Abstract
Rationale
Recent work in animal models and humans has demonstrated the presence of organ-specific progenitor cells required for the regenerative capacity of the adult heart. In response to tissue injury, progenitor cells differentiate into specialized cells, while their numbers are maintained through mechanisms of self-renewal. The molecular cues that dictate the self-renewal of adult progenitor cells in the heart, however, remain unclear.
Objective
Herein, we investigate the role of canonical Wnt signaling on adult cardiac side population (CSP) cells under physiological and disease conditions.
Methods and Results
CSP cells isolated from C57BL/6J mice were utilized to study the effects of canonical Wnt signaling on their proliferative capacity. The proliferative capacity of CSP cells was also tested following injection of recombinant Wnt3a protein (r-Wnt3a) in the left ventricular free wall. Wnt signaling was found to decrease the proliferation of adult CSP cells, both in vitro and in vivo, through suppression of cell cycle progression. Wnt stimulation exerted its anti-proliferative effects through a previously unappreciated activation of insulin-like growth factor binding protein 3 (IGFBP3), which requires intact IGF binding site for its action. Moreover, injection of r-Wnt3a following myocardial infarction in mice showed that Wnt signaling limits CSP cell renewal, blocks endogenous cardiac regeneration and impairs cardiac performance, highlighting the importance of progenitor cells in maintaining tissue function after injury.
Conclusions
Our study identifies canonical Wnt signaling and the novel downstream mediator, IGFBP3, as key regulators of adult cardiac progenitor self-renewal in physiological and pathological states.
Keywords: cardiac side population cells, Wnt signaling, cardiac regeneration, stem cells, proliferation
Introduction
Accumulating evidence over the past decade in both humans and animal models has documented the presence of endogenous progenitor cells in adult myocardium.1-6 In response to local tissue injury, cardiac progenitor cells differentiate into specialized cells, while the pool of progenitor cells is maintained, in part, through self-renewal and enhanced proliferation.7, 8 However, the molecular cues and signaling pathways that dictate the homeostasis of adult progenitor cells and in particular their self-renewal, in physiological and pathological states, remain unclear.
Wnt ligands constitute a family of 19 secreted glycoproteins that act as key regulators of cellular function during development, adulthood and disease.9, 10 Several reports have proposed time and context dependent roles for Wnt signaling in cardiogenesis and progenitor cell biology.11 Studies in chick and Xenopus embryos have demonstrated that inhibition of Wnt signaling is required for cardiac differentiation,12, 13 whereas, Wnt signaling has also been found to promote cardiomyogenesis in Drosophila and embryonic carcinoma P19 cells.14, 15 Moreover, early in gastrulation, Wnt ligands activate a cardiac differentiation program, whereas at later stages they act as potent inhibitors of the cardiomyogenic differentiation.16, 17 Recent genetic studies have demonstrated that the canonical Wnt signaling cascade promotes the proliferation of neonatal and embryonic Isl-1+ cardiac progenitors in vitro and in vivo.18-20 Little is known, however, regarding the role of Wnt signals in modulating adult progenitor cell populations.
Side population (SP) cells were initially identified based on their unique ability to efflux the DNA binding dye Hoechst 33342,21 and were found to retain the long term regenerative potential of bone marrow. Subsequently, SP cells were identified in various adult tissues/organs including adult myocardium.22 Cardiac SP (CSP) cells are enriched in Sca1 but do not express c-kit or Isl-1.6 More importantly, CSP cells are found to be capable of differentiation into functional cardiac myocytes in vitro or in vivo following myocardial infarction (MI).6, 23-25 Herein, we demonstrate that in contrast to previous work in embryonic or early post-natal stem cells, Wnt-signaling negatively regulates the proliferation of adult CSP progenitor cells, both in vitro and in vivo, through suppression of cell cycle progression. Moreover, Wnt activation exerts its anti-proliferative effects through a previously unappreciated activation of IGFBP3, which is found to require intact IGF binding site for its action. Importantly, activation of Wnt signaling was found to limit CSP cell renewal post-MI, impair endogenous regenerative capacity and worsen post-MI structural and functional remodeling. Overall, our study represents the first investigation of the role of canonical Wnt pathway in the homeostasis of adult cardiac progenitor cells in both physiological and pathological states and highlights the importance of cardiac progenitor cells in maintaining tissue function after injury.
Methods
An expanded Methods section describing all procedures and protocols is available in the Online Data Supplement at http://circres.ahajournals.org.
Animals
C57BL/6J male and female mice (8-12 weeks old) were purchased from Jackson Laboratory (Jackson East, MP 15). All animal procedures and handling were performed under the guidelines of Harvard Medical School, the Longwood Medical Area Institutional Animal Care and Use Committee (IACUC), and the National Society for Medical Research. MI was generated in C57BL/6J female mice as previously described.26 MI size was estimated using histologic imaging of three transverse myocardial sections (base, mid-papillary and apex) following staining with Masson's trichrome, as previously described.27
Intra-myocardial injection of recombinant Wnt3a (r-Wnt3a) or IGFBP3 (r-IGFBP3)
10 μl of r-Wnt3a (400 ng) or r-IGFBP3 proteins (1000 ng) (R&D) or Vehicle (PBS) were injected intra-myocardially into three sites of the left ventricular free-wall of non-surgically operated female mice (r-Wnt3a) or into the infarct/border zone area of mice immediately following myocardial infarction (r-Wnt3a or r-IGFBP3).
Heart fixation and histology
One day following echocardiographic measurements, animals were sacrificed and hearts were fixed at an end-diastolic pressure of 5 mmHg using a Langendorff apparatus. For details, see Methods in the online Data Supplement.
Echocardiography
Echocardiography was performed 1 day prior to MI and 7 days following MI using a hi-resolution, hi-frequency digital imaging system (Vevo 2100, VisualSonics), as previously described.28 For details, see Methods in the online Data Supplement.
FACS analysis
FACS was performed using a FACSAria (Becton Dickinson, BD) equipped with three lasers (488-nm, 633-nm, 355-nm). Hoechst 33342 dye was excited by an UV (355-nm) laser. Acquired data were analyzed by FACSDIVA software (BD Biosciences).
RNA isolation and RT-PCR
RNA was extracted from CSP cells using Trizol reagent (Invitrogen) followed by RNeasy Mini Kit (Qiagen). Genomic DNA was removed using Turbo-DNA free kit (Ambion). cDNA was generated using a reverse transcription synthesis kit (Bio-Rad) and RT-PCR was performed in a MyiQ cycler (Bio-Rad). Primer sequences are available on request.
Luciferase assays
Luciferase activity was measured using a dual luciferase kit (Promega). Cell lysates were prepared from trypsinized CSP cells and used to determine the values of Firefly and Renilla luciferases, with a 20/20n Luminometer (Turner Biosystems).
