Abstract
Streptococcus pyogenes is the causative agent of numerous diseases ranging from benign infections (pharyngitis and impetigo) to severe infections associated with high mortality (necrotizing fasciitis and bacterial sepsis). As with other bacterial infections, there is considerable interest in characterizing the contribution of interleukin-17A (IL-17A) responses to protective immunity. We here show significant il17a up-regulation by quantitative real-time PCR in secondary lymphoid organs, correlating with increased protein levels in the serum within a short time of S. pyogenes infection. However, our data offer an important caveat to studies of IL-17A responsiveness following antigen inoculation, because enhanced levels of IL-17A were also detected in the serum of sham-infected mice, indicating that inoculation trauma alone can stimulate the production of this cytokine. This highlights the potency and speed of innate IL-17A immune responses after inoculation and the importance of proper and appropriate controls in comparative analysis of immune responses observed during microbial infection.
Keywords: bacterial immunity, infection, interleukin-17A, natural killer cells, Streptococcus pyogenes, T helper type 17 cells
Introduction
Interleukin-17A (IL-17A) was first described as a pro-inflammatory cytokine at the end of the twentieth century.1 Much interest in this cytokine has been generated by the description in humans and mice of a discrete T helper type 17 (Th17) subset (IL-17A-producing CD4+ T cells), initially believed to be critical for experimental allergic encephalomyelitis and then linked to other autoimmune diseases as well as to responses to infection.2,3 Although much attention has centred on its production by T cells (CD8+, CD4+ and γδ subsets), it is also secreted by other innate populations such as neutrophils, natural killer (NK) cells and invariant natural killer T (iNKT) cells.4
The precise role of IL-17A in responses to infection remains controversial. Most studies have focused on a physiological role of Th17 cells in defence against bacteria.5 Production of IL-17A by CD4+ T cells during Streptococcus pneumoniae or Klebsiella pneumoniae infection has been shown to have a protective effect in mice through the rapid recruitment of neutrophils that aid bacterial clearance.6,7 Such a role has also been confirmed by antibody-mediated IL-17A or CD4+ T-cell depletion experiments, which showed reversal of the recruitment of neutrophils and monocytes to the mucosal surface leading to increased bacterial colonization and poor survival outcome.6 Over-expression of IL-17A in the pulmonary compartments has been shown to be beneficial, enhancing survival after lethal challenge with K. pneumoniae.8
Although a role for Th17 cells during infection has been demonstrated, innate immune cells such as γδ T, NK and NKT cells also produce IL-17A in the face of infection and are considered to constitute the first line of host defence, acting before adaptive immunity can be initiated.9–11 Interleukin-17A is secreted by γδ T cells during Escherichia coli infection: upon cytokine depletion, decreased neutrophil recruitment to the site of infection is observed, resulting in impaired microbial clearance and increased bacterial burden.12 A similar pattern of response by γδ T cells has been described in Listeria monocytogenes and Salmonella enterica serovar enteritidis infection models.13,14 The NK cells are an early source of IL-17A during toxoplasmosis and depletion of this immune population with anti-asialo-GM1 decreased serum IL-17A levels.11 Taken together, these studies provide evidence for the protective role of IL-17A in the early immune response to serious bacterial infection.
However, production of IL-17A during infection has not always been correlated with protection. In the murine caecal ligation puncture model of polymicrobial sepsis, IL-17A from γδ T cells was detected, but depletion of this cytokine led to a decrease in bacteraemia and a reduction in systemic pro-inflammatory cytokines [tumour necrosis factor-α (TNF-α), IL-1β and IL-6] and chemokines.15 This offers a somewhat conflicting view suggesting that the consequence of IL-17A release in different disease models varies, depending on the nature and magnitude of the infection, conferring different survival outcomes.
Streptococcus pyogenes is a Group A Streptococcus (GAS),16 the causative agent of diverse diseases, ranging from non-invasive (‘strep throat’, impetigo) to severely invasive (necrotizing fasciitis and bacterial sepsis). Many symptoms of sepsis have been attributed to a so-called ‘cytokine storm’, classically characterized by an excessive release of pro-inflammatory cytokines (TNF-α and IL-1β) leading to heightened systemic inflammatory responses observed in patients.17–19 However, this may be an over-simplified view because administration of anti-cytokine antibodies, either in the clinical setting or in murine models, does not offer protection from toxic shock.20
Streptococcus pyogenes has a wide array of virulence factors implicated in pathogenesis and immune evasion, the most widely studied being the superantigens21–23 and M proteins.24,25 To date, only a handful of papers have been published relating to aspects of IL-17A release during S. pyogenes infection. Purified superantigens from S. pyogenes and superantigen-contaminated preparations of peptidoglycan are potent inducers of IL-17A from T cells.26 Patients with GAS infections including streptococcal toxic shock syndrome show elevated levels of γδ T cells, though IL-17A release was not analysed.27 Tonsil cultures from patients with recurrent GAS-associated tonsillitis can be stimulated with heat-killed M1 serotype GAS to produce IL-17A together with transforming growth factor-β, which suggests the possible differentiation of Th17 cells.28
With a potential role for adaptive Th17 cells during S. pyogenes infection previously reported, the innate source of IL-17A has often been overlooked. We sought to clarify the contribution of IL-17A from innate cell types during GAS infection. Using a murine model of acute sepsis, we demonstrated rapid up-regulation of both il17a transcript and serum IL-17A levels in S. pyogenes-infected mice. To our surprise, we also detected IL-17A responses in sham-treated animals; with NK and CD4+ T cells representing the main producers of this pro-inflammatory cytokine in both PBS-treated and GAS-treated mice. This study provides evidence that early IL-17A responses are initiated by tissue damage and trauma caused by the route of inoculation or by bacterial infection. Th17 responses are also observed within hours, suggesting that the CD4/Th17 cells are closer to the innate/adaptive interface than previously recognized and play a more essential role in the acute response to trauma and infection.
