Abstract
Dengue is a mosquito-borne viral disease of humans, and animal models that recapitulate human immune responses or dengue pathogenesis are needed to understand the pathogenesis of the disease. We recently described an animal model for dengue virus (DENV) infection using humanized NOD-scid IL2rγnull mice (NSG) engrafted with cord blood haematopoietic stem cells. We sought to further improve this model by co-transplantation of human fetal thymus and liver tissues into NSG (BLT-NSG) mice. Enhanced DENV-specific antibody titres were found in the sera of BLT-NSG mice compared with human cord blood haematopoietic stem cell-engrafted NSG mice. Furthermore, B cells generated during the acute phase and in memory from splenocytes of immunized BLT-NSG mice secreted DENV-specific IgM antibodies with neutralizing activity. Human T cells in engrafted BLT-NSG mice secreted interferon-γ in response to overlapping DENV peptide pools and HLA-A2 restricted peptides. The BLT-NSG mice will allow assessment of human immune responses to DENV vaccines and the effects of previous immunity on subsequent DENV infections.
Keywords: dengue, human, T cells, transgenic mice, viral
Introduction
Dengue virus (DENV) is a mosquito-borne member of the Flavivirus genus and includes four serotypes (DENV-1, DENV-2, DENV-3 and DENV-4). The virus infects approximately 50 million individuals each year, leading to over 500 000 hospitalizations. Infection results in a range of symptoms from mild fever to acute febrile illness (dengue fever). In a small percentage of cases, however, individuals develop a severe capillary leakage syndrome, dengue haemorrhagic fever and dengue shock syndrome, which can be life-threatening.1,2 Studies in humans suggest that dengue haemorrhagic fever and dengue shock syndrome are more likely to occur in individuals experiencing their second DENV infections and in infants born to DENV-immune mothers. Experimental manipulation of in vivo immune responses to DENV is a critical step in exploration of the role of previous immunity in subsequent DENV infection and testing of candidate vaccines and therapeutics.
Progress in understanding the pathogenesis of dengue haemorrhagic fever has come largely from controlled well-designed clinical studies of patients with mild and severe forms of dengue disease in endemic areas.3–10 Most patients who present to hospital live in endemic areas and are experiencing a secondary infection; however, the serotype of the previous DENV infection is difficult to determine. Furthermore, controlled virus challenge studies are not feasible in humans, and it is difficult to assess the contribution of antibodies or T cells to DENV pathogenesis. Immunodeficient mice bearing components of a human immune system (humanized mice) present a novel approach for studying human immune responses to DENV.11 The ability to assess the function of human antibodies and T cells during primary DENV infection and to control the dose and serotype of DENV used for a second infection would be a significant advance in understanding the fine specificity of the adaptive immune response and their involvement in protection or subsequent secondary dengue disease. Furthermore, practical and predictive humanized animal models would be beneficial to evaluate the induction of human immune responses, at both cellular and humoral levels by candidate dengue vaccines in development.12
Our group and several others have shown that humanized mice provide a tractable animal model that permits in vivo infection of human cells with DENV and elicits human DENV-specific immune responses.13–16 Using cord blood haematopoietic stem cell (HSC)-engrafted NOD-scid IL2rγnull (NSG) mice we previously showed that the engrafted mice support DENV infection. Human T cells from infected NSG mice expressing the HLA-A2 transgene produced interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) upon stimulation with DENV peptides. These mice also developed moderate levels of IgM antibodies directed against the DENV envelope protein.14 We speculated that suboptimal positive selection of HLA-restricted human T cells on murine thymus in NSG mice may have led to reduced human T-cell and B-cell responses.
Humanized fetal liver/thymus (BLT-NSG) mice were developed to provide a microenvironment for human T-cell development.17 In these mice, human fetal liver and thymus tissue are implanted under the kidney capsule to produce a thymic organoid that allows the education of human T cells on autologous thymus. Then, HSC from the same liver and thymus donor are injected intravenously into the transplanted mice. Engrafted BLT-NSG mice develop robust populations of functional human T lymphocytes within mouse lymphoid tissues. Following infection of BLT-NSG mice with Epstein–Barr virus and HIV, antigen-specific cellular and humoral immune responses have been detected.17–20 In this manuscript we tested the hypothesis that the education and maturation of human T cells on autologous human thymic tissue in the BLT model and subsequent infection of BLT-NSG mice with DENV would lead to heightened DENV-specific cellular and humoral immune responses.
Materials and methods
Generation of BLT-NSG mice
The NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice (NSG) were bred at The Jackson Laboratory and subsequently maintained in the animal facilities at the University of Massachusetts Medical School. All experiments were performed in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School and the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996). NSG mice at 6–8 weeks of age were irradiated (200 cGy) and received surgical implants under the kidney capsule of 1-mm3 fragments of HLA-A2-positive or negative human fetal thymus and liver on the same day as the tissues were received. Tissues were purchased from Advanced Bioscience Resources (Alameda, CA). On the same day as the tissue transplant, CD34+ cells (1 × 105 to 5 × 105), isolated from autologous fetal liver using anti-human CD34+ microbeads (Miltenyi, Auburn, CA), were injected intravenously as a source of HSC to develop BLT-NSG mice. Human cells were allowed to engraft and to generate an immune system in recipient mice for at least 12 weeks, at which time human haematolymphoid engraftment was validated by flow cytometry on peripheral blood as described previously.6,10 Successfully engrafted mice were then randomized based on engraftment levels for use in experiments.