Statistical analysis
Statistical differences were evaluated using one-way ANOVA analysis and Student's unpaired t-test, using GraphPad Prism (Version 5.03). Data are presented as mean ± s.e.m. A p-value ≤ 0.05 was considered statistically significant.
Results
Wnt3a negatively regulates the growth potential of CSP cells both in vitro and in vivo
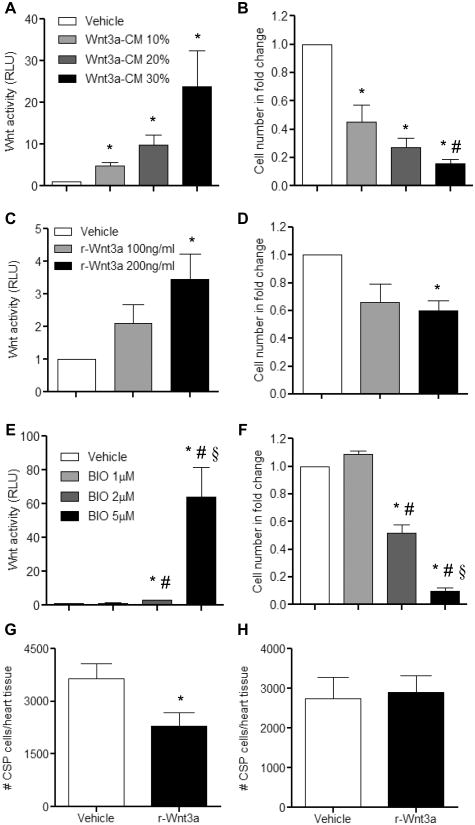
Given the context dependency of Wnt signaling, we examined the role of Wnt signals in mediating the proliferative capacity of adult CSP cells. Under unstimulated conditions, application of recombinant SFRP2 (Soluble Frizzled-related Protein 2), a known Wnt antagonist29, did not affect the proliferation capacity of CSP cells in vitro suggesting that Wnt activity in baseline CSP cells in vitro is relatively low. (Online Figure I). Treatment of CSP cells with increasing doses of Wnt3a-conditioned medium (Wnt3a-CM) or Vehicle-conditioned medium (Vehicle) resulted in a gradual activation of the canonical Wnt signaling pathway, as assessed by a TCF-controlled luciferase reporter assay30 and a respective decrease in CSP cell number (Figure 1A-B). A similar decrease in cell proliferation in response to activation of canonical Wnt signaling was also observed in CSP cells treated with recombinant Wnt3a protein (r-Wnt3a) (Figure 1C-D). Likewise, BIO ((2′Z, 3′E)-6-Bromoindirubin-3′-oxime), a GSK-3 inhibitor, caused a comparable decline in CSP cell number with corresponding activation of the Wnt pathway (Figure 1E-F). We further examined the anti-proliferative effects of Wnt signaling on CSP cells in vivo, with direct intra-myocardial injection of r-Wnt3a protein into the LV free wall of normal adult mouse hearts. Injection of r-Wnt3a substantially reduced the number of CSP cells (Figure 1G) in the injected area relative to Vehicle, whereas the number of CSP cells remote to the injection site (atria, septum and right ventricle) remained unchanged (Figure 1H).
Figure 1. Canonical Wnt signaling pathway mediates anti-growth effects on CSP cells.
Treatment of CSP cells with increasing concentrations of (A) Wnt3a-CM (n=3), (C) r-Wnt3a (n=3), and (E) BIO (n=6) activated the canonical Wnt signaling pathway, as measured by the TCF-luciferase reporter assay. Corresponding proliferation assays using the same dosages of (B) Wnt3a-CM (n=4), (D) r-Wnt3a (n=4) and (F) BIO (n=6) revealed decreased growth capacity of CSP cells. TCF-luciferase activity is measured in relative light units (RLU). Analysis of CSP cell number in (G) the Vehicle or r-Wnt3a injected areas (left ventricular free wall) (Vehicle, n=14; r-Wnt3a, n=16) and in (H) the remote areas from the injection sites (atria, septum, right ventricle) (Vehicle, n=12; r-Wnt3a, n=13). Data are mean ± s.e.m. * P≤0.05 all samples to respective Vehicle, # P≤0.05 Wnt 10% to Wnt 30%, BIO 1μM to BIO 2μM and BIO 5μM, § P≤0.05 BIO 2μM to BIO 5μM. Cell number in fold change presented in B, D, and F is normalized to respective Vehicle group.
Wnt3a directly alters the cell cycle progression of adult CSP cells
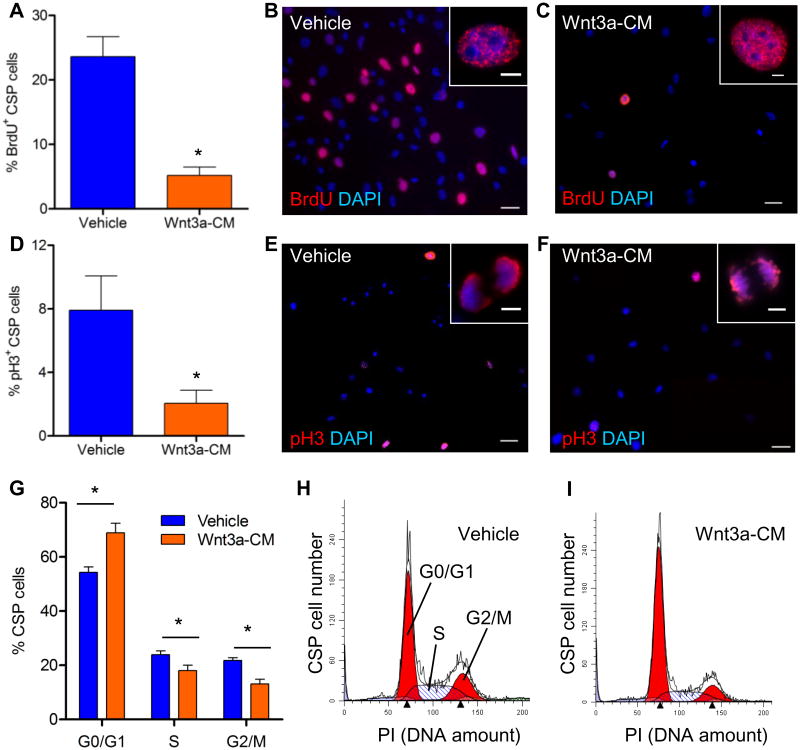
To further investigate the anti-proliferative effect of canonical Wnt signaling on CSP cells, we examined the effect of Wnt3a on cell cycle progression in CSP cells. Treatment of CSP cells with Wnt3a-CM led to a drastic reduction of cells residing in the cell cycle S phase, as evidenced by decreased BrdU incorporation (Figures 2A-C). Moreover, activation of canonical Wnt signaling decreased the fraction of CSP cells residing in the cell cycle M phase, as shown by immunostaining for the phosphorylated form of histone H3 (p-H3) (Figures 2D-F). The altered cell cycle profile in Wnt3a-CM-treated CSP cells was further tested by direct measurement of DNA content with propidium iodide staining. As demonstrated in Figures 2G-I, Wnt3a-CM treatment considerably increased the amount of cells residing in G0/G1-cell cycle phases while decreasing the fraction of cells residing in S- and G2/M-cell cycle phases, in comparison to Vehicle treated CSP cells.
