07 | Neg |
Tumor and cirrhotic grade according to AJCC cancer staging Handbook 6th edition.
The genomic DNA level was determined using quantitative PCR.
The relative level of YAP mRNA was determined using quantitative RT-PCR.
The YAP stain level was determined as described in Materials and Methods.
Indicate a gene amplification in the genomic DNA level, or an increase of RNA or protein levels when compared to the adjacent tissues.
Nuclear YAP expression correlates with nuclear Survivin expression in hepatocellular carcinoma
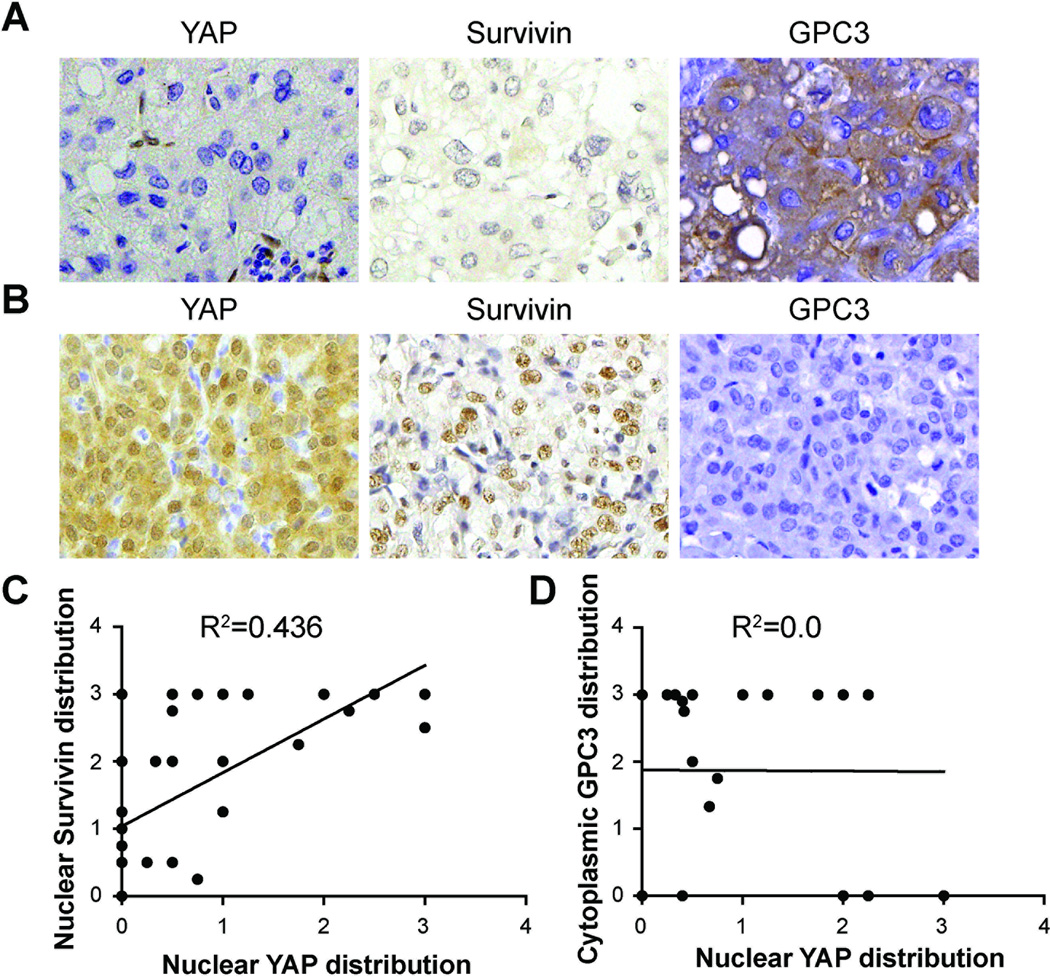
Survivin expression is also increased in HCC and predicts poor prognostic outcome (27, 28). Survivin expression in HCC specimens was evaluated with immunohistochemistry. Correlation analysis revealed a significant positive association between nuclear YAP and nuclear Survivin expression (n=35, R2=0.436, p<0.0001) (Figure 4). However, the expression of Glypican-3, another gene reported to be YAP regulated (4); a marker of poor prognosis; and a potential therapeutic target for HCC (29), does not correlate with YAP expression in HCC (n=25, R2=0, p=0.9809). These findings suggested that YAP may regulate Survivin expression in both HCC and ICC.