Materials and methods
Mice
HLA-DQ8.Aβ0 transgenic mice, used for their heightened sensitivity to streptococcal superantigen and GAS infection,29 were bred on-site and maintained in accordance with UK Home Office guidelines. Female mice used in the experiments were aged between 10 and 21 weeks, and age-matched in any given infection experiment.
Acute sepsis infection model
Streptococcus pyogenes (NCTC8198) was cultured in Todd–Hewitt broth (Oxoid, Basingstoke, UK) overnight at 37° in 5% CO2. The next day, the culture was washed three times in sterile PBS by centrifugation at 3000 g, for 15 min at 4° to remove contaminating broth and resuspended in injection-grade sterile saline. To study IL-17A responses during the time–course of infection, 50 μl bacterial suspension or sterile saline (as sham control) was given via the intramuscular (i.m.) route into the right thigh of female mice at t = 0 hr. The bacterial inoculum was quantified by serial plating on to columbia blood agar plates (Oxoid) and was between 108 and 109 colony-forming units per dose. Sterile saline inoculum was also plated out and no bacterial contamination was detected after 24 hr of incubation. At per-determined time-points post-infection, groups of 10 infected mice or groups of five sham-treated mice were killed for analysis (carried out as part of two independent experiments). Tissue was also obtained from five non-inoculated naive female mice for normalization of quantitative reverse transcription (qRT-) PCR data. For ex vivo IL-17A cytokine flow cytometry, female mice were inoculated with S. pyogenes (n = 4) or PBS (n = 3) as described above, and killed at 24 hr along with untreated naive mice (n = 3).
Quantitative RT-PCR
Spleen and inguinal draining lymph nodes were harvested from naive, sham-infected or infected mice at 4, 8, 12 or 24 hr and stored in RNAlater™ RNA Stabilisation Reagent (Qiagen, Crawley, UK) at −80° until ready for use. Total RNA was extracted using the acid phenol method with TRIzol (Invitrogen, Paisley, UK) as per the manufacturer’s instructions before resuspension in RNase-free water containing RNaseOUT Recombinant Ribonuclease Inhibitor (Invitrogen). Concentration of RNA and the nucleic acid : protein ratio were analysed using a NanoDrop spectrophotometer (NanoDrop, Wilmington, DE). One microgram of total RNA was reverse transcribed into cDNA using Superscript® III Reverse Transcriptase (Invitrogen). A qRT-PCR was run on cDNA samples in triplicates using in-house-designed target-gene-specific primers and hydrolysis probes (Table 1) on an MX3000P real-time PCR thermocycler (Agilent Technologies, Inc., Santa Clara, CA). Reactions were performed in 20 μl total volume with a thermal profile of 50° for 2 min, followed by 10 min at 95° and then 50 cycles of 15 seconds at 95°, 15 seconds at 60° and 15 seconds at 72°. The fold changes in gene expression levels, relative to unimmunized naive samples, were normalized to two tissue-specific reference genes and calculated based on the ΔΔCT method,30 along with differential amplification efficiencies and randomization statistical analysis using the Relative Expression Software Tool (rest) (Qiagen, Hilden, Germany).31
Table 1.