Figure 2. Activation of the canonical Wnt signaling pathway in CSP cells blocks cell cycle progression.
Immunocytochemical analysis of (A) BrdU incorporation (n=5) and (D) expression of p-H3 (n=4) in CSP cells, following treatment with Wnt3a-conditioned-medium (Wnt3a-CM) or Vehicle. Representative images of (B, C) BrdU+ and (E, F) p-H3+ CSP cells, in low (scale bars, 50μm) and high magnification (inset, scale bars, 5μm). (G) Flow cytometric analysis of CSP cells stained with propidium iodide (PI) (n=4). Representative examples of PI analysis following treatment with (H) Vehicle or (I) Wnt3a-CM. Data are mean ± s.e.m. * P≤0.05.
To further clarify the role of Wnt signaling in the regulation of CSP cell cycle progression, we examined the expression pattern of known cell cycle regulator genes. Consistent with the impaired proliferative capacity, treatment of CSP cells with Wnt3a-CM, in comparison to Vehicle, down-regulated the expression of several positive cell-cycle regulators including Ki67, PCNA and Brca2 while up-regulating negative cell-cycle regulators such as Gadd45a and p16 (Cdkn2a), within 8 hours (Online Table I). Notably, this gene expression pattern persisted throughout later time points, and was accompanied by further down-regulation of additional cell cycle regulators, including c-myb, Ccnf, Ccnb1, Cdk4, Mcm3, Mcm2, Cdc25a, Brca1 and Wee1 (Online Table I). These findings suggest that canonical Wnt stimulation impairs the proliferation of adult cardiac progenitor cells potentially through modulation of cell-cycle regulators.
IGFBP3 is up-regulated in response to activated Wnt signaling in CSP cells
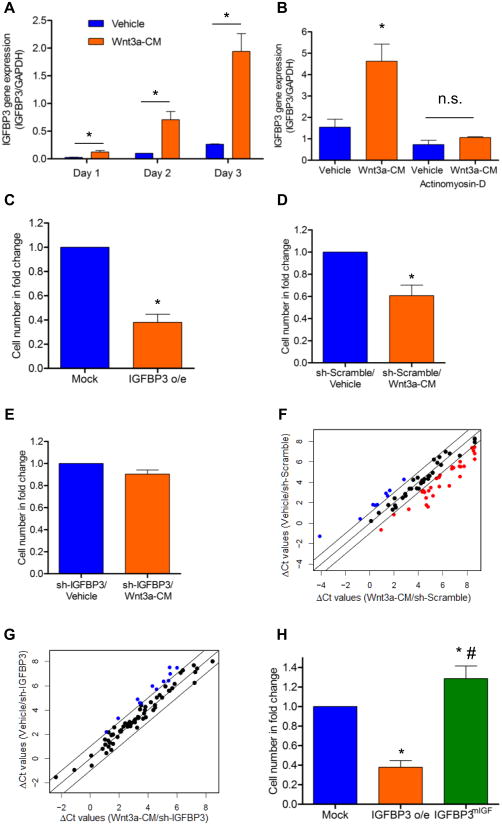
Using a RT-PCR-based array to monitor several key intracellular signaling pathways, we sought to identify possible mediators for the anti-proliferative action of Wnt signaling. Several genes previously identified to be associated with Wnt signaling activation (Wnt2, Tcf7, Lef1) and with cell cycle regulation (p16) were found to be significantly up-regulated in Wnt3a-CM treated CSP cells in comparison to Vehicle treated cells (Online Figure IIA and Online Table II). Interestingly, among those genes examined, IGFBP3 exhibited the most robust up-regulation with Wnt activation, increasing more than 40-fold (Online Figure IIA). Furthermore, IGFBP3 gene expression was up-regulated by Wnt3a-CM in CSP cells in a time-dependent manner (Figure 3A). Consistent to the increased gene expression, IGFBP3 protein levels were found increased in CSP cells treated with Wnt3a-CM (Online Figure IIB). To examine whether increased IGFBP3 was derived from de novo transcription, stabilization of IGFBP3 mRNA, or by alternative mechanisms, CSP cells were treated with actinomyosin-D, an inhibitor of de novo transcription. Actinomyosin-D abolished Wnt-mediated up-regulation of IGFBP3, suggesting that the IGFBP3 induction following Wnt3a treatment was mediated predominantly by transcriptional mechanisms (Figure 3B). These experiments identify IGFBP3 as a potential mediator of Wnt3a signaling in adult cardiac progenitor cells.
Figure 3. IGFBP3 mediates the anti-proliferative effects of the Wnt signaling pathway on CSP cells.
(A) Time course gene expression analysis of IGFBP3 in CSP cells following treatment with Vehicle or Wnt3a-CM (n=3). (B) IGFBP3 gene expression analysis in CSP cells following pre-treatment with Actinomyosin-D (30 minutes) and subsequent application of Vehicle or Wnt3a-CM (24 hours) (n=3). (C) Proliferation assay of Mock and IGFBP3 (IGFBP3 o/e) infected CSP cells (n=5). Proliferation assay of (D) sh-Scramble and (E) sh-IGFBP3 infected CSP cells (n=4) following treatment with Vehicle or Wnt3a-CM. Scatter plot analysis of the ΔCt values of cell cycle related genes, in (F) sh-Scramble and (G) sh-IGFBP3 infected CSP cells, following treatment with Vehicle or Wnt3a-CM for 5 days. Outer diagonal lines indicate 2-fold changes. Black, blue and red circles represent unchanged, up-regulated and down-regulated genes following Wnt activation, respectively. (H) Proliferation assay of Mock, IGFBP3 o/e and IGFBP3mIGF infected CSP cells (n=3). Data are mean ± s.e.m. * P≤0.05 to respective Vehicle or Mock, # P≤0.05 IGFBP3mIGF to IGFBP3 o/e. Cell number in fold change presented in B, C, D and G was normalized to respective Vehicle or Mock.