Figure 4. Nuclear YAP expression correlates with nuclear Survivin but not Glypican-3 expression in human hepatocellular carcinoma.
(A) Representative HCC tumor stained with YAP, Survivin and Glypican-3 (GPC3). Note the lack of nuclear staining for both YAP and Survivin, and the strong cytoplasmic signal for GPC3. (B) Representative HCC tumor stained with YAP, Survivin and GPC3. Note the strong nuclear staining for both YAP and Survivin but negative signal for GPC3. (C) Correlation curve between nuclear YAP distribution and nuclear Survivin distribution. (n=35, Pearson test, R2=0.436, p < 0.0001). (D) Correlation curve between nuclear YAP distribution and cytoplasmic GPC3 distribution (n=25, Pearson’s test, R2=0, P =0.9809).
YAP regulates Survivin mRNA levels in moue livers
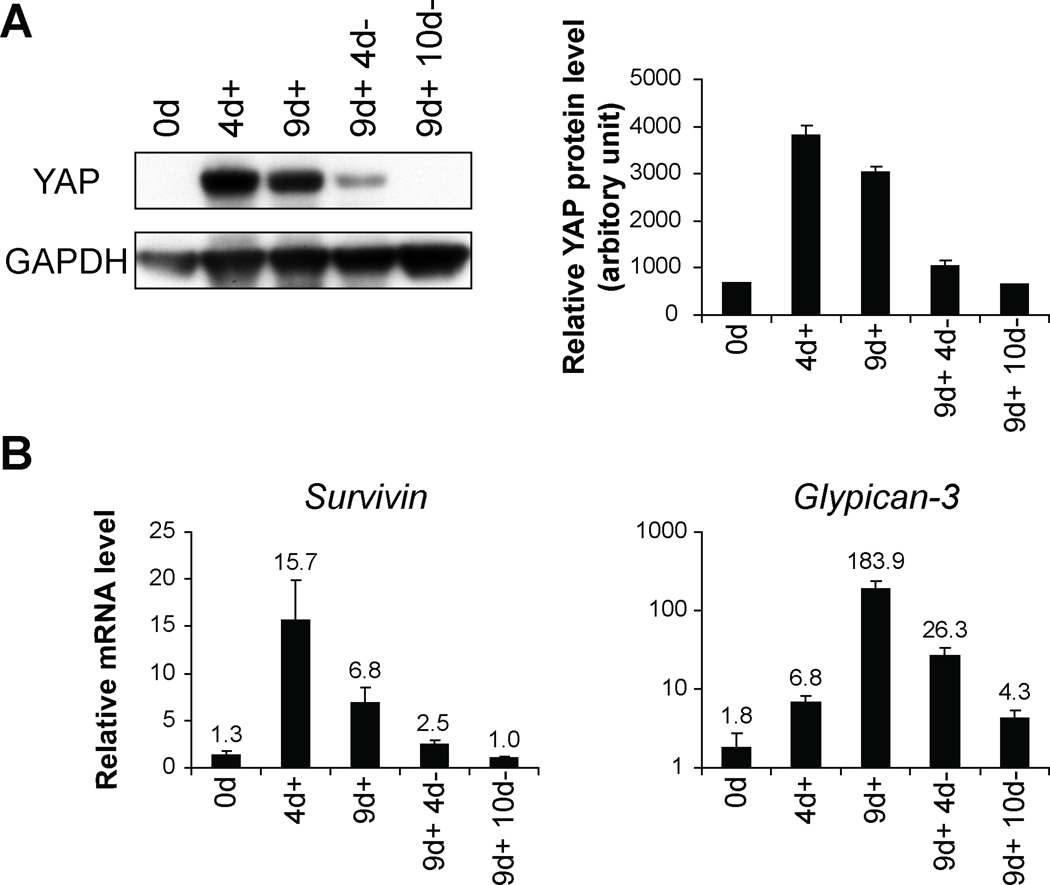
To confirm the relationship between YAP and Survivin expression in ICC and HCC tumors, we turned to a transgenic mouse model capable of inducing YAP in the mouse liver (4). We exposed the ApoE/rtTA-YAP mice to doxycyline (Dox), which leads to YAP expression in the mouse liver, for up to 9 days followed by withdrawal of Dox. The mouse livers were harvested 4 (4d+) and 9 (9d+) days after Dox exposure. Another two groups were exposed to Dox for 9 days and then withdrawn from Dox for an additional 4 (9d+ 4d−) or 10 days (9d+ 10d−). The YAP protein levels at specific time points in the liver are shown in Figure 5A. The YAP protein levels peaked after 4 days of Dox treatment even though one group was treated for 9 days. After withdrawing Dox, YAP protein levels fell quickly. Survivin and Glypican-3 mRNA levels measured at the same time points showed that Survivin mRNA levels strongly matched YAP protein levels while Glypican-3 mRNA levels showed some delay compared to YAP protein levels (Figure 5B). These results support a role for YAP in regulating Survivin expression in the liver.
