Abstract
OBJECTIVES
The Cystic Fibrosis Foundation recently deemed the use of extended-interval dosing (EID) of aminoglycosides acceptable for the treatment of cystic fibrosis (CF) pulmonary exacerbations, but current practices across United States (US) pediatric CF accredited care centers and affiliate programs are unknown. The objectives of this research are to characterize the practice trends, dosing strategies, therapeutic drug monitoring practices, and adverse drug reaction monitoring of EID of aminoglycosides in the treatment of pulmonary exacerbations across US pediatric CF programs.
METHODS
A 38-question online survey was distributed on behalf of the author by the CF Foundation to all US pediatric CF accredited care centers and affiliate programs.
RESULTS
Of the 70 participating CF programs (42.2% survey response rate), 94.3% reported using EID of aminoglycosides (as once-daily or twice-daily dosing), whereas 84.3% reported using once-daily EID in their pediatric CF population. The frequency of EID use increased with patient age. Tobramycin dosed 10 mg/kg per day every 24 hours, infused over the course of 30 minutes, in combination with an antipseudomonal beta-lactam, was the most commonly cited regimen. Monitoring of aminoglycoside serum concentrations was reported by 98.5% of programs, with a tobramycin peak of 25 to 30 mg/L and trough of less than 1 mg/L targeted most frequently. Nephrotoxicity was commonly monitored through serum creatinine measurements, whereas ototoxicity was monitored by audiometry in approximately one-half of programs.
CONCLUSIONS
This study indicates that the use of EID of aminoglycosides across US pediatric CF accredited care centers and affiliate programs is common, particularly among adolescents, with tobramycin being the preferred agent.
INDEX TERMS: aminoglycoside, cystic fibrosis, extended-interval dosing, pediatric
INTRODUCTION
Extended-interval dosing (EID) of aminoglycosides enhances bactericidal activity by increasing the peak serum concentration to minimum inhibitory concentration ratio.1 This dosing method traditionally combines the total daily dose into a single dose that is administered once daily. However, EID regimens using twice-daily dosing have been used in some patient populations.1
EID of aminoglycosides has been evaluated in both children and adults.2,3 Its use within health care institutions across the United States (US) increased from 19% in 1993 to 75% in 1998.4,5 Its use is now commonplace in adults, with 99.5% of institutions reporting the use of EID in those ages 19 to 65 years. In the pediatric population, its use is less common; 23.1% of institutions reported using EID to treat children and adolescents ages 1 to 18 years, whereas only 1.3% reported use in infants younger than 1 year.4 The use of EID in pediatric populations is most common in the general pediatric acute care setting.6 The frequency of EID use in pediatrics must be interpreted with caution because these data are derived from American Hospital Association institutions, which are primary adult hospitals that contain a pediatric ward or children's hospital.4
Compared with the general pediatric population, patients with cystic fibrosis (CF) generally have a relative increase in the volume of distribution and clearance of the aminoglycoside antibiotics.7 Possibly because of this altered pharmacokinetic profile, previous reports indicate the use of EID in patients with CF to be less common than in the general population.5,6,8–10 In Australia and the United Kingdom, 34% to 77% of centers use EID, with once- and twice-daily dosing being used by 17% to 54% and 17% to 23% of centers, respectively.8–10 More recent research from the United Kingdom indicates that 40% of practitioners who were not using EID prior to 2005 have since implemented this dosing strategy in their CF centers.10 In the US, 61% of CF accredited care centers and affiliated programs were using once-daily dosing of tobramycin as of January 2008.11 In light of the emergence of safety and efficacy data in the CF population, the CF Foundation deemed once-daily aminoglycoside dosing as acceptable for Pseudomonas aeruginosa (grade C recommendation) in its September 2009 pulmonary exacerbation guidelines.12–21 Although empiric evidence suggests the use of EID of aminoglycosides in the US CF population to be increasingly common, the specific age groups in which it is being prescribed and the dosing and monitoring practices associated with this regimen following publication of the CF pulmonary exacerbation guideline remain to be elicited.
The aim of this study is to characterize the practice trends, dosing strategies, therapeutic drug monitoring practices, and adverse drug reaction monitoring of EID (once-daily and twice-daily dosing) of aminoglycosides in the treatment of pulmonary exacerbations in the pediatric CF population across CF programs in the US. To do so, a survey pertaining to the use of EID in pediatric patients with CF was distributed in cooperation with the CF Foundation to all US pediatric CF accredited care centers and affiliate programs (hereafter referred to as pediatric CF programs).