DENV production and infection of BLT-NSG mice
Dengue virus serotype-2 strain New Guinea C (DENV-2 NGC) was propagated in C6/36 Aedes albopictus cells cultured in RPMI-1640 (Invitrogen, Grand Island, NY) containing 5% heat-inactivated fetal calf serum (Gibco, Grand Island, NY) at 28° as previously described.14 Dengue virus serotype-2 strain S16803 was kindly provided by Dr Robert Putnak at Walter Reed Army Institute of Research. Virus titres were determined by focus-forming assay on Vero cells. Groups of BLT-NSG mice were inoculated by the subcutaneous route with approximately 106 plaque-forming units (PFU) DENV-2 NGC or increasing doses of DENV-2 S16803 (106−108 PFU). Clinical assessments (weight loss and signs of illness including ruffling and hunching) were monitored for 30 days. Organs (spleen, liver and bone marrow) were surgically removed from mice killed at different times post-infection. Aliquots of the sera, liver, bone marrow and spleen cells were immediately frozen at −80° for RNA analysis. A piece of the spleen, was depleted of red blood cells using an RBC lysis buffer (Sigma, St Louis, MO) and processed to make single-cell suspensions for T-cell and B-cell assays.
Quantification of viral RNA
Sera and bone marrow were tested for the presence of DENV-2 RNA by reverse transcription (RT-) PCR. Serum viral RNA was extracted and purified using the QIAamp Viral RNA Mini kit (Qiagen, Valencia, CA). RNA from bone marrow cells was isolated using the Qiagen RNeasy mini kit (Qiagen) and subjected to reverse-transcription and amplification using a Qiagen One-Step RT-PCR Kit (Qiagen) with DENV-2-specific primers D1 and TS2 as described by Lanciotti et al.21 Viral RNA copy numbers in sera were measured by using a quantitative real-time RT-PCR-based TaqMan system (Applied Biosystems, Foster City, CA). The RNA was subjected to reverse transcription and amplification using a TaqMan One-Step RT-PCR Master Mix Reagents Kit (Applied Biosystems, Foster City, CA) with DENV-2 consensus primers (forward, 5′AAGGTGAGATGAAGCTGTAGTCTC-3′, and reverse, 5′CATTCCATTTTCTGGCGTTCT-3′) and DENV-2 consensus TaqMan probe (6FAM-5′CTGTCTCCTCAGCATCATTCCAGGCA-3′-TAMRA). Probed products were quantitatively monitored by their fluorescence intensity with the ABI 7300 Real-Time PCR system (Applied Biosystems). DENV-2 viral RNA was used as control RNA for quantification. Viral RNA in sera was calculated based on the standard curve of control RNA. All assays were carried out in triplicate.
Detection of DENV specific T-cell responses
Spleens from DENV-2-infected mice were surgically removed at different time-points and single cell suspensions were prepared. Approximately 0·5 × 106 to 1 × 106 spleen cells were incubated with either 10 μg/ml of the indicated peptide, RPMI-1640 medium (Gibco) or PMA (0·1 μg/ml) + ionomycin (1 μg/ml). Golgi plug (BD Biosciences, San Jose, CA) was added to each of the above samples and incubated at 37° for 6 hr. Cells were washed with FACS buffer, blocked with Fc block (2.4G2) for 10 min and then surface stained with peridinin chlorophyll protein-Cy5.5-hCD45 (clone 2D1), phycoerythrin-Cy7-mCD45 (clone 30-F11), FITC-hCD3 (clone UCHT1), phycoerythrin-hCD8 (clone H1T8a) and Pacific Blue-hCD4 (clone RPAT4) antibodies for 20 min at room temperature. Cells were washed with FACS buffer, then permeabilized using Cytofix/Cytoperm buffer (BD Biosciences) and stained with allophycocyanin-hIFN-γ (clone B27) and Alexa700-TNF-α for 20 min at room temperature. In all experiments the viability marker LIVE/DEAD® Aqua (Molecular Probes, Eugene, OR) was added to exclude dead cells. All cell preparations were fixed with Cytofix (BD Biosciences). Cytokine levels were also assessed by ELISA; 0·5 × 106 to 1 × 106 spleen cells from DENV-2-infected mice were incubated with either 10 μg/ml of the indicated peptide, RPMI-1640 medium (Gibco) or PMA (0·1 μg/ml) + ionomycin (1 μg/ml) and incubated at 37° for 96 hr. Culture supernatants were collected and IFN-γ level was determined by IFN-γ ELISA (R&D Systems, Minneapolis, MN).