IGFBP3 is sufficient to inhibit the proliferative capacity of CSP cells
To test whether the expression of IGFBP3 was sufficient to mimic the anti-proliferative effects of Wnt3a, we constructed a lentiviral-vector over-expressing IGFBP3 (IGFBP3 o/e) in CSP cells (Online Figure III). We found that the proliferative capacity of CSP cells over-expressing IGFBP3 was markedly decreased (Figure 3C). Consistent with this observation, over-expression of IGFBP3 resulted in a cell cycle gene expression pattern similar to that of canonical Wnt signaling, with approximately 80% of examined genes (66 genes out of a total of 84 genes tested) similarly regulated in both experimental conditions (Online Table III). Overall, our data suggest that IGFBP3 acts as a mediator of Wnt signaling that is sufficient to recapitulate the phenotypic response to Wnt activation in CSP cells.
IGFBP3 is required for Wnt3a-mediated anti-proliferative effects in CSP cells
To determine whether IGFBP3 is required for the anti-proliferative effects of Wnt3a, we decreased IGFBP3 protein expression in CSP cells by utilizing lentiviral-mediated expression of a shRNA targeting IGFBP3 (sh-IGFBP3) (Online Figure IV) prior to Wnt activation. Scramble-infected (sh-Scramble) CSP cells treated with Wnt3a-CM exhibited decreased proliferation (Figure 3D), similar to uninfected cells. In contrast, silencing of IGFBP3 prevented Wnt mediated inhibition of CSP cell proliferation (Figure 3E). To further investigate the role of IGFBP3 in mediating the anti-proliferative effects of Wnt signaling at the gene level, we compared the expression profile of cell cycle-related genes in sh-Scramble and sh-IGFBP3 CSP cells following treatment with Vehicle or Wnt3a-CM. In sh-Scramble CSP cells, 40 of the 75 (53%) expressed cell-cycle related genes were altered (up- or down-regulated) by more than 2-fold following treatment with Wnt3a-CM (Figure 3F and Online Table IV), including up-regulation of cell-cycle inhibitors (Cdkn2a and Cdkn1a) and down-regulation of positive mediators of cell-cycle progression (Ccna2, Ccnb1, E2f3, Ki67, PCNA, Mcm2, Mcm3, Mcm4 and Wee1). In sh-IGFBP3 CSP cells only 12 out of the 75 examined cell cycle regulators (16%) were altered by more than 2-fold following treatment with Wnt3a-CM (Figure 3G and Online Table IV). In sh-IGFBP3 infected CSP cells, Wnt signaling did not result in altered expression of negative cell cycle regulators and even up-regulated the expression of several positive regulators (Ccna2, Ccnb1, Wee1). Overall, our data suggest that silencing of IGFBP3 results in normalization of cell cycle gene expression in response to Wnt signaling activation and that IGFBP3 is necessary for the anti-proliferative effects of Wnt3a activation in adult CSP cells.
Integrity of IGF-binding site is required for the anti-proliferative effect of IGFBP3 on CSP cells
IGFBP3 is an abundant circulating IGF-binding protein and acts as a modulator of cell survival, cell proliferation and cell metabolism via IGF-dependent or IGF-independent mechanisms.31 IGFBP3 is known to bind IGFs both in vitro and in vivo.31 IGF-1 is a potent stimulant of several cell types including adult cardiac stem cells.32 We found that IGF-1 stimulates CSP cell proliferation in vitro (Online Figure VA). However, the expression of IGF-1 receptors did not change following Wnt3a treatment (Online Figure VB-C). To determine whether IGF binding to IGFBP3 is required for the anti-proliferative effect of IGFBP3, we over-expressed wild type (WT) IGFBP3 or mutated IGFBP3 with loss of the IGF binding site (IGFBP3mIGF) in CSP cells.33 Over-expression of WT IGFBP3 decreased cell proliferation while over-expression of IGFBP3mIGF increased cell division (Figure 3H), suggesting that the IGF binding site is critical for the anti-proliferative function of IGFBP3 in CSP cells.
Administration of r-Wnt3a protein post-MI diminishes the pool of endogenous cardiac progenitors
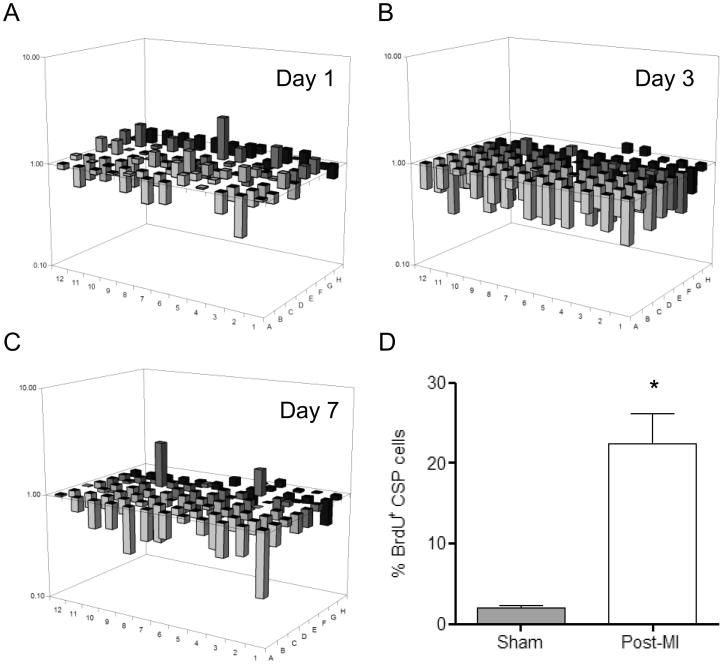
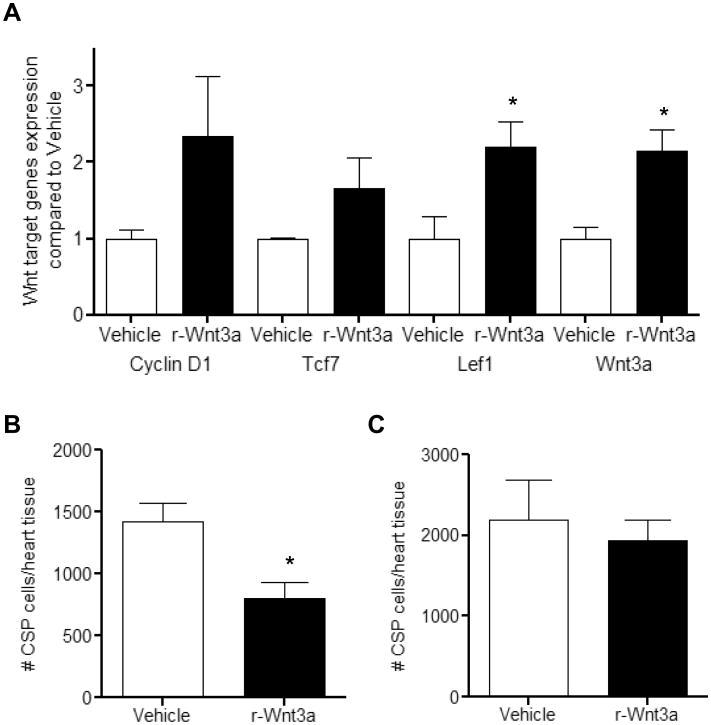
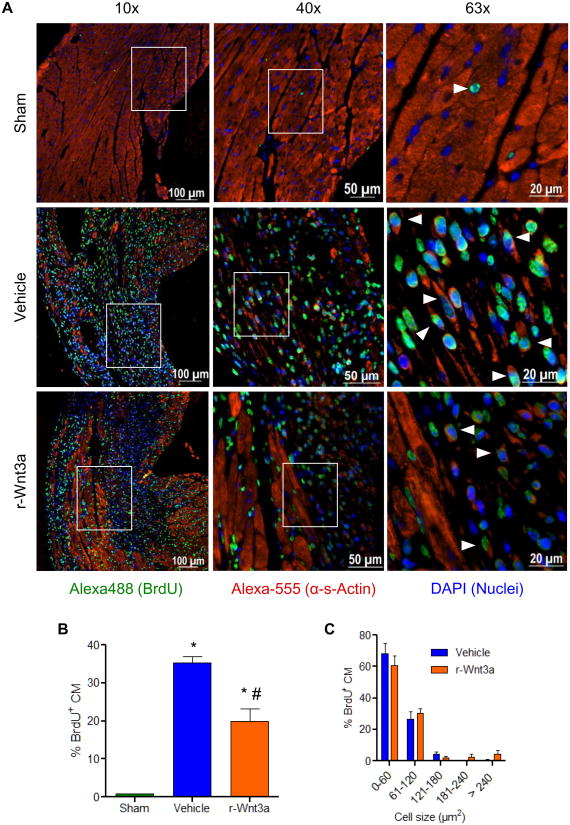
