Abstract
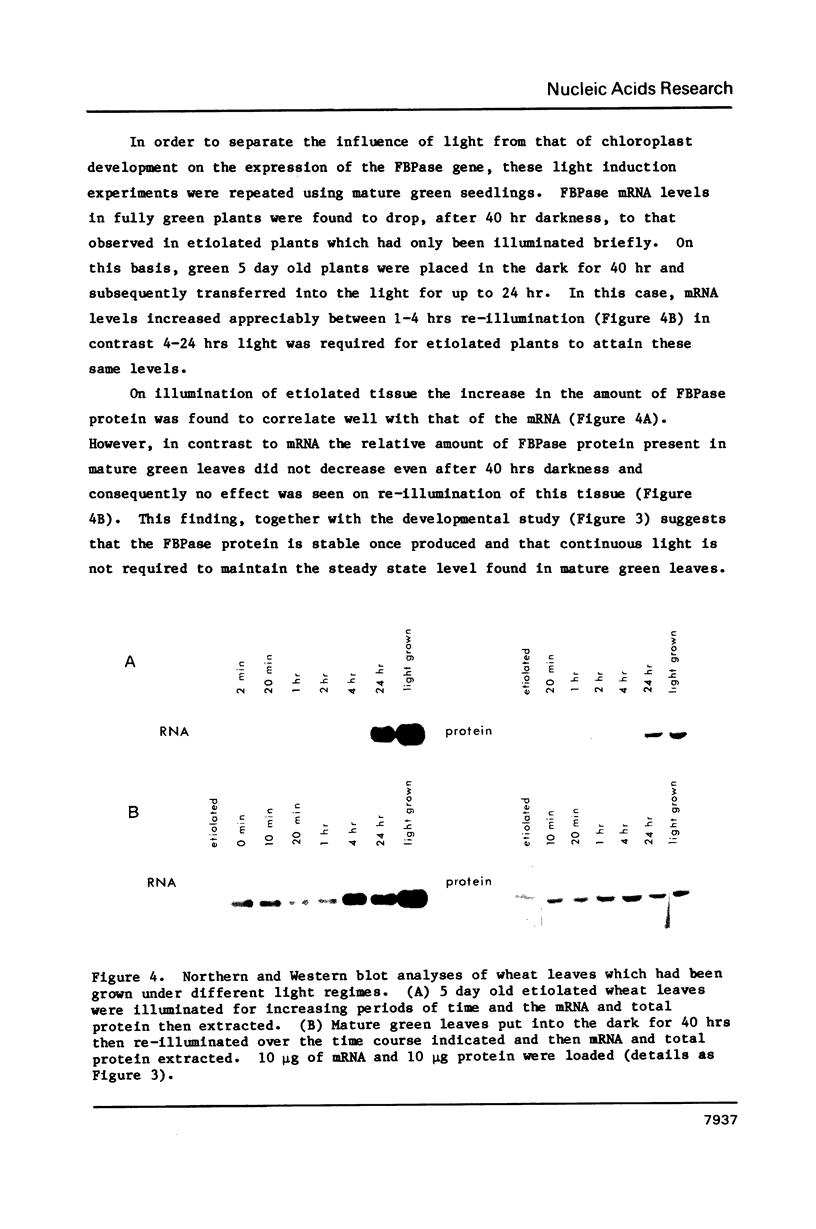
We show here that light stimulates the expression of nuclear genes in wheat leaves for chloroplast fructose-1,6-bisphosphatase (FBPase) and describe a sequence of amino acids in this enzyme which may be responsible, via thioredoxin, for the light regulation of its activity. This data results from (a) our isolation and characterization of a cDNA of this enzyme which contains its entire coding sequence, and (b) our use of this cDNA as a probe to detect mRNA levels in wheat plants subjected to different light regimes. The similarity in amino acid sequence of the encoded enzyme from diverse sources suggests that the FBPase genes all had a common origin. However, their control sequences have been adjusted so that they are appropriately expressed and their coding sequences modified so that the enzymic activity of their products are suitably regulated in the particular cellular environment in which they must function. The light-activated regulatory sequences in the gene for the chloroplast protein have probably come together by a shuffling of DNA segments.
Full text
PDF










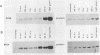
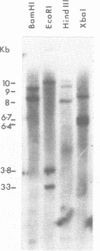
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boffey S. A., Ellis J. R., Selldén G., Leech R. M. Chloroplast Division and DNA Synthesis in Light-grown Wheat Leaves. Plant Physiol. 1979 Sep;64(3):502–505. doi: 10.1104/pp.64.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Leech R. M. Genome Expression during Normal Leaf Development : I. CELLULAR AND CHLOROPLAST NUMBERS AND DNA, RNA, AND PROTEIN LEVELS IN TISSUES OF DIFFERENT AGES WITHIN A SEVEN-DAY-OLD WHEAT LEAF. Plant Physiol. 1982 Apr;69(4):904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher W. K., Thompson E. O. Amino acid sequence studies on sheep liver fructose-bisphosphatase. II. The complete sequence. Aust J Biol Sci. 1983;36(3):235–250. doi: 10.1071/bi9830235. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Harrsch P. B., Kim Y., Fox J. L., Marcus F. Amino acid sequence similarity between spinach chloroplast and mammalian gluconeogenic fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1985 Dec 17;133(2):520–526. doi: 10.1016/0006-291x(85)90937-4. [DOI] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Tobin E. M. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986 Jan;5(1):9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Fructose-bisphosphatase from spinach leaf chloroplast and cytoplasm. Methods Enzymol. 1982;90(Pt E):371–378. doi: 10.1016/s0076-6879(82)90158-6. [DOI] [PubMed] [Google Scholar]
- Kornberg A. DNA replication. J Biol Chem. 1988 Jan 5;263(1):1–4. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Reardon I., Heinrikson R. L. Complete amino acid sequence of pig kidney fructose-1,6-bisphosphatase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7161–7165. doi: 10.1073/pnas.79.23.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Gontero B., Harrsch P. B., Rittenhouse J. Amino acid sequence homology among fructose-1,6-bisphosphatases. Biochem Biophys Res Commun. 1986 Mar 13;135(2):374–381. doi: 10.1016/0006-291x(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Marcus F., Harrsch P. B., Moberly L., Edelstein I., Latshaw S. P. Spinach chloroplast fructose-1,6-bisphosphatase: identification of the subtilisin-sensitive region and of conserved histidines. Biochemistry. 1987 Nov 3;26(22):7029–7035. doi: 10.1021/bi00396a026. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Soulié J. M., Pradel J., Ricard J. Substrate-binding isotherms of spinach chloroplastic fructose-1,6-bisphosphatase and the photoregulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):513–520. doi: 10.1111/j.1432-1033.1981.tb05093.x. [DOI] [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Rittenhouse J., Harrsch P. B., Kim J. N., Marcus F. Amino acid sequence of the phosphorylation site of yeast (Saccharomyces cerevisiae) fructose-1,6-bisphosphatase. J Biol Chem. 1986 Mar 25;261(9):3939–3943. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Purification and properties of spinach leaf cytoplasmic fructose-1,6-bisphosphatase. J Biol Chem. 1978 Sep 10;253(17):5952–5956. [PubMed] [Google Scholar]