Detection of DENV-specific antibody responses
Levels of DENV-2 envelope (E) protein-specific antibody in the serum of DENV-infected engrafted, uninfected engrafted and non-engrafted mice were determined using a standard ELISA. Ninety-six-well microplates were coated overnight with 100 ng/well of DENV-2 E protein (Hawaii Biotech, Aiea, HI) or 1 : 40 dilution of DENV-2-infected Vero cell lysate. The plates were blocked with 1% bovine serum albumin for 90 min and a 1 : 20 dilution of sera diluted with PBS was added to the wells for 1 hr. Plates were washed with PBS containing 0·1% Tween-20. Horseradish peroxidase-labelled goat anti-human IgM or IgG (Bethyl Laboratories Inc., Montgomery, TX) was added as the secondary antibody. TMB Solution (Sigma-Aldrich Inc., St Louis, MO) was used as the substrate. The enzyme reaction was stopped by addition of 1 m HCl and the plates were read at 450 nm. Positive controls included sera from a known DENV-positive human. Sera from uninfected engrafted and non-engrafted mice were used as negative controls. All assays were carried out in duplicate or triplicate.
FACS-based neutralization assay
Splenocytes from DENV-2 S16803-infected or naive mice were stimulated in vitro with CpG (2·5 μg/ml) + interleukin-2 (1 μg/ml) and Epstein–Barr virus (50 μl/ml). Supernatants of stimulated splenocytes collected 14 days after in vitro stimulation were tested for DENV-specific IgM antibodies and for DENV neutralization activity. Vero cells were seeded in 48-well plates at a density of 0·5 × 105 cells per well and incubated overnight. The culture supernatants were serially diluted in minimal essential medium containing 1% bovine serum albumin supplemented with penicillin and streptomycin. DENV-2 was added to the diluted supernatant and incubated at 4° for 1 hr. The virus and supernatant mixture was added to the Vero cells to achieve a multiplicity of infection of 0·2. Each dilution was assayed in duplicate. The plates were incubated at 37° in 5% CO2 for 1 hr. One millilitre of minimum essential medium containing 5% fetal bovine serum was added to each well, and the plates were incubated at 37° in 5% CO2 for 24 hr. Each well was washed with 1 ml PBS. Plates were incubated with 0·2 ml trypsin/well at 37° for 5 min and washed with1 ml PBS containing 10% fetal bovine serum. The cells were pipetted to break up any clumps and centrifuged at 1000 g for 5 min. Cells were permeabilized using Cytofix/Cytoperm and stained with a 1 : 100 dilution of DENV-specific antibody 2H2 (Millipore, Billerica, MA) followed by 1 : 200 dilution of FITC-conjugated anti-mouse IgG as a secondary antibody (Sigma). Approximately 20 000 cells were analysed for each sample. The per cent neutralization in the number of infected cells was calculated for each dilution using the formula: 100 – [(Frequency of infected cells in the presence of antibody × 100)/Frequency of infected cells in the absence of antibody].
Statistical analysis
All statistical calculations were performed using graph pad prism version 5 (Graph Pad software, La Jolla, CA). Mann–Whitney U-tests (two-tailed) were performed to determine statistically significant differences between median values of each data set. P-values < 0.05 were considered significant.
Results
Human cell engraftment in BLT-NSG mice
The BLT-NSG mice were implanted with HLA-A2-positive or -negative human fetal thymus and liver under the kidney capsule. CD34+ cells isolated from autologous fetal liver were injected intravenously as a source of HSC. BLT-NSG mice were validated for levels of human haematopoietic engraftment at 12 weeks by flow cytometry of peripheral blood, spleen and bone marrow as described previously.14 We found that BLT-NSG mice had high-level engraftment of multiple human T-cell and B-cell populations in their bone marrow and spleen, which was superior to reconstitution in cord blood-engrafted mice (Fig. 1). The total percentages of human CD45+ ranged between 13 and 75% (median 50%, n = 16) in the spleen and 16–84% (median 53%, n = 16) in the bone marrow (Fig. 1b). Similarly high percentages of human CD45+ CD3+ T cells and CD19+ CD20+ human B cells were detected in the periphery of the BLT-NSG mice (Fig. 1c,d).
Figure 1.
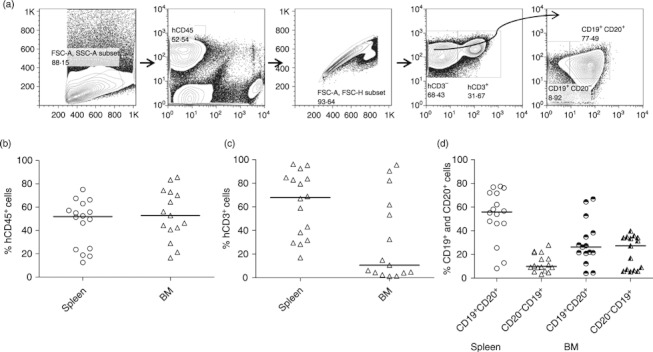
Human haematolymphoid cells in organs of engrafted BLT-NSG mice. (a) Approximately 3 months after engraftment of BLT-NSG mice with human fetal liver and thymus tissue, engraftment was assessed in target organs using flow cytometry. Initial gating strategy to identify cells in the lymphocyte gate was based on forward and side scatter profiles. Human CD45+ cells were next selected using antibodies directed against mouse and human CD45. Viable hCD45+ cells were gated by exclusion of the viability marker LIVE DEAD AQUA. T cells were next selected for by identifying CD3+ cells within the lymphocyte gate. B cells were identified using the phenotypic markers CD19 and CD20 on the CD3-negative population within the lymphocyte gate. Values on the y-axis represent frequencies of (b) hCD45+, (c) hCD3+ and (d) hCD19+ CD20+ and CD19+ CD20− cells within the lymphocyte gate in the spleen (n = 16) and all cells in the bone marrow (n = 16).