Following cardiac injury, CSP cell pools are acutely depleted and later renewed, in part, through enhanced proliferation of local CSP cells.26 Given the context and cell type dependency of Wnt activation in response to cardiac injury, we measured Wnt related genes in CSP cells isolated from the myocardium 1, 3, and 7 days following coronary ligation. Using a Wnt pathway focused gene array, we established that Wnt related genes were down-regulated in CSP cells early post-MI and continued to decline over 7 days. Conversely, a corresponding increase in Wnt pathway inhibitor genes was detected (Figure 4A-C and Online Tables V-VIII). During this early post-MI period, CSP proliferation increased, as shown by BrdU labeling (Figure 4D). To determine whether activation of Wnt signaling may inhibit the growth capacity of CSP cells following tissue injury, r-Wnt3a was injected into the infarct and border zone of mouse hearts following MI. Injection of r-Wnt3a post-MI up-regulated Wnt target genes, including Ccnd1, Tcf7, Lef-1 and Wnt3a (Figure 5A), confirming the activation of canonical Wnt in vivo. Administration of r-Wnt3a resulted in a significant reduction in CSP cell number in the region of injection (Figure 5B), but did not alter CSP cell number in areas remote to the injection site (atria, right ventricle and septum) relative to Vehicle treated animals (Figure 5C). To further determine whether the Wnt-mediated decrease in CSP pools alters myocardial regeneration post-MI, we determined the formation of new cardiomyocytes by pulsing BrdU, a thymidine analog, via Alzet mini-osmotic pumps for a period of one week. BrdU positive cardiomyocytes were found to reside primarily in the infarct and border zone areas of the injured myocardium (Figure 6A) and were significantly decreased after r-Wnt3a administration compared to Vehicle injected counterparts (Figure 6B), suggesting an impairment in new cardiomyocyte generation. Notably, the majority of BrdU positive cardiomyocytes were smaller in size in both r-Wnt3a and Vehicle injected hearts as compared to non-dividing, BrdU negative cardiomyocytes (Figure 6C). A representative cluster of BrdU positive cardiomyocytes located in the infarct/border zone area is presented in Online Figure VI. Our data show that activation of Wnt signaling pathway diminishes the endogenous repair mechanisms of the heart following MI.
Figure 4. Wnt signaling pathway expression profile in CSP cells post-MI.
3-D diagrams show expression profile of Wnt-signaling related genes in CSP cells at (A) 1 day post-MI, (B) 3 days post-MI and (C) 7 days post-MI (n=3). The gene corresponding to each bar can be found in Online Table VIII. The position (well) A1 in (A-C) is located in the bottom right corner of each diagram. (D) Analysis of CSP cell proliferation (BrdU incorporation assay) at 4 days following MI (n=3). Data are mean ± s.e.m. * P≤0.05.
Figure 5. Administration of r-Wnt3a protein decreases the amount of CSP cells following MI.
(A) Gene expression analysis of Wnt target genes (Cyclin-D1, Tcf7, Lef1 and Wnt3a) in the infarct/border zone area (injection site) following intra-myocardial injection of r-Wnt3a protein (n=3). Analysis of CSP cell number 3 days following MI, in (B) the infarct/border zone area (injection site) and (C) the remote areas (atria, septum, right ventricle) of Vehicle or r-Wnt3a injected hearts (Vehicle, n=10 and r-Wnt3a, n=9). Data are mean ± s.e.m. * P≤0.05.
Figure 6. Injection of r-Wnt3a following MI inhibits the formation of new cardiomyocytes (CM).
(A) Representative fluorescent microscope images of BrdU+ CM located in the infarct/border zone area 1 week following myocardial infarction, presented in low (10×), medium (40×) and high (63×) magnification power. White arrowheads, shown in 63× magnification, indicate BrdU+ CM. (B) Quantification of BrdU incorporation in CM at 1 week post-MI in Sham (n=3), Vehicle (n=5) and r-Wnt3a (n=4) injected hearts. (C) Cell size distribution frequency of BrdU+ CM in the infarct/border zone area of Vehicle (n=3) and r-Wnt3a injected (n=4) animals. Data are mean ± s.e.m. * P≤0.05 all samples to Sham, # P≤0.05 r-Wnt3a to Vehicle.