Figure 5. YAP regulates Survivin mRNA in the transgenic mouse liver.
(A) Western blotting analysis. Protein extracts from livers of YAP transgenic mice (ApoE/rtTA-YAP) without doxycycline (Dox) (0d), Dox treatment for 4 (4d+) and 9 days (9d+), Dox treatment for 9 days followed by withdrawal of Dox for 4 (9d+4d−) and 10 days (9d+10d−) were probed with the indicated antibodies. The YAP protein levels were quantified in the graphs to the right. (B) Real-time PCR analysis. mRNA from livers of YAP transgenic mice (ApoE/rtTA-YAP) exposing to Dox at above time periods in (A) were probed with Survivin and Glypican-3. Data are means ± SEM, n=3–4.
Discussion
In this study, we showed that YAP could be found at all levels of the intrahepatic and extrahepatic biliary tract. We found nuclear (active) YAP expression was significantly elevated in BECs of ICC tumors compared to BECs in normal interlobular bile ducts. YAP activity is regulated by the Hippo signaling pathway, a tumor suppressor pathway. Conditional deletion of the Hippo signaling pathway tumor suppressors Nf2 or Sav1 in mouse livers increased YAP activity levels (6, 8) and resulted in aberrant proliferation of BECs which can lead to ICC (6, 8, 30, 31). In contrast, Yap deficiency in mouse livers compromised bile duct development, leading to bile duct paucity in the adult phase (8). Furthermore, deletion of Yap is able to suppress the BEC over-proliferation in Nf2-deficient livers (8). Taking these studies together with our current observations, elevated YAP activity may contribute to the malignant transformation of BECs in ICC tumors. Thus YAP may be a promising therapeutic target in this devastating cancer.
Our findings further support YAP’s potential role in the development of HCC (4, 10, 32). Specific deletion of the Hippo signaling pathway tumor suppressors Mst1&Mst2 increased activity of YAP and resulted in the development of HCC (7, 9, 31). We have previously shown that Yap-deficient primary hepatocytes have much lower viability compared to wild type primary hepatocytes when cultured in vitro (8). The elevated nuclear active form of YAP in some HCC tumors could explain enhanced hepatocyte proliferation and survival in these patients. This study and previous reports showed that increased nuclear YAP protein is present in about 50% of human HCCs (10). However, the amplification of the Yap locus appears to be relatively uncommon, restricted to about 5% to 10% of tumors, which prompted us to define the mechanisms of YAP overexpression in HCC. Through measuring DNA, RNA and protein levels of YAP in HCC tumors, we found that the YAP protein upregulation may due to gene amplification, mRNA upregulation and post-transcription mechanisms. However, gene amplification and mRNA upregulation do not necessarily result in increased YAP protein levels. Therefore, the levels of YAP protein may be controlled by multiple mechanisms.