Quantitative real-time reverse transcription-PCR primers and hydrolysis probes
| Gene | Sense primer | Anti-sense primer | 6FAM-probe-TAMRA |
|---|---|---|---|
| b2m | CTACTGGGATCGAGACATTGTGAT | TGTGTACATTGCTATTTCTTTCTGC | TGCTCTGAAGATTCATTTGAACCTGCT |
| gapdh | GAGAAACCTGCCAAGTGTGATGAC | AGACAACCTGGTCCTCAGTGTAG | TCAAGAAGGTGGTGAAGCAGGCATC |
| tfrc | AATGGTAACTTAGACCCAGTGGAG | ATTAGCATGGACCAGTTTACCAGA | TCCCGAGGGTTATGTGGCATTCAGT |
| tbp | CAGTGCCCAGCATCACTATTT | GCATCCTCTGAATATCTCCTTAGAA | CATGGTGTGAAGATAACCCAGAACA |
| il17a | CTGTGTGTGTGATGCTGTTGCT | AAGGGAGTTAAAGACTTTGAGGTTG | AGCTCAGCGTGTCCAAACACTGAGG |
| rorc il6 tgfb | GTCTGCAAGTCCTTCCGAGAG GTTCCTCTCTGCAAGAGACTTCC ATGTTCTTCAATACGTCAGACATTC | ATCTCCCACATTGACTTCTG GTATCCTCTGTGAAGTCTCCTCTCC TTGCTATATTTCTGGTAGAGTTCCA | CTGCGACTGGAGGACCTTCTACGGC CTTGGGACTGATGCTGGTGACAACC GCAGAGCTGCGCTTGCAGAGATTAA |
Sequences of sense, anti-sense and probes used for quantification of fold changes in gene expression. b2m, gapdh, tfrc and tbp were used as tissue-specific reference genes to normalize the fold change in il17a, rorc, il6 and tgfb expression.
ELISA
Blood from naive, infected or sham-infected mice was collected by cardiac puncture. Serum IL-17A concentrations were determined by sandwich ELISA (MABTECH AB, Nacka Strand, Sweden) and optical densities of samples against an IL-17A standard curve were measured using an ELISA plate reader (μQuant BIO-Tek Instruments, Inc., Winooski, VT) and KC Junior software.
Ex vivo intracellular IL-17A flow cytometry
Female mice inoculated with S. pyogenes or with PBS or left untreated (naive) were given 50 μg brefeldin A (BFA; Sigma-Aldrich, Poole, UK) or PBS via an intraperitoneal (i.p.) route in a volume of 100 μl at 20 hr to stop the release of cytokines from cells in the last 4 hr of infection.32 Mice were killed at 24 hr and inguinal draining lymph nodes and spleens were harvested and homogenized into a single cell suspension in PBS containing 10% fetal calf serum using cell strainers (BD, Oxford, UK). Cells were washed twice in cold PBS (10% fetal calf serum) before blocking with Fc Block (eBioscience, San Diego, CA) for 10 min on ice. Surface staining was carried out with anti-mouse CD3 V500-conjugated, anti-mouse T-cell receptor-γδ phycoerythrin-conjugated, anti-CD4 allophycocyanin-H7-conjugated and anti-mouse NK1.1 phycoerythrin-Cy7-conjugated (all BD) for 20 min at 4°. Cells were washed and resuspended in 1× Fix/Perm solution (eBioscience) for 30 min at 4° before being washed twice in 1× permeabilization buffer (eBioscience) and intracellular IL-17A was stained using AlexaFluor 647-conjugated anti-mouse IL-17A (BD) for 30 min at 4° before washing and fixation in 1% paraformaldehyde. The IL-17A Fluorescence Minus One (FMO) controls were used to determine positive populations (Fig. S1) and samples were run on a BD FACSAria II™ flow sorter (BD, Mountain View, CA) and analysed using FlowJo software (Treestar, Ashland, OR).
Statistical analysis
Statistical analysis of qRT-PCR data was performed using rest software. Data are presented as mean ± SEM. Any significant differences between treatment groups for ELISA and ex vivo IL-17A flow cytometry were determined with a Kruskal–Wallis significance test using Graphpad prism 4.0 software (Graphpad Inc., La Jolla, CA).
Results
Rapid induction of il17a expression after inoculation
Female HLA-DQ8.Aβ0 transgenic mice were used in this study because of their increased susceptibility to S. pyogenes infection and superantigen sensitivity.29 Female mice received either S. pyogenes (n = 10 per time-point) or PBS (n = 5 per time-point) using the intramuscular route to mimic acute septic infection and were killed at defined time-points. Naive, untreated mice (n = 5 females) were also killed, one at each time-point, and pooled together to form the normalization group (t = 0). From qRT-PCR analysis, it was observed that there was rapid up-regulation in il17a expression in the draining lymph nodes after GAS infection, peaking at 4 hr, then slowly declining and remaining steady from 12 to 24 hr post-infection compared with unimmunized mice (Fig. 1a). Interestingly, sham infection with sterile saline also produced swift expression of il17a in the lymph nodes and expression remained higher than in infected samples even 24 hr after treatment (Fig. 1a). Increased transcription of il17a was also observed in the spleen (Fig. 1b) but with no significant difference between sham-treated and naive mice at 8 and 12 hr post-treatment. This suggested a return to basal levels of il17a expression in the spleen at the intermediate time-points, whereas S. pyogenes-infected mice displayed more sustained up-regulation of il17a transcription throughout the time–course of infection (Fig. 1b). Hence, inoculation with either saline or S. pyogenes induced a dramatic increase in il17a expression in the secondary lymphoid organs analysed.