Administration of Wnt or IGFBP3 adversely affects post-MI cardiac remodeling
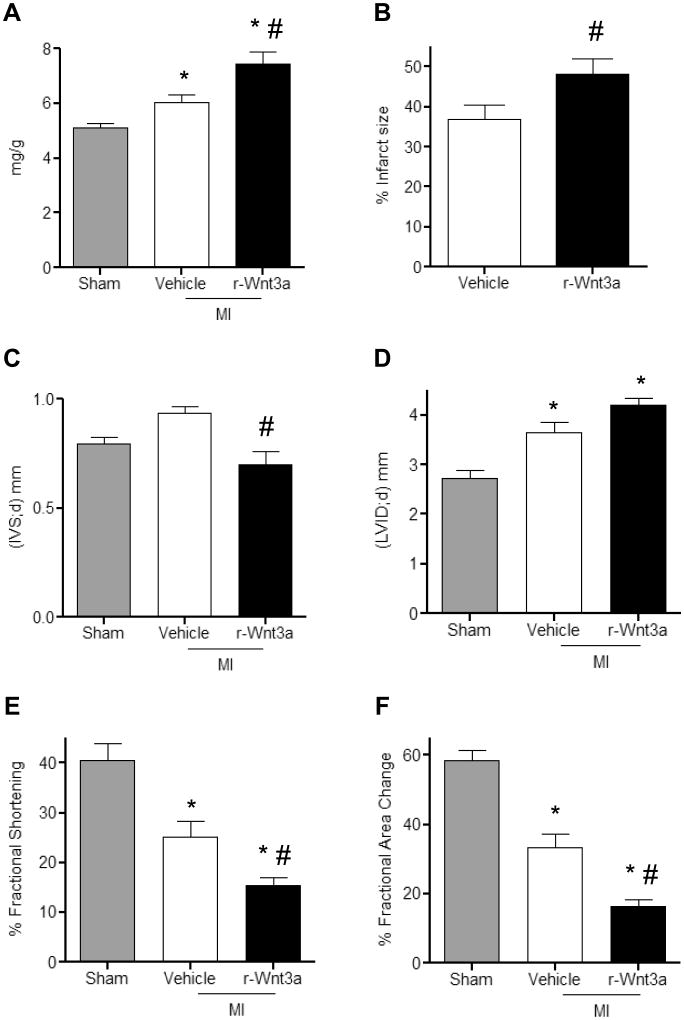
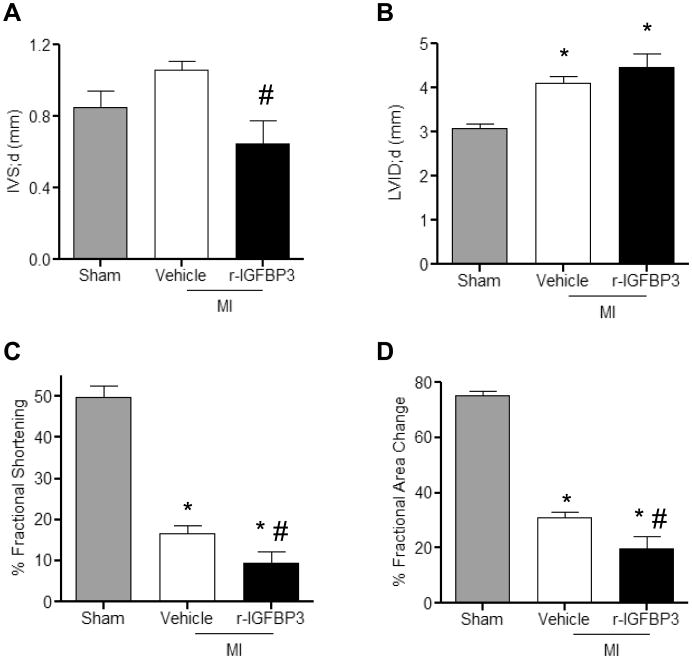
We further determined whether diminished CSP pools and myocardial regeneration post-MI influence the development of adverse cardiac remodeling. One week post-MI HW/BW (Figure 7A) and infarct size (Figure 7B) were significantly increased with r-Wnt3a injection. Moreover, injection of r-Wnt3a protein and impaired myocardial regeneration were associated with decreased intra-ventricular septal (IVS) wall thickness, increased left ventricular chamber dimension (LVID) and impaired cardiac performance, marked by reduced ratio of fractional shortening (FS) and fractional area change (FAC), compared to Vehicle injected mice (Figure 7C-F). Taken together, our data suggest that activation of Wnt signaling worsens the post-MI structural and functional remodeling. Similarly, administration of r-IGFBP3 into the infarct/border zone area acutely after coronary artery occlusion resulted in a decrease in IVS thickness and an increase in LVID, as well as a corresponding reduction in LV FS and FAC (Figure 8A-D). Collectively, these data suggest that IGFBP3 mimics the effects of r-Wnt3a on cardiac function in the presence of ischemic myocardial injury.
Figure 7. Administration of r-Wnt3a following MI leads to impaired cardiac performance.
(A) Quantification of heart weight to body weight ratios in Sham (n=8), Vehicle (n=12) and r-Wnt3a injected animals (n=12). (B) Measurement of infarct size in Vehicle and r-Wnt3a injected hearts (n=8). Echocardiographic assessment of (C) intra-ventricular septal thickness in diastole (IVS;d), (D) left ventricular internal dimension in diastole (LVID;d), (E) fractional shortening and (F) fractional area change (Sham, n=7; Vehicle, n=9; r-Wnt3a, n=10). All measurements were performed at 1 week post-MI. Data are mean ± s.e.m. * P≤0.05 all samples to Sham, # P≤0.05 r-Wnt3a to Vehicle.
Figure 8. Administration of r-IGFBP3 after MI compromises cardiac function.
Echocardiographic assessment of (A) intra-ventricular septal thickness in diastole (IVS;d), (B) left ventricular internal dimension in diastole (LVID;d), (C) fractional shortening and (D) fractional area change (Sham, n=3; Vehicle, n=6; r-IGFBP3, n=6). All measurements were performed at 1 week post-MI. Data are mean ± s.e.m. * P≤0.05 all samples to Sham, # P≤0.05 r-IGFBP3 to Vehicle.
Discussion
Detailed knowledge of the molecular cues that regulate progenitor cell fate decisions in physiological and pathological states is of the utmost importance for achieving the overarching goal of therapeutic cardiac regeneration. Wnt signaling is a pivotal factor in the regulation of organogenesis from embryonic development to aging, as well as in various disease conditions.9, 10 While Wnt signaling is undoubtedly established to be critical for cardiogenesis during cardiac development11, its role in adult cardiac progenitor cells and post-MI remodeling remains poorly understood. In this study, we define the role of canonical Wnt signaling in regulating the function of adult cardiac progenitor cells in vitro and in vivo. Furthermore, we identify a previously unknown link between canonical Wnt signaling and IGFBP3 and demonstrate an important role for IGFBP3 in mediating Wnt signaling effects in adult cardiac progenitor cells. Lastly, our study emphasizes the functional significance of adult cardiac progenitor cells in tissue regeneration following cardiac injury such as MI.