Our data showed that YAP and Survivin levels significantly correlate in both ICC and HCC patients and mice. Since Survivin is a known prognostic marker (25), our observation provides the first clinically relevant target that is regulated by YAP. Survivin is a member of the inhibitor of apoptosis protein (IAP) family which inhibits caspase activity and cell death in response to apoptotic stimuli. Survivin is primarily found in developmental and cancerous tissues, but not in normal, terminally differentiated tissues (33). The molecular function of Survivin is to inhibit apoptosis while at the same time promote cell division by interfering with Aurora-B kinase (34), exhibiting similar cellular roles of YAP (3). Since the expression of both YAP and Survivin increased in a wide spectrum of human cancers (3, 35), it is particular interest to determine if YAP correlates with Survivin in non-hepatic cancer types. Several direct and indirect Survivin inhibitors have undergone investigations and clinical trials (35) and these inhibitors may be useful in targeting YAP-overexpressed hepatic cancer cells.
Although Glypican-3 mRNA levels do not perfectly match YAP protein levels in the ApoE/rtTA-YAP mice, Glypican-3 mRNA levels were indeed elevated significantly after transgenic YAP induction, so we are not able to exclude Glypican-3 from the list of YAP target genes. However, our correlation studies indicate there is no positive correlation between nuclear YAP expression and Glypican-3 expression in 25 HCC tumors. There are several possibilities for this contradiction. First, Glypican-3 expression can be regulated by multiple mechanisms during HCC formation; or secondly, YAP may regulate Glypican-3 expression in special subsets of HCC or during early stages of tumor formation. Although Gypican-3 is an important diagnostic and prognostic marker and potential therapeutic target of HCC, the mechanisms of its regulation in HCC have not been illustrated. Further investigation on relationship of YAP and Glypican-3 is needed to explore the regulatory mechanism for Glypican-3.
Acknowledgement
Grant support: This study was combined supported by grants from National Institute of Health R01DK080736 (R.A.A.); R01DK081417 (R.A.A.); Michael Rolfe Foundation for Pancreatic Cancer Research (R.A.A.); Biliary Cancer Research Fund (P. A.); National Natural Science Foundation of China (No.81030038, 81071661); Shanghai Rising-Star Follow-up Program Funds (No. 10QH1400500); National Key Sci-Tech Special Project of Infectious Diseases (No.2008ZX10002-022), D.P. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594–603. doi: 10.1016/j.jamcollsurg.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, George J, Deb S, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Dong Q, Zhang Q, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orr BA, Bai H, Odia Y, et al. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashyam MD, Bair R, Kim YH, et al. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005;7:556–562. doi: 10.1593/neo.04586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Z, Zhu WG, Morrison CD, et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet. 2003;12:791–801. doi: 10.1093/hmg/ddg083. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez L, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imoto I, Yang ZQ, Pimkhaokham A, et al. Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 2001;61:6629–6634. [PubMed] [Google Scholar]
- 19.Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of, YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snijders AM, Schmidt BL, Fridlyand J, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 24:4232–4242. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 21.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Ji JY, Yu M, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu MZ, Chan SW, Liu AM, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javle MM, Tan D, Yu J, et al. Nuclear survivin expression predicts poor outcome in cholangiocarcinoma. Hepatogastroenterology. 2004;51:1653–1657. [PubMed] [Google Scholar]
- 26.Obama K, Ura K, Li M, et al. Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma. Hepatology. 2005;41:1339–1348. doi: 10.1002/hep.20718. [DOI] [PubMed] [Google Scholar]
- 27.Fields AC, Cotsonis G, Sexton D, et al. Survivin expression in hepatocellular carcinoma: correlation with proliferation, prognostic parameters, and outcome. Mod Pathol. 2004;17:1378–1385. doi: 10.1038/modpathol.3800203. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Shiraki K, Sugimoto K, et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 29.Zou ZQ, Ding YP, Long B, et al. Gpc-3 is a notable diagnostic, prognostic and a latent targeted therapy marker in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:1285–1290. [PubMed] [Google Scholar]
- 30.Benhamouche S, Curto M, Saotome I, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Wolfe A, Septer S, et al. Deregulation of Hippo kinase signalling in Human hepatic malignancies. Liver Int. 2011 doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 34.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Cheung CH, Cheng L, Chang KY, et al. Investigations of survivin: the past, present and future. Front Biosci. 2011;16:952–961. doi: 10.2741/3728. [DOI] [PubMed] [Google Scholar]