Figure 1.
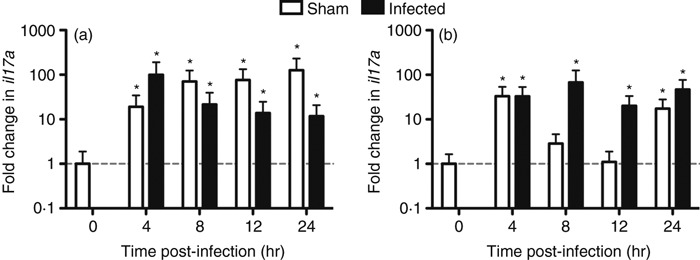
Fold change in expression of il17a after Streptococcus pyogenes infection or sham infection. HLA-DQ8.Aβ0 mice were injected intramuscularly with 1·1 × 109 colony-forming units S. pyogenes (n = 10 per time-point) or PBS (n = 5 per time-point) at t = 0 hr. Draining lymph nodes (a) or spleens (b) were taken at t = 0, 4, 8, 12 and 24 hr for quantitative real-time reverse transcription-PCR. Sham and infected samples were normalized to naive uninfected mice (n = 5) and to two tissue-specific reference genes. Fold change in il17a expression was determined using rest software and any significant differences (*P < 0·05) relative to naive samples are indicated.
Elevated IL-17A levels in the serum were detected post-inoculation
The rapid up-regulation in il17a gene expression was matched by serum IL-17A concentrations. Serum IL-17A was markedly elevated 4 hr after infection with S. pyogenes, but was reduced at 8 hr before rising again during the later stages of infection. The data were in contrast to those for naive controls (t = 0 hr), which displayed low background levels of IL-17A in the serum (Fig. 2). Sham infection with saline resulted in strong production of IL-17A, which followed a similar bimodal pattern to GAS-infected samples (Fig. 2). Taken together, inoculation with either saline or S. pyogenes resulted in elevated levels of IL-17A in the serum.
Figure 2.
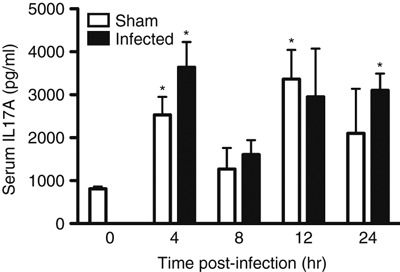
Serum interleukin-17A (IL-17A) released after Streptococcus pyogenes infection or sham infection. HLA-DQ8.Aβ0 mice were injected intramuscularly with 1·1 × 109 colony-forming units S. pyogenes (n = 6 per time-point) or PBS (n = 3 per time-point) or naive (n = 4) at t = 0 hr. Serum was collected from mice at t = 0, 4, 8, 12 and 24 hr by cardiac puncture for serum IL-17A analysis by ELISA. Any significant differences between sham or infected samples compared with naive mice are shown above (*P < 0·05). No significant differences were found between time-matched sham or infected samples).
Effects of inoculation on rorc gene expression
Quantitative RT-PCR analysis was also carried out on the draining inguinal lymph node and spleen tissue to analyse any changes in rorc, a hallmark transcription marker of Th17 cells, to determine the contribution of this cell type during immunization.33 Paradoxically, this transcript was significantly down-regulated, both in sham-infected and S. pyogenes-infected lymph node samples (Fig. 3a) relative to naive uninfected controls, despite an increase in il17a transcription (Fig. 1a). This suggested that increased IL-17A in draining lymph nodes may not be the result of rapid induction of Th17 cells, but may be produced from some other cell-type. In contrast to changes in the draining lymph node, transcription of rorc increased in the spleen following both GAS infection and sham infection (Fig. 3a). These changes in the spleen may represent a delayed, systemic Th17 response to GAS infection or injection trauma and could contribute to the released IL-17A in the serum as observed in Fig. 2.
Figure 3.