Assessment of viral loads after in vivo infection
To determine whether BLT-NSG mice could be infected with DENV, immunization was carried out with laboratory and vaccine strains of DENV-2 by the subcutaneous route. We monitored infected mice for signs of illness. More than 50% of mice experienced weight loss by day 13 and had ruffled fur and hunched back posture, suggesting that BLT-NSG mice exhibited clinical signs of DENV infection. We assessed the presence of DENV-2 in the sera by RT-PCR. Viral RNA was detected in the sera of 19/35 mice 7 days after infection with DENV-2 NGC or DENV-2 S16803. By quantitative PCR assay with a detection limit of 1000 copies per reaction, viral titres detected in the sera of DENV-2 S16803 infected mice ranged from 1·2 × 104 to 5·7 × 107/μg of RNA at day 7 post-infection (Table 1). In mice infected with 108 PFU DENV-2 S16803 the titre peaked by day 14 and no viral RNA was detected by day 35 in any mice tested (data not shown).
Table 1.
Detection of dengue virus serotype 2 (DENV-2) in infected BLT mice
| Strain | Day post infection | Viral dose | Serum | Copies based on quantitative PCR in serum |
|---|---|---|---|---|
| NGC | 7 | 106 | 10/23 | Not done |
| S16803 | 7 | 106 | 2/3 | 5·8 × 104 to 5·7 × 107 copies/μg RNA |
| S16803 | 7 | 107 | 2/2 | 7·5 × 104 to 2 × 105 copies/μg RNA |
| S16803 | 7 | 108 | 5/7 | 1·20 × 104 to 6·5 × 106 copies/μg of RNA |
BLT-NSG mice were inoculated with the indicated doses of DENV-2NGC or DENV-2 S16803 by the subcutaneous route. Mice were bled or killed at day 7. RNA was isolated from serum and subjected to one-step reverse transcription (RT-) PCR and quantitative PCR as described in the Materials and methods. Values represent number of mice that were positive by PCR and copy number of viral RNA present in serum detected by quantitative RT-PCR.
DENV-specific T-cell responses in BLT-NSG mice
We next determined whether DENV-infected BLT-NSG mice generated antigen-specific T-cell responses. Seven days after infection, splenocytes from infected mice were collected and stimulated with overlapping peptide pools (14 peptide pools containing 511 peptides; BEI Resources, Manassas, VA) that spanned the entire DENV-2 genome to measure cytokine responses in an intracellular cytokine staining assay (Fig. 2a). T cells that develop in engrafted BLT-NSG mice have the potential to be restricted by multiple HLA alleles because they are educated on autologous thymus. Therefore experiments were performed to examine total antigen-specific T-cell responses regardless of HLA-restriction. We found that splenocytes from acutely infected mice responded to multiple peptide pools by producing IFN-γ. Five peptide pools, containing peptides from the NS2B, NS3, NS4A and NS4B proteins, significantly stimulated human CD8+ T cells from DENV-infected BLT-NSG mice to produce IFN-γ. To evaluate memory T-cell responses, DENV-2-immunized BLT-NSG mice were re-infected with DENV-2 NGC 2 months after primary infection. Seven days after a second immunization we assessed IFN-γ levels in supernatants of peptide-stimulated spleen cells by ELISA. Our results indicate that peptide pools NS2B and NS5 pool 2 (P = 0·06) stimulated T cells to secrete IFN-γ (Fig. 2b).
Figure 2.
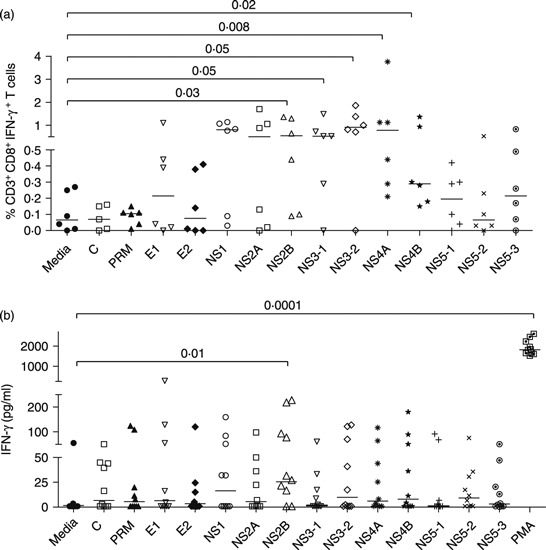
Human T-cell responses in BLT-NSG mice. (a) Interferon-γ (IFN-γ) response after primary subcutaneous infection with 1 × 106 plaque-forming units (PFU) of dengue virus serotype 2 (DENV-2) NGC. Splenocytes from BLT-NSG mice (day 7 post-infection) were stimulated with overlapping peptide pools spanning the entire DENV-2 genome (BEI Resources). Cytokine production was assessed by intracellular cytokine staining. A representative graph indicates the frequency of hCD45+ CD3+ CD8+ IFN-γ-producing T cells. (b) IFN-γ levels in supernatants of splenocytes obtained after second immunization with 1 × 106 PFU of DENV-2 NGC. Splenocytes from DENV-2-infected mice were stimulated with DENV-2 peptide pools for 5 days. Culture supernatants were assessed for human IFN-γ by ELISA (Quantikine; R&D Systems).