MATERIALS AND METHODS
Questionnaire
The survey consisted of 38 multiple-choice and short-answer questions regarding the demographics of the pediatric CF program and the use of EID in patients with CF at that program; the clarity of question composition was validated by a pediatric pulmonologist with experience using EID in the treatment of pulmonary exacerbations associated with CF. If the CF program used EID, questions were asked regarding which aminoglycoside(s) is/are used, the dose and dosing interval used, the method(s) of administration, the method(s) for therapeutic drug monitoring, target serum concentrations, and the method(s) of monitoring for toxicity. The survey tool was approved by the Social and Behavioral Sciences Institutional Review Board at the University at Buffalo on December 2, 2009.
Data Collection
All pediatric CF accredited care centers and affiliate programs in the US were eligible for inclusion. Using the CF Foundation database, 166 pediatric CF programs were identified. Although the survey was sent to both pediatric and adult CF accredited care centers and affiliate programs, the current publication only presents data from surveys obtained from pediatric programs. Survey Monkey (Portland, OR) was used to distribute, collect, and analyze survey responses. The CF Foundation distributed an electronic hyperlink to the survey to all medical directors and clinical coordinators at CF programs in the US on December 17, 2009. Although the survey was directed to CF center medical directors and clinical coordinators, the responsibility for completing the survey could be delegated to a knowledgeable representative (i.e., a CF center physician, CF center nurse or nurse practitioner, CF center physician assistant, or CF center pharmacist). To optimize survey response rate, a reminder e-mail was distributed by the CF Foundation on February 4, 2010. The survey remained open for 3 months, and was closed on March 17, 2010.
Data Analysis
Surveys and responses were numerically coded and entered into the computer database using Microsoft Office Excel. To generalize the results, the response rate and distribution of responses were determined. Demographic data were grouped into 9 divisions based on geographic location as defined by the US Census Bureau (http://www.census.gov/geo/www/us_regdiv.pdf). Frequency and descriptive statistics were used to characterize the population and survey responses. The practice trends of EID use in the treatment of CF pulmonary exacerbations across pediatric CF programs in the US were characterized using 95% confidence intervals (95% CIs).
RESULTS
Demographic Characteristics
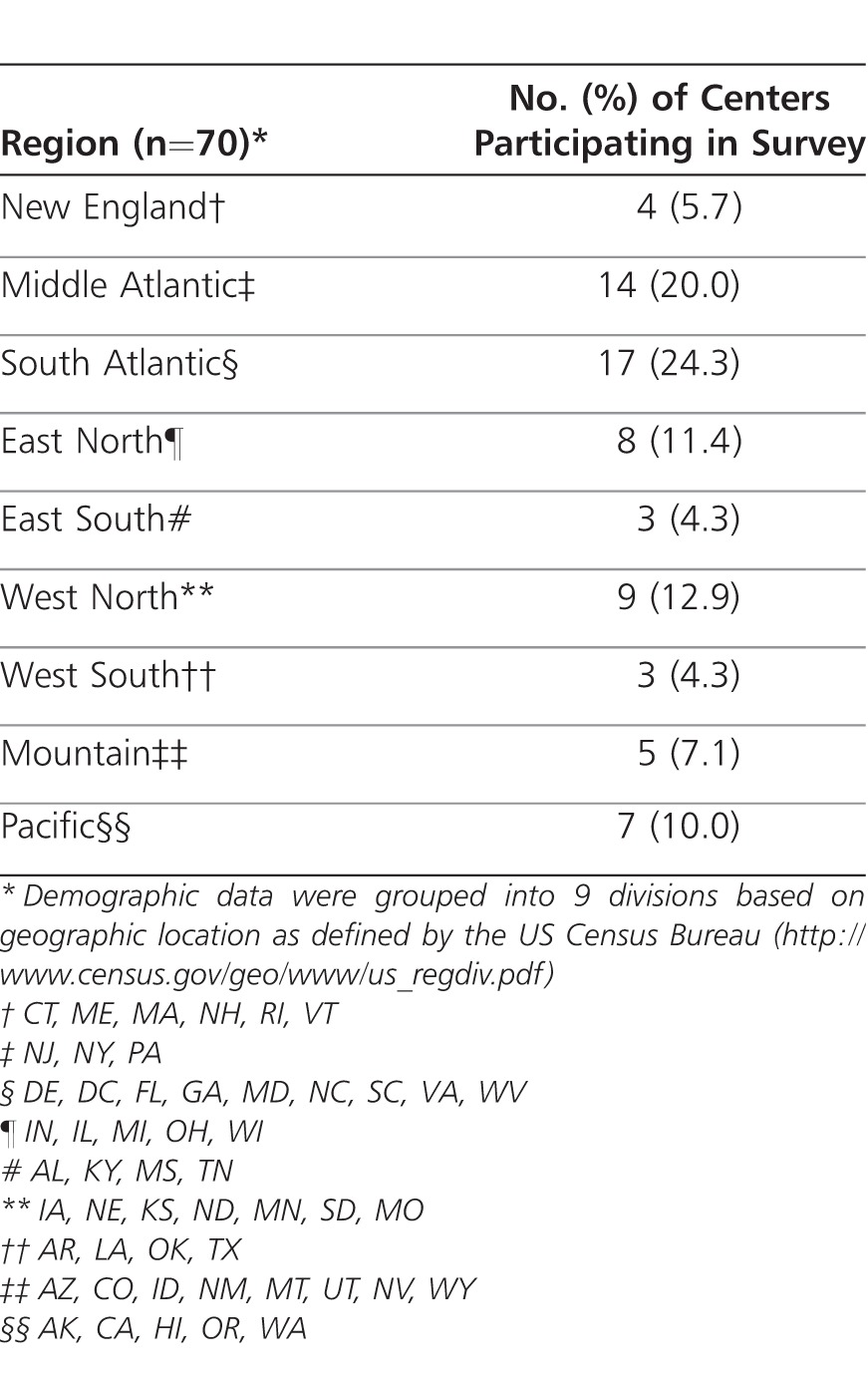