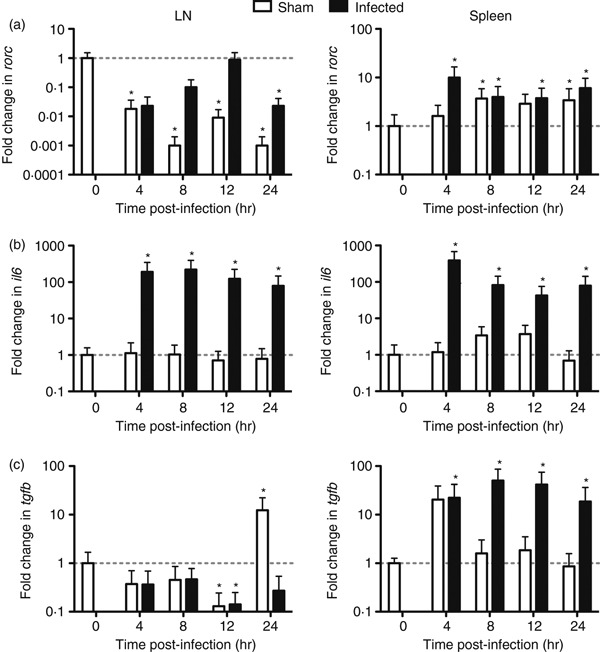
Fold change in expression of rorc during Streptococcus pyogenes infection or sham infection. HLA-DQ8.Aβ0 mice were injected intramuscularly with 1·1 × 109 colony-forming units S. pyogenes (n = 10 per time-point) or PBS (n = 5 per time-point) at t = 0 hr. Draining lymph nodes and spleens were taken at t = 0, 4, 8, 12 and 24 hr for quantitative real-time reverse transcription-PCR analysis. Fold change in rorc (a), il6 (b) and tgfb (c) expressions was determined using rest software and any significant differences (*P < 0·05) relative to naive samples are indicated.
The qRT-PCR analysis of fold changes in il6 and tgfb expression between GAS-infected and sham-infected samples was also performed as these two cytokines are essential for RORγt induction and hence Th17 differentiation.34,35 Expression of the il6 gene was significantly up-regulated only in S. pyogenes-infected samples relative to naive controls in the draining lymph node and spleen (Fig. 3b). This was also mirrored by tgfb expression in GAS-infected spleen (Fig. 3c). As a result, up-regulation of il6 and tgfb expression in the spleen seems to correlate with induction of rorc expression, whereas up-regulation of il6 in the draining lymph nodes and in the absence of tgfb expression was not sufficient to induce rorc expression and Th17 cell differentiation.
Identification of innate and adaptive sources of IL-17A post-inoculation
The cellular source of IL-17A was determined by ex vivo intracellular cytokine flow cytometry, allowing identification of IL-17A producing cell types following either GAS or sham infection. IL-17A+ cellular populations were determined in the draining lymph node of naive, sham-infected or GAS-infected mice. In the last 4 hr of i.m. immunization, BFA was given i.p. as part of the ex vivo staining protocol and PBS was used as control for this injection. The percentage of IL-17A+ cells from the total population was not significantly different in the naive controls given either BFA or PBS. This suggests that the administration of chemicals into the peritoneal cavity did not affect the percentage of cells producing IL-17A (Fig. 4). Interestingly, mice treated with i.m. immunization (either sham-infected or GAS-infected) and BFA i.p. exhibited a slight (but not significant) decrease in the percentage of IL-17A+-expressing cells compared with mice given PBS i.p. (Fig. 4). Infected mice given BFA contained a significantly lower percentage of IL-17A+ cells compared with naive mice also treated with BFA (Fig. 4).
Figure 4.
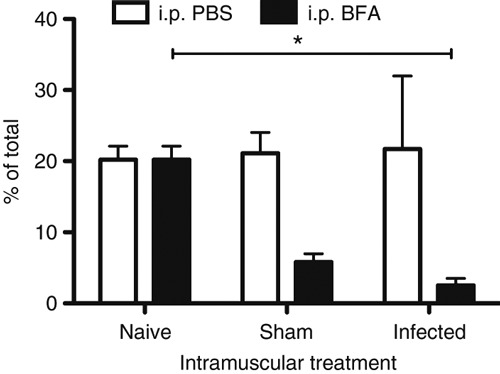
Flow cytometric staining of interleukin-17A-positive (IL-17A+) cells after infection or immunization. Inguinal draining lymph nodes were harvested from untreated naive HLA-DQ8.Aβ0 mice (n = 3) or Streptococcus pyogenes-infected (n = 4) or PBS-immunized (n = 3) mice at t = 24 hr. In the last 4 hr of immunization, PBS or brefeldin A (BFA) was given intraperitoneally before mice were culled and cells were stained for IL-17A production and analysed by flow cytometry. Fluorescence Minus One (FMO) controls were used to determine IL-17A+ populations. The percentage of IL-17A+ cells from total cells are shown for naive, sham and infected mice. Any significant differences are indicated (*P < 0·05) using the Kruskal–Wallis statistical test.