To determine whether CD8 T cells in BLT-NSG mice could respond to HLA-A2-restricted DENV epitopes previously identified in humans, we selected mice that were engrafted with HLA A2+ tissues. We assessed IFN-γ responses in splenocytes from BLT-NSG A2+ mice stimulated with three HLA-A2-restricted peptides NS4B2353, NS4B2423 and NS4A2148 identified in our laboratory.22 We detected elevated frequencies of CD8+ T cells that responded to ex vivo stimulation with all three peptides by secreting IFN-γ (Fig. 3b) and a novel epitope on NS52582–2598 that was identified in screening assays by deconvoluting the NS5 pool. There were no significant differences between the frequencies of CD8 T cells that responded to HLA-A2-restricted peptides in BLT NSG A2 mice used in this study and the frequencies detected in cord-blood-engrafted NSG-A2 mice in our previous study.14 The frequencies of CD8 T cells that responded to the HLA-A2-restricted peptides in BLT-NSG mice engrafted with A2-negative tissues were low (0·09% NS4B2423, 0·04% NS4B2353 and 0·02% NS4A2148; n = 3). We also found that splenocytes obtained from BLT-NSG mice 1 week after a second immunization with DENV-2 produced IFN-γ in response to these HLA-A2-restricted peptides (Fig. 3c). The results were obtained in two independent groups of BLT-NSG mice engrafted with HLA-A2+ thymus and liver. Together our data indicate that T cells obtained from BLT-NSG mice during acute infection and in the memory phase secrete cytokines in response to stimulation with multiple DENV peptide pools as well as known HLA-A2-restricted DENV peptides.
Figure 3.
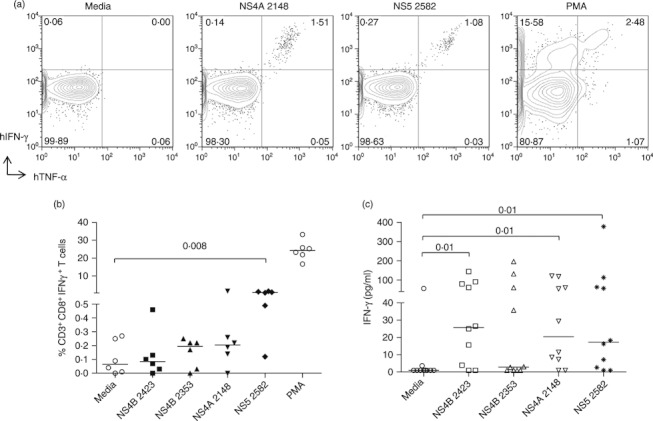
HLA-A2-restricted human T-cell responses in HLA-A2+ BLT-NSG mice. (a) Representative dot plots indicating the frequencies of interferon-γ-positive, tumour necrosis factor-α-positive (IFN-γ+ TNF-α+) T cells by intracellular cytokine staining of splenocytes from BLT-NSG mice infected by the subcutaneous route with 1 × 106 plaque-forming units (PFU) dengue virus serotype 2 (DENV-2) NGC. Splenocytes were stimulated with 10 μg/ml of NS4A2148, NS52582 peptides, PMA or media and cytokine production was assessed in an intracellular cytokine staining assay. (b) Frequencies of hCD45+ CD3+ CD8+ IFN-γ+ secreting T cells at day 7 post-infection after ex vivo stimulation with HLA-A2-restricted peptides NS4A2148, NS4B2353, NS4B2423, NS52582, PMA and media. (c) IFN-γ levels in supernatants of splenocytes stimulated with HLA-A2-restricted DENV-2 peptides and peptide NS52582 after a second immunization with 1 × 106 PFU DENV-2 NGC.
DENV-specific antibody responses in BLT-NSG mice
We next assessed the generation of DENV-2-specific antibodies in DENV-infected BLT-NSG mice by sandwich ELISA. Sera from DENV-2 NGC-infected BLT-NSG mice had significantly higher IgM antibody responses against the DENV-2 envelope protein compared with responses detected in HLA-A2-transgenic NSG mice engrafted with human cord blood HSC (Fig. 4a) and previously published data in HLA-A2 cord blood HSC-engrafted NSG mice.14 High IgM responses were consistently validated in the sera of mice up to 8 weeks post-infection (Fig. 4b). Little or no DENV-specific IgG was detected even 8 weeks post-infection with DENV-2 NGC (Fig. 4b). We assessed whether multiple immunizations with DENV-2 NGC would enhance antibody responses and found a modest increase in IgM antibodies in the sera of mice that were infected more than once with DENV (Fig. 4c). No IgG responses were detected in the sera of mice immunized multiple times (data not shown).