Responses were received from 70 pediatric CF programs, representing a survey response rate of 42.2%. The New England, Middle Atlantic, South Atlantic, East North, and East South regions accounted for 65.7% of responding programs, whereas the West North, West South, Mountain, and Pacific regions accounted for 34.3% of responding programs (Table 1). The survey was completed by 41 CF center medical directors (58.6%), 3 clinical coordinators (4.3%), 4 CF center physicians (5.7%), 5 CF center nurse or nurse practitioners (7.1%), 9 CF center pharmacists (12.9%), and a combination of the above in 8 CF programs (11.4%).
Table 1.
Demographics of Cystic Fibrosis Foundation Accredited Pediatric Care Centers and Affiliate Programs
EID Use in Pediatric CF
A total of 66 of the 70 participating pediatric CF programs (94.3%; 95% CI, 88.9%-99.7%) reported the use of EID (as once-daily or twice-daily dosing) in their pediatric population, whereas 59 of the 70 participating pediatric CF programs (84.3%; 95% CI, 75.8%-92.8%) reported the use of once-daily EID. The frequency of use was classified as occasional by 13 programs (19.7%), frequent by 27 (40.9%), always by 25 (37.9%), and was not reported by 1 (1.5%). Among the 66 pediatric CF programs using EID, 7 (10.6%) used this regimen in neonates younger than 1 month, 21 (31.8%) in infants ages 1 month to 1 year, 39 (59.1%) in young children ages 1 to 5 years, 59 (89.4%) in older children ages 6 to 12 years, and 64 (97.0%) in adolescents ages 13 to 17 years. The most commonly used aminoglycoside was tobramycin (65 of 66 [98.5%]). In contrast, amikacin and gentamicin were used by 28 of 66 (42.4%) and 17 of 66 (25.8%) pediatric CF programs, respectively. One program reported using amikacin but did not indicate if it did or did not use tobramycin or gentamicin, and was therefore assumed to not use either aminoglycoside. A total of 63 of 66 pediatric CF programs (95.5%) used EID in combination with an antipseudomonal beta-lactam, whereas 1 pediatric CF program (1.5%) used EID in combination with ciprofloxacin. Two programs did not respond and were assumed to not use EID in combination with an antipseudomonal beta-lactam or ciprofloxacin.
EID Dosing in CF
Among patients naive to an aminoglycoside (i.e., patients who had not been treated previously and in whom the dosage could not be individualized based on previous therapeutic drug monitoring), empiric dosages of 10 mg/kg per day of tobramycin, 30 mg/kg per day of amikacin, and 10 mg/kg per day of gentamicin were used (Table 2). Among patients treated with an aminoglycoside previously and in whom the dosage could be based on prior therapeutic drug monitoring, 28 of 65 pediatric CF programs (43.1%) individualized dosing in patients receiving tobramycin, 8 of 28 programs (28.6%) did this in patients receiving amikacin, and 5 of 17 programs (29.4%) did this in patients given gentamicin. Regardless of the availability of prior pharmacokinetic data, the remainder deferred to empiric dosing.
Table 2.
Dosing of Extended-Interval Aminoglycosides Across Cystic Fibrosis Foundation Accredited Pediatric Care Centers and Affiliate Programs