To determine the precise cells capable of producing IL-17A, a further ex vivo flow cytometric analysis was conducted (Fig. 5a). In naive animals, the predominant cell types in the draining lymph node to produce IL-17A upon treatment with PBS i.p. appeared to be NK and CD4+ T cells (Fig. 5b). This was also true for sham-infected and GAS-infected mice given PBS i.p. (Fig. 5b). Mice treated with BFA i.p. had a reduced percentage of IL-17A+ expression cells compared with mice given PBS (Fig. 5b). Significantly fewer NK cells and γδ T cells expressed IL-17A in the sham-infected mice treated with BFA compared with the PBS control group (Fig. 5b). Naive mice treated with BFA had significantly fewer γδ T cells compared with infected mice given BFA (Fig. 5b). This pattern of reduced cell types expressing IL-17A appears to agree with the decrease in the percentage of IL-17A+ cells of the total population (Fig. 4). Overall, IL-17A synthesis in either naive, sham-infected or GAS-infected mice treated with PBS was primarily from NK and CD4+ T cells.
Figure 5.
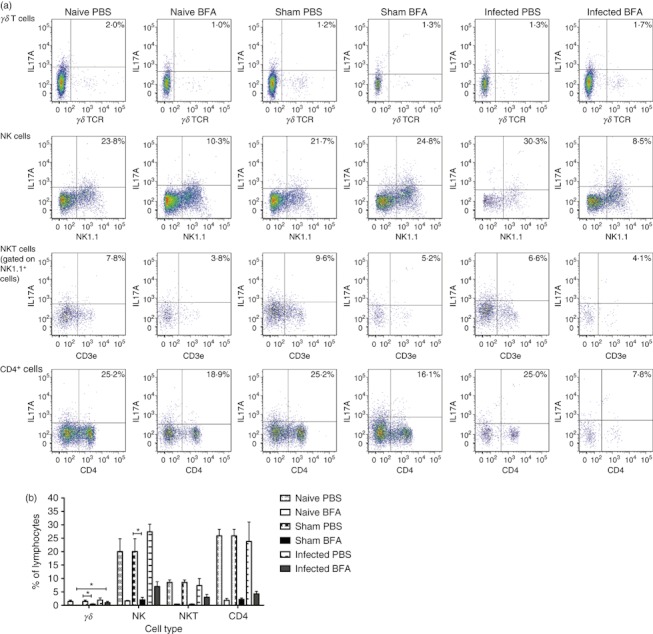
Immune cell populations producing interleukin-17A (IL-17A) during Streptococcus pyogenes infection or sham infection. Inguinal draining lymph nodes were taken from naive (n = 3) HLA-DQ8.Aβ0 mice or from mice inoculated intramuscularly with 6·5 × 108 CFU S. pyogenes (n = 4) or PBS (n = 3) for 24 hr. In the last 4 hr, PBS or brefeldin A (BFA) was given intraperitoneally before mice were culled and cells were stained for IL-17A production in various cell types and analysed by flow cytometry. Representative dot-plots are shown above (a) with the percentage of IL-17A+ cells in the lymphocyte gate show in the top-right corner of the plot. Collective data are shown in (b) and any significant differences are indicated (*P < 0·05) using the Kruskal–Wallis statistical test.
Discussion
Interleukin-17A is the most widely studied from a family of five related cytokines from the IL-17 family (A–F).36 Interleukin-17A has 50% homology with IL-17F and shares signalling via the heterodimeric IL-17RA and IL-17RC subunit receptor complex ubiquitously expressed on many different cells.37 Hence, IL-17A can bring about immunological effects on various cell types (including non-lymphoid cells such as epithelial and mesenchymal cells) via a diverse array of target genes mediated by signal transduction downstream of its receptor.38 Key examples of target genes include pro-inflammatory chemokines (CXCL1, CXCL8), cytokines (TNF-α, IL-6 and IL-1), anti-microbial peptides and tissue remodelling factors.39
Different roles in immunity have been postulated for IL-17A since its discovery in the early 1990s. One potent source of this pro-inflammatory cytokine was found to be adaptive CD4+ T cells, termed Th17 cells in line with the Th1 and Th2 subsets postulated by Mosmann et al. in 1986.40 Since then, most research has been dedicated to the role of Th17 cells in host defence in infectious diseases or contribution in the development of autoimmunity (e.g. experimental allergic encephalomyelitis, rheumatoid arthritis, inflammatory bowel disease).5,41 However, in the past few years, interest has been generated as innate cell types such as γδ T, NK and NKT cells were found to also secrete IL-17A in response to microbial stimuli.4 Despite our current understanding, the precise interplay between innate and adaptive sources of, or the role of, IL-17A and downstream consequences during the early stages of acute bacterial infection have not been fully elucidated. In this study we sought to characterize both adaptive and innate sources of IL-17A during a time–course of acute S. pyogenes infection using a humanized murine model.
In this study, up-regulated il17a gene expression and protein production were detected following S. pyogenes infection in the inguinal draining lymph node, spleen and serum, respectively. However, using a stringent negative control not always included in infection protocols, it was noted that sham infection with sterile saline could induce changes of similar magnitude in il17a expression (Fig. 1) and protein production (Fig. 2) despite the absence of pathogen or antigen. Sham infection induced a marginally larger and longer lasting effect in il17a gene expression compared with infected samples.