The anti-proliferative effects of Wnt signaling on CSP cells appear to be mediated through its negative effects on the cell cycle progression. Activation of Wnt signaling leads to accumulation of CSP cells in the early non-proliferating G0/G1 cell-cycle phases, while altering substantially the expression profile of various cell cycle regulators. In contrast, prior reports suggest that Wnt signaling potentiates the expansion of embryonic and neonatal Isl-1+ cardiac progenitor cells.18-20 The distinct response of CSP cells to Wnt signals in adult vs embryonic progenitor cells is likely due to intrinsic differences of each cell type and to the highly time and context dependent nature of Wnt signaling.11, 17 The milieu-dependent role of Wnt signaling is also supported by recent evidence in the cancer biology field. Although Wnt signaling is associated with oncogenic transformation, it has recently been suggested that activation of Wnt signaling may decrease proliferation in melanoma cancerous cells.34
Further, we demonstrated that IGFBP3 is transcriptionally up-regulated by Wnt signaling and that it is the critical determinant of the anti-proliferative effects of Wnt on CSP cells. Importantly, this phenomenon depends on a functional IGF binding site in IGFBP3. Analysis of the IGFBP3 gene promoter sequence has revealed a number of conserved transcriptional factor binding sites for factors such as REPIN1, MYOD1, and NFATC.35-37 ChIP-chip and ChIP-seq experiments have recognized a number of other transcriptional factors that may bind to the promoter region of IGFBP3.38 Recent reports suggest IGFBP5 is a potential target of Wnt-mediated transcriptional activity.39, 40 Among the members of the IGFBP family, IGFBP3 has a high structural and functional resemblance to IGFBP541, 42, raising the possibility that IGFBP3 is up-regulated in CSP cells through a direct Wnt-mediated transcriptional mechanism.
The role of IGFBP proteins in the adult myocardium is largely unknown. The insulin-like growth factor axis (ratio of IGF-1 to IGFBP3) has been introduced as a predictor of clinical outcomes in heart failure patients.43 Moreover, mice over-expressing IGFBP3 exhibited cardiac organomegaly.44 More recently, a direct link between cardiomyogenesis, IGFBP proteins and Wnt signaling was identified.45 IGFBP4 increases the cardiomyogenic differentiation of P19CL6 cells and embryonic stem cells through inhibition of canonical Wnt.45 However, IGFBP4 activates Wnt/β-catenin transcriptional activity in a renal cancer cell line.46 Thus, a context- and cell type- dependent specificity may define the interaction between IGFBP proteins and Wnt.
Activation of Wnt signaling decreases resident cardiac progenitor cell renewal and negatively affects the myocardial response to infarction. SFRP1 and SFRP2, two well-documented Wnt signaling inhibitors, exert a potent cardioprotective effect following ischemic myocardial injury.47-50 Similarly, depletion of β-catenin attenuates post-MI cardiac remodeling and improves animal survival by stimulating the resident cardiac stem cell pool.51 Wnt signaling has been reported to be enhanced within the infarct-border zone, but Wnt activation was limited to endothelial cells, smooth muscle cells, CD31+/Sca1+ cells, c-kit+/CD45+ cells and fibroblasts.52, 53 Results from the current study, however, indicate that Wnt pathway genes were decreased in CSP cells isolated from the infarcted heart, whereas Wnt inhibitors, such as SFRP2, increased. Consistent with this observation, we found that CSP cell proliferation was enhanced early following MI. Conversely, activation of Wnt signaling in CSP cells through the delivery of recombinant Wnt protein decreased the number of CSP cells. Based on in vitro data, it is reasonable to speculate that down-regulation of Wnt signaling in CSP cells may promote the proliferation of CSP cells after cardiac injury. In addition to the effects on cardiac progenitor cells, r-Wnt3a may influence other cell types, including cardiomyocytes. Indeed we found that r-Wnt3a leads to an increase in cardiomyocyte death (Online Figure VIIA), but no change is observed in cell death when all cardiac cells are considered (Online Figure VIIB). Importantly, recombinant Wnt3a results in an increase in heart weight to body weight ratio, although cardiomyocyte cross sectional area remains unchanged. These results do not exclude an increase in myocyte length, which may contribute to the expansion in cavitary volume (Online Figure VIII). While we cannot exclude that Wnt impacts on other cell types, our findings point to a potential interaction between impaired progenitor cell function and negative outcome after myocardial infarction. Importantly, future research is necessary to determine those cell types which underlie the in vivo effects of recombinant Wnt administration and whether targeting Wnt may be a viable therapeutic option.
In summary, our work reveals a novel role of Wnt signaling pathway in adult cardiac progenitor cells and shows that canonical Wnt ligands compromise the self-renewal properties of CSP cells in vitro and in vivo. This phenomenon, in turn, may lead to impaired cardiac recovery after ischemic injury. Furthermore, we have identified a previously unrecognized link between Wnt signaling and IGFBP3, which together regulate cardiac progenitor cell function. Canonical Wnt contributes to negative LV remodeling by interfering with the endogenous myocardial regeneration. Understanding the molecular signals that modulate tissue homeostasis and repair is important for the design of novel therapeutic strategies for the failing heart.
Supplementary Material
Novelty and Significance.
What is known?
Cardiac Side Population (CSP) cells represent an endogenous pool of progenitor cells in the adult heart.
The molecular mechanisms that dictate the homeostasis and cell fate of CSP cells remain elusive.
Wnt signals are key molecules that govern cardiac development and proliferation of embryonic stem cells, though their role in regulating adult cardiac progenitor cells remains unknown.
What new information does this article contribute?
Activation of Wnt signaling pathway negatively regulates the proliferation capacity of adult CSP cells and compromises the endogenous regenerative capacity of the heart following myocardial infarction (MI).
Wnt stimulation exerts its anti-proliferative effects through a previously unappreciated transcriptional activation of insulin-like growth factor binding protein 3 (IGFBP3)
Designing novel therapeutic approaches to modulate the endogenous regenerative capacity of the failing heart represents a desirable, although challenging, task. Understanding the basic mechanisms that control homeostasis of cardiac precursors cells, such as cardiac side population (CSP) cells, is of fundamental importance in achieving this goal. The Wnt signaling pathway is a key modulator of cardiac development and a major factor that determines cell-fate, particular in embryonic stem cells populations. However, the role of Wnt signaling in adult cardiac progenitor cells remains unclear. Our work reveals an anti-proliferative effect for Wnt signaling in adult CSP cells through a previously unappreciated transcriptional interaction with IGBFP3 and direct regulation of CSP cell cycle progression. Following cardiac injury in vivo, Wnt signaling is down-regulated in CSP cells with corresponding increase in CSP cell proliferation. Increasing Wnt stimulation following myocardial infarction in CSP cells compromises the endogenous regenerative capacity of the heart and is associated with worsening of cardiac remodeling. Our findings demonstrate that canonical Wnt signaling is a potent modulator of endogenous adult cardiac progenitor cells, and suggest that modulation of Wnt signaling or IGFBP3 may could be an effective therapeutic strategy for promoting cardiac regeneration.