Figure 4.
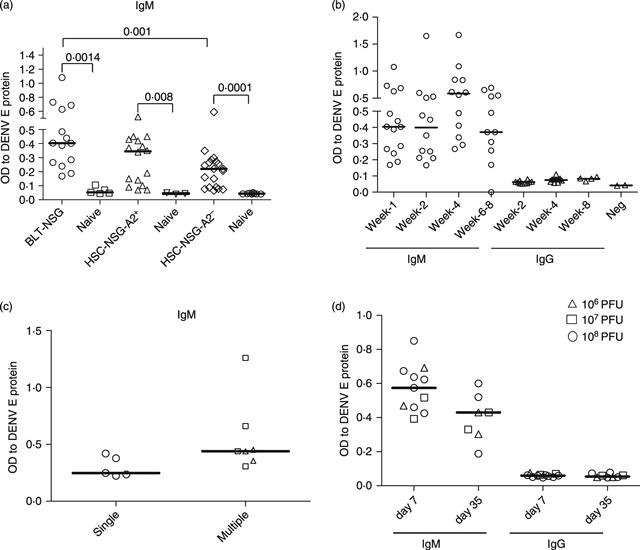
Detection of dengue virus (DENV) -specific IgM antibodies in the sera of infected engrafted BLT-NSG mice. Sera were obtained from DENV-2-infected and mock-infected mice. IgM or IgG antibodies against DENV-2 E protein (Hawaii Biotech) were detected by ELISA. (a) Comparison of IgM responses in the sera of infected BLT-NSG, NSG HLA-A2+ and NSG HLA-A2– mice 7 days after infection with 1 × 106 plaque-forming units (PFU) of DENV-2 NGC. (b) Kinetics of IgM and IgG responses in the sera of BLT-NSG mice infected with DENV-2 NGC. (c) DENV-specific IgM responses in the sera of mice immunized once (open circles), twice (open triangles) or three times (open squares) with 1 × 106 PFU of DENV-2 NGC. (d) IgM and IgG response in BLT-NSG mice infected by the subcutaneous route with 108 (open circles), 107 (open squares) or 106 (open triangles) PFU DENV-2 S16803.
To determine whether the strain and dose of DENV influenced antibody responses, we infected mice with increasing doses of DENV-2 S16803 (a live-attenuated vaccine strain) (Fig. 4d). We found similar IgM antibody responses in the sera of mice infected with DENV-2 NGC and DENV S16803. Irrespective of the inoculation dose IgM responses were similar and in all cases we detected low DENV-specific IgG responses. Our data indicate that IgM antibodies, which are neither viral strain-dependent nor dose-dependent, are the predominant isotype produced in response to dengue viral infection in BLT-NSG mice.
Specificity and neutralizing activity of antibodies from BLT-NSG mice
Experiments were conducted next to determine whether splenic B cells from BLT-NSG mice were able to secrete DENV-specific antibodies. We used culture supernatants from stimulated splenocytes as a source of DENV-specific antibodies. We were able to detect antibodies in the supernatants of immune but not naive splenocytes from BLT-NSG mice that bound an inactivated lysate of DENV-2 and the DENV-2 E protein (Fig. 5a). We next tested the neutralizing activity of DENV-2-specific antibodies generated by B cells in infected mice. We found that supernatants obtained from stimulated splenocytes of DENV-2-infected mice inhibited DENV-2 infection of Vero cells whereas supernatants obtained from stimulated naive splenocytes were unable to reduce infection (Fig. 5b). A summary of DENV-specific neutralizing activity (41–97% neutralization at 1 : 5 dilution) (n = 6) in supernatants obtained from splenocytes of infected mice is shown (Fig. 5c). Together, these data indicate that B cells generated following DENV infection of BLT-NSG mice can secrete DENV-specific antibodies that neutralize DENV infection.
Figure 5.
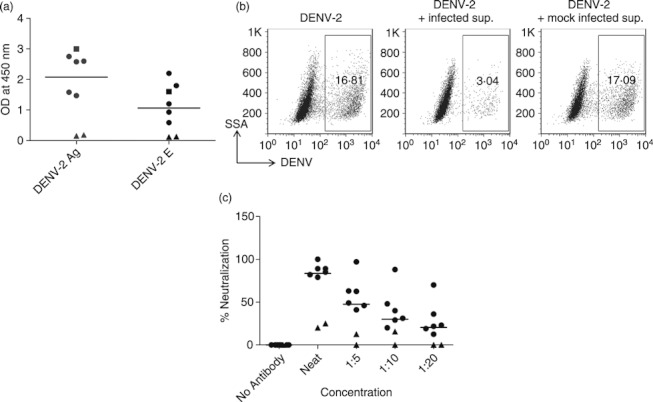
Neutralization activity of dengue virus serotype 2 (DENV-2) -specific antibodies. Splenocytes from DENV-2 S16803-infected or naive mice were stimulated with CpG + interleukin-2 and Epstein–Barr virus. Culture supernatants obtained 14 days later were used as a source of antibodies to perform ELISA and neutralization assays. (a) IgM responses to DENV-2 antigen and DENV-2 envelope protein in culture supernatants from splenocytes of naive mice (closed triangles) and infected mice [108 plaque-forming units (PFU) closed circles; 107 PFU closed squares]. (b) Representative dot plots show the frequencies of DENV-2-infected cells in the presence or absence of DENV-2-specific antibodies. (c) Percent neutralization activity of serially diluted supernatants from stimulated splenocytes obtained from BLT-NSG mice infected with 108 PFU of DENV-2 S16803 (closed circles) and naive mice (closed triangles).