The main sources of IL-17A in naive, sham or infected mice treated with PBS i.p. were NK and CD4+ T cells, implying that both innate and unexpectedly early adaptive immune cells are involved in the production of IL-17A (Fig. 5). Interestingly, rorc transcription actually decreased following S. pyogenes infection or sham infection in the local draining lymph node when normalized to unimmunized naive controls, indicative of fewer Th17 cells migrating to the site of trauma or infection or even of Th17 differentiation. This was supported by down-regulation of expression of one of the key cytokines; tgfb required for Th17 differentiation in both sham-treated and GAS-treated samples compared with naive tissue. The CD4+ T cells detected by ex vivo IL-17A staining in the draining lymph nodes of infected mice may be pre-existing memory cells, reminiscent of an IL-17A-producing memory Th17 population identified using superantigen-secreting Staphylococcus aureus isolates.42
The decreased percentage of IL-17A+ cells of the total population in the sham-infected and GAS-infected samples treated with BFA i.p. compared with naive controls as shown by ex vivo IL-17A staining may be caused by increased cell death caused by the trauma of inoculation. This is particularly the case for S. pyogenes-infected mice, whereby this pathogen has been demonstrated to induce dramatic apoptosis in immune cell types.43 The increased detection of IL-17A+ cells in naive controls without treatment with BFA may represent a store of this pro-inflammatory cytokine and suggests that these cells are poised for quick release in response to trauma or infection.
As no increase in rorc or tgfb gene expression was detected in the inguinal draining lymph node, it cannot be ruled out that Th17 cells may differentiate elsewhere upon sham or GAS infection. It has been shown that skin-migratory Langerhans cells can induce Th17 differentiation from naive CD4+ T cells in the presence of IL-6 and IL-15.44 Differentiated CD4+ T cells were able to home to the skin and produce IL-17 and also interferon-γ to exert tissue damage. Therefore, it is not implausible to suggest that the trauma caused at the site of injection in our model may activate skin-migratory Langerhans cells to induce the differentiation of Th17 cells in a similar fashion and the production of IL-17A may contribute to the observed tissue damage and cell death. However, this would need to be confirmed.
The small difference between sham-infected and S. pyogenes-infected samples in terms of serum IL-17A levels is indicative of the very strong and volatile innate IL-17A response to local trauma. It has been long established that tissue damage and trauma can lead to the production of pro-inflammatory cytokines, such as IL-1, IL-2, TNF-α, IL-6, IL-12 and interferon-γ.45 These cytokines and also chemokines act as danger signals, recruiting cells, such as NKT cells, to the site of injury to create a microenvironment suitable for tissue repair and wound healing46 and also to guard against risk of infection.47 Interleukin-17A is also associated with trauma and tissue damage. Systemic increases have been described in post-trauma patients. However, this was associated with Th17 cells as IL-23 induced by local macrophages and dendritic cells maintained the survival of this T-cell subset.48 Elevated IL-17A has also been detected after severe burn injuries in both clinical patients and murine models.49,50γδ T cells have been demonstrated to sense stress signals from dying cells and secrete IL-17A in response51 as a form of immune surveillance.52 In our acute sepsis model we found a significantly increased percentage of IL-17A+γδ T cells in infected BFA-treated mice relative to naive BFA controls, indicating that this innate immune cell type may be playing a role in immune surveillance.
The major sources of IL-17A in this model were NK cells, CD4+ cells, and to a lesser extent NKT cells, following S. pyogenes infection in terms of percentage of lymphocytes. Both pathogenic and protective roles for NK cells during bacterial sepsis have been demonstrated by depletion experiments using the caecal ligation puncture animal model of polymicrobial sepsis and other models of trauma.53–56 Clearly, a potential mechanism for the interaction between S. pyogenes and immune cells leading to IL-17A production may be via secreted superantigens. For example, hepatic NKT cells can be activated by Kupffer cells in the presence of superantigens to produce interferon-γ and IL-12 and show enhanced cytotoxic activity.57 We are currently defining the relative contributions of antigenicity and superantigenicity using superantigen-knockout GAS isolates.
Our experiments have shown potent and rapid release of IL-17A by both innate and adaptive cell types in response to trauma and infection. This appears to be an exquisitely reactive mechanism, poised to prime the host against potential infection and inflammation as perceived through tissue damage at the site of injury. Hence, extreme care is required in choosing appropriate controls for infection models.
Acknowledgments
This work was supported by MRC grant (G0700153).