Acknowledgments
We thank G. Losyev at the Cardiovascular FACS Core for assistance with SP cells sorting, S. Ngoy at the Cardiovascular Physiology Core for mouse surgery and C. Mbah for assistance with echocardiography measurements. We also thank B. Jiang and members of Liao laboratory for discussion.
Sources of Funding: This study was supported in part by NIH grants (HL086967, HL093148, and HL099173) to R.L. and American Heart Association fellowship awards to A.O. (predoctoral) and J-K.H. and M.B. (postdoctoral). G.F. and A.F.B are supported by Sarnoff Cardiovascular Research Foundation.
Non-standard Abbreviations and Acronyms
- SP
Side Population
- CSP
Cardiac Side Population
- TCF
T-cell factor
- IGF
Insulin Growth Factor
- IGFBP3
Insulin Growth Factor Binding Protein 3
- LV
Left Ventricle
- RV
Right Ventricle
- MI
Myocardial Infarction
- HW
Heart Weight
- BW
Body Weight
- FS
Fractional Shortening
- FAC
Fractional Area Change
- IVS
Intra-Ventricular Septal wall
- LVID
Left-Ventricular Internal Dimension
Footnotes
Disclosures: No disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 4.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. Cd31- but not cd31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien AJ, Conrad WH, Moon RT. A wnt survival guide: From flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 11.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 12.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura T, Sano M, Songyang Z, Schneider MD. A wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Golden K, Bodmer R. Heart development in drosophila requires the segment polarity gene wingless. Dev Biol. 1995;169:619–628. doi: 10.1006/dbio.1995.1174. [DOI] [PubMed] [Google Scholar]
- 16.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of isl-1-positive cardiac progenitor cells through regulation of fgf signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of isl1+ cardiovascular progenitors are controlled by a wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human isl1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Challen GA, Little MH. A side order of stem cells: The sp phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 23.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 24.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, Mouquet F, Westerman K, Liao R. Role of the atp-binding cassette transporter abcg2 in the phenotype and function of cardiac side population cells. Circ Res. 2008;103:825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 27.Jain M, DerSimonian H, Brenner DA, Ngoy S, Teller P, Edge AS, Zawadzka A, Wetzel K, Sawyer DB, Colucci WS, Apstein CS, Liao R. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. doi: 10.1161/01.cir.103.14.1920. [DOI] [PubMed] [Google Scholar]
- 28.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking-based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SE, Jomary C. Secreted frizzled-related proteins: Searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 30.Biechele TL, Moon RT. Assaying beta-catenin/tcf transcription with beta-catenin/tcf transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 31.Yamada PM, Lee KW. Perspectives in mammalian igfbp-3 biology: Local vs. Systemic action. Am J Physiol Cell Physiol. 2009;296:C954–976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- 32.D'Amario D, Cabral-Da-Silva MC, Zheng H, Fiorini C, Goichberg P, Steadman E, Ferreira-Martins J, Sanada F, Piccoli M, Cappetta D, D'Alessandro DA, Michler RE, Hosoda T, Anastasia L, Rota M, Leri A, Anversa P, Kajstura J. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circulation research. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Buckway CK, Wilson EM, Ahlsen M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the n-terminal domain of igf-binding protein-3 essential for high affinity igf binding. J Clin Endocrinol Metab. 2001;86:4943–4950. doi: 10.1210/jcem.86.10.7936. [DOI] [PubMed] [Google Scholar]
- 34.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu XF, Jiang XG, Lu YB, Bai JH, Mao ZB. Characterization of a novel positive transcription regulatory element that differentially regulates the insulin-like growth factor binding protein-3 (igfbp-3) gene in senescent cells. The Journal of biological chemistry. 2005;280:22606–22615. doi: 10.1074/jbc.M412073200. [DOI] [PubMed] [Google Scholar]
- 36.Paquette J, Bessette B, Ledru E, Deal C. Identification of upstream stimulatory factor binding sites in the human igfbp3 promoter and potential implication of adjacent single-nucleotide polymorphisms and responsiveness to insulin. Endocrinology. 2007;148:6007–6018. doi: 10.1210/en.2006-1729. [DOI] [PubMed] [Google Scholar]
- 37.Schweighofer B, Testori J, Sturtzel C, Sattler S, Mayer H, Wagner O, Bilban M, Hofer E. The vegf-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thromb Haemost. 2009;102:544–554. doi: 10.1160/TH08-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCabe CD, Spyropoulos DD, Martin D, Moreno CS. Genome-wide analysis of the homeobox c6 transcriptional network in prostate cancer. Cancer Res. 2008;68:1988–1996. doi: 10.1158/0008-5472.CAN-07-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Guo Y, Ge X, Itoh H, Watanabe A, Fujiwara T, Kodama T, Aburatani H. Elevated expression and potential roles of human sp5, a member of sp transcription factor family, in human cancers. Biochem Biophys Res Commun. 2006;340:758–766. doi: 10.1016/j.bbrc.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 41.Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of igfbp-3 and igfbp-rp1 in breast cancer. Eur J Cancer. 2005;41:1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (igfbp) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe S, Tamura T, Ono K, Horiuchi H, Kimura T, Kita T, Furukawa Y. Insulin-like growth factor axis (insulin-like growth factor-i/insulin-like growth factor-binding protein-3) as a prognostic predictor of heart failure: Association with adiponectin. Eur J Heart Fail. 2010;12:1214–1222. doi: 10.1093/eurjhf/hfq166. [DOI] [PubMed] [Google Scholar]
- 44.Murphy LJ, Molnar P, Lu X, Huang H. Expression of human insulin-like growth factor-binding protein-3 in transgenic mice. J Mol Endocrinol. 1995;15:293–303. doi: 10.1677/jme.0.0150293. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I. Igfbp-4 is an inhibitor of canonical wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 46.Ueno K, Hirata H, Majid S, Tabatabai Z, Hinoda Y, Dahiya R. Igfbp-4 activates the wnt/beta-catenin signaling pathway and induces m-cam expression in human renal cell carcinoma. Int J Cancer. 2011 doi: 10.1002/ijc.25899. [DOI] [PubMed] [Google Scholar]
- 47.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The wnt modulator sfrp2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (sfrp2) is the key akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergmann MW. Wnt signaling in adult cardiac hypertrophy and remodeling: Lessons learned from cardiac development. Circ Res. 2010;107:1198–1208. doi: 10.1161/CIRCRESAHA.110.223768. [DOI] [PubMed] [Google Scholar]
- 50.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing frza. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 51.Zelarayan LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, Zafiriou MP, van der Nagel R, Dietz R, de Windt LJ, Balligand JL, Bergmann MW. Beta-catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19762–19767. doi: 10.1073/pnas.0808393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP. Active wnt signaling in response to cardiac injury. Basic Res Cardiol. 2010;105:631–641. doi: 10.1007/s00395-010-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.