Discussion
Previous immunity to DENV is a major risk factor for developing severe dengue disease in humans.23 A small reliable animal model that supports functional human innate and adaptive immune responses that will further our knowledge of protective and pathological immune responses to dengue virus is therefore clearly important. Researchers have detected measurable signs of dengue disease after infection of cord-blood-engrafted NSG mice with virulent low-passage clinical strains of DENV-2.13,16 However, robust human anti-DENV adaptive immune responses were not thoroughly assessed in those studies. We have shown DENV-specific HLA-A2-restricted T-cell function and modest antibody responses in cord blood HSC-engrafted NSG mice.14 The main objective of the current study was to determine whether we can detect improved adaptive immune responses to primary DENV infection in BLT-NSG mice. Here we show HLA-A2-restricted T-cell responses to multiple non-structural proteins in BLT-NSG mice at frequencies similar to those detected in humans. We show heightened antibody responses in BLT-NSG mice compared with cord blood HSC-engrafted mice. Furthermore, B cells maintained long-term in immunized BLT-NSG mice were able to secrete DENV-specific neutralizing antibodies. We have not assessed germinal centre formation or somatic hypermutation of immunoglobulin genes in B cells from BLT-NSG mice; therefore it is unclear whether these B cells can be considered bona fide memory B cells.
We and others have noted that levels of haematolymphoid engraftment in BLT-NSG mice are increased compared with levels in cord blood HSC-engrafted NSG mice.24–26 Humanized mice have demonstrated some evidence of human adaptive immune responses to Epstein–Barr virus infection, toxic shock syndrome toxin-1 and HIV infection.17,18,27,28 Human T cells are educated on autologous human thymic tissue in the BLT-NSG mice, so we speculated that DENV-specific T cells restricted by multiple HLA alleles expressed by the donor should develop in the mice following infection. We therefore used overlapping peptide pools that encompass the entire genome to assess the breadth, magnitude and quality of DENV-specific T-cell responses. Our results demonstrate that non-structural proteins are the predominant targets of CD8 T cells. These findings are similar to findings in humans,29–31 further validating BLT-NSG mice as an animal model that can be used to measure human T-cell responses to DENV during acute infection and in memory.
We detected elevated serum IgM responses, which persist for several weeks in DENV-infected BLT-NSG mice during acute infection. Furthermore, B cells obtained from splenocytes of BLT-NSG mice immunized several weeks earlier were able to secrete DENV-specific antibodies capable of neutralizing DENV infectivity in vitro. To our knowledge, this is the first report of neutralizing antibodies being elicited in BLT-NSG mice in response to infection with a virus. As severe dengue disease is associated with a second infection with a heterologous serotype of DENV, it would be of interest to determine the magnitude, quality, serotype specificity and enhancing activity of antibodies generated following a second infection with other serotypes and strains of DENV.
There is variability in the infection rate and immunological responses detected in BLT-NSG mice. We were unable to find a correlation between the degree of reconstitution of hCD45+ cells in the blood before infection and the ability of BLT-NSG mice to become productively infected (data not shown). Robust IgM immune responses also did not correlate with viral titres in these mice. Another limitation of the BLT-NSG model is variable and low DENV-specific IgG responses, which may reflect multiple deficiencies in this model. The inability of mouse cytokines such as B-lymphocyte-stimulating factor to signal effectively to human B cells in the xenogeneic environment may account for poor B-cell development.26,32 Generation of B-lymphocyte-stimulating factor-transgenic NSG mice is currently underway and should enhance both human B-cell and T-cell immune function in humanized mice. Human IgG concentrations in the serum are on average lower in BLT-NSG mice compared with human adults.33,34 Inadequate T-cell help and lack of human follicular dendritic cell engraftment may also contribute to ineffective class switching in these mice. Providing adequate human HLA expression as well as supporting B-cell maturation by addition of human stromal cells with follicular dendritic cell engraftment differentiation capacity should lead to improved humoral responses.