Disclosure
The authors have no financial disclosures.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Comparison of an isotype control and Fluorescence Minus One (FMO) control in the analysis of flow cytometry data of IL-17A positive cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 2.Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159:109–19. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 5.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–85. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien RL, Roark CL, Born WK. IL-17-producing γδ T cells. Eur J Immunol. 2009;39:662–6. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. γδ T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776–83. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata K, Yamada K, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–72. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 13.Hamada S, Umemura M, Shiono T, et al. IL-17A produced by γδ plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–63. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegemund S, Schütze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, Sabat R, Alber G. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis. Int Immunol. 2009;21:555–65. doi: 10.1093/intimm/dxp025. [DOI] [PubMed] [Google Scholar]
- 15.Flierl MA, Rittirsch D, Gao H, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 18.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 19.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008;214:211–23. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:S121–5. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 21.Sriskandan S, Faulkner L, Hopkins P. Streptococcus pyogenes: insight into the function of the streptococcal superantigens. Intl J Biochem Cell Biol. 2006;39:12–9. doi: 10.1016/j.biocel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol. 2003;133:299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llewelyn M, Cohen J. Superantigens: microbial agents that corrupt immunity. Lancet Infect Dis. 2002;2:156–62. doi: 10.1016/s1473-3099(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 24.Pählman LI, Olin AI, Darenberg J, Mörgelin M, Kotb M, Herwald H, Norrby-Teglund A. Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell Microbiol. 2008;10:404–14. doi: 10.1111/j.1462-5822.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JH, Case MC, Kehoe MA. Characterization of a conserved helper-T-cell epitope from group A Streptococcal M proteins. Infect Immun. 1993;61:1062–8. doi: 10.1128/iai.61.3.1062-1068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Nooh MM, Kobt M, Re F. Commercial peptidoglycan preparations are contaminated with superantigen-like activity that stimulates IL-17 production. J Leukoc Biol. 2008;83:409–18. doi: 10.1189/jlb.0807588. [DOI] [PubMed] [Google Scholar]
- 27.Arvand M, Schneider T, Jahn HU, Hahn H. Streptococcal toxic shock syndrome associated with marked γδ T cell expansion: case report. Clin Infect Dis. 1996;22:362–5. doi: 10.1093/clinids/22.2.362. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Dileepan T, Briscoe S, Hyland KA, Kang J, Khoruts A, Cleary PP. Induction of TGF-β1 and TGF-β1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci USA. 2010;107:5937–42. doi: 10.1073/pnas.0904831107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faulkner L, Altmann DM, Ellmerich S, Huhtaniemi I, Stamp G, Sriskandan S. Sexual dimorphism in superantigen shock involves elevated TNF-α and TNF-α induced hepatic apoptosis. Am J Respir Crit Care Med. 2007;176:473–82. doi: 10.1164/rccm.200611-1712OC. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–40. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korn T, Mitsdoerffer M, Croxford AL, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:18460–5. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–73. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Gaffen S. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–21. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 41.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islander U, Andersson A, Lindberg E, Adlerberth I, Wold AE, Rudin A. Superantigenic Staphylococcus aureus stimulates production of interleukin-17 from memory but not naive T cells. Infect Immun. 2010;78:381–6. doi: 10.1128/IAI.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–19. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 44.Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, Morelli AE, Larregina AT. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–33. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 45.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma injury. Injury. 2007;38:1336–45. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Schneider DF, Palmer JL, Tulley JM, Speicher JT, Kovacs EJ, Gamelli RL, Faunce DE. A novel role for NKT cells in cutaneous wound repair. J Surg Res. 2011;168:325–33. doi: 10.1016/j.jss.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi K, Tsuda Y, Yoshida T, Takeuchi D, Utsunomiya T, Takahashi H, Suzuki F. Bacterial sepsis and chemokines. Curr Drug Targets. 2006;7:119–34. doi: 10.2174/138945006775270169. [DOI] [PubMed] [Google Scholar]
- 48.Frangen TM, Bogdanski D, Schinkel C, Roetman B, Kälicke T, Muhr G, Köller M. Systemic IL-17 after severe injuries. Shock. 2008;29:462–7. doi: 10.1097/shk.0b013e3181598a9d. [DOI] [PubMed] [Google Scholar]
- 49.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 50.Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–5. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 53.Schneider DF, Glenn CH, Faunce DE. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res. 2007;28:365–79. doi: 10.1097/BCR.0B013E318053D40B. [DOI] [PubMed] [Google Scholar]
- 54.Barkhausen T, Frerker C, Putz C, Pape H, Krettek C, van Griensven M. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30:401–10. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]
- 55.Goldmann O, Chhatwal GS, Medina E. Contribution of natural killer cells to the pathogenesis of septic shock induced by Streptococcus pyogenes in mice. J Infect Dis. 2005;191:1280–6. doi: 10.1086/428501. [DOI] [PubMed] [Google Scholar]
- 56.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–9. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Dobashi H, Seki S, Habu Y, Ohkawa T, Takeshita S, Hiraide H, Sekine I. Activation of mouse liver natural killer cells and NK1.1+ T cells by bacterial superantigen-primed Kupffer cells. Hepatology. 1999;30:430–6. doi: 10.1002/hep.510300209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