We have begun to phenotype human B cells in engrafted BLT-NSG mice and speculate that poor IgG production may be related to high frequencies of immature B cells in the periphery, as has been shown by other groups.35,36 Biswas et al.37 indicate that 50% of the human B cells in the periphery, but not in the bone marrow, also express the CD5 antigen, which is found only infrequently on mature follicular B cells in humans. Immunization with recombinant viral envelope antigens (HIV-gp140 and West Nile virus envelope proteins) stimulated production of antigen-specific human antibodies to West Nile virus and HIV gp140 that were predominantly of the IgM isotype. Transgenic expression of human-specific molecules and cytokines should better recapitulate immune responses observed in immunocompetent individuals.11,24
In conclusion, we have demonstrated improved DENV-specific adaptive immune responses in BLT-NSG mice. There are still some limitations with current models and further improvement in human engraftment and DENV-specific immune responses are required before these models can be used routinely and reproducibly in vaccine development. Given the potential interest that humanized mice represent, this should reinforce the efforts being undertaken to optimize the humoral immune response in these models. The data presented set the stage for investigating both host-specific and virus-specific mechanisms that control primary and sequential DENV infections. Previous immunity is a major risk factor for dengue haemorrhagic fever, so these mice could potentially be used to study the role of cross-reactive sub-neutralizing antibodies and T cells during sequential DENV infections as well as to test drugs and vaccines against dengue. Increased understanding of the contribution of host components to severe dengue disease will lead to the development of effective therapeutics and vaccines.
Acknowledgments
We thank Dr Alan L. Rothman for carefully reading this manuscript and Kim West for technical assistance. This project was supported by grant U19 AI57319 and U19 AI057234 from the National Institute of Allergy and Infectious Diseases, a grant from the Juvenile Diabetes Research Foundation and the Helmsley Foundation, National Institutes of Health (NIH) grant CA34196, an NIH Diabetes Endocrinology Research Center (DERC) grant DK52530 and support from USAMRID.
Glossary
- BLT-NSG
humanized bone marrow/liver/thymus NOD-scid IL2rγnull mice
- DENV
dengue virus
- HSC
cord blood haematopoietic stem cell
- IFN-γ
interferon-γ
- NSG
NOD-scid IL2rγnull mice
- PFU
plaque-forming units
- RT-PCR
reverse transcription-PCR
- TNF-α
tumour necrosis factor-α
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ, Clark GG. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis. 1995;1:55. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of pre-existing dengue virus (DV) neutralizing antibody levels to viremia and disease severity in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 4.Laoprasopwattana K, Libraty DH, Endy TP, et al. Antibody dependent cellular cytotoxicity in pre-secondary dengue virus serotype 3 (DV3) but not in DV2 infection plasma samples inversely correlated with viremia levels. J Infect Dis. 2007;195:1108–16. doi: 10.1086/512860. [DOI] [PubMed] [Google Scholar]
- 5.Libraty DH, Acosta LP, Tallo V, et al. A prospective nested case–control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libraty DH, Endy TP, Houng HH, et al. Differing influences of viral burden and immune activation on disease severity in secondary dengue 3 virus infections. J Infect Dis. 2002;185:1213–21. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 7.Chau TN, Hieu NT, Anders KL, et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J Infect Dis. 2009;200:1893–900. doi: 10.1086/648407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dung NT, Duyen HT, Thuy NT, et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J Immunol. 2010;184:7281–7. doi: 10.4049/jimmunol.0903262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khor CC, Chau TN, Pang J, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet. 2011;43:1139–41. doi: 10.1038/ng.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2010;85:410–21. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 12.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 13.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–9. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rγnull mice. PLoS One. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2–/–γ(c)–/– (RAG-hu) mice. Virology. 2007;369:143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009;83:8638–45. doi: 10.1128/JVI.00581-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 18.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–21. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denton PW, Olesen R, Choudhary SK, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–4. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149–65. doi: 10.1007/978-3-540-75647-7_10. [DOI] [PubMed] [Google Scholar]
- 21.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashyam HS, Green S, Rothman AL. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol. 2006;176:2817–24. doi: 10.4049/jimmunol.176.5.2817. [DOI] [PubMed] [Google Scholar]
- 23.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–13. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 24.Brehm MA, Cuthbert A, Yang C, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγ(null) mutation. Clinical Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–5. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe Y, Takahashi T, Okajima A, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γc(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–58. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 27.Becker PD, Legrand N, van Geelen CM, et al. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS One. 2010;5:e13137. doi: 10.1371/journal.pone.0013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yajima M, Imadome K, Nakagawa A, et al. A new humanized mouse model of Epstein–Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. 2008;198:673–82. doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- 29.Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Nat Acad Sci USA. 2010;107:16922–7. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew A, Kurane I, Green S, et al. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J Virol. 1998;72:3999–4004. doi: 10.1128/jvi.72.5.3999-4004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew A, Kurane I, Rothman AL, Zeng LL, Brinton MA, Ennis FA. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J Clin Invest. 1996;98:1684–94. doi: 10.1172/JCI118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traggiai E, Becker S, Subbarao K, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–5. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baenziger S, Tussiwand R, Schlaepfer E, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2–/–γc–/– mice. Proc Nat Acad Sci USA. 2006;103:15951–6. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ-chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas S, Chang H, Sarkis PT, Fikrig E, Zhu Q, Marasco WA. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5+ B cells. Immunology. 2011;134:419–33. doi: 10.1111/j.1365-2567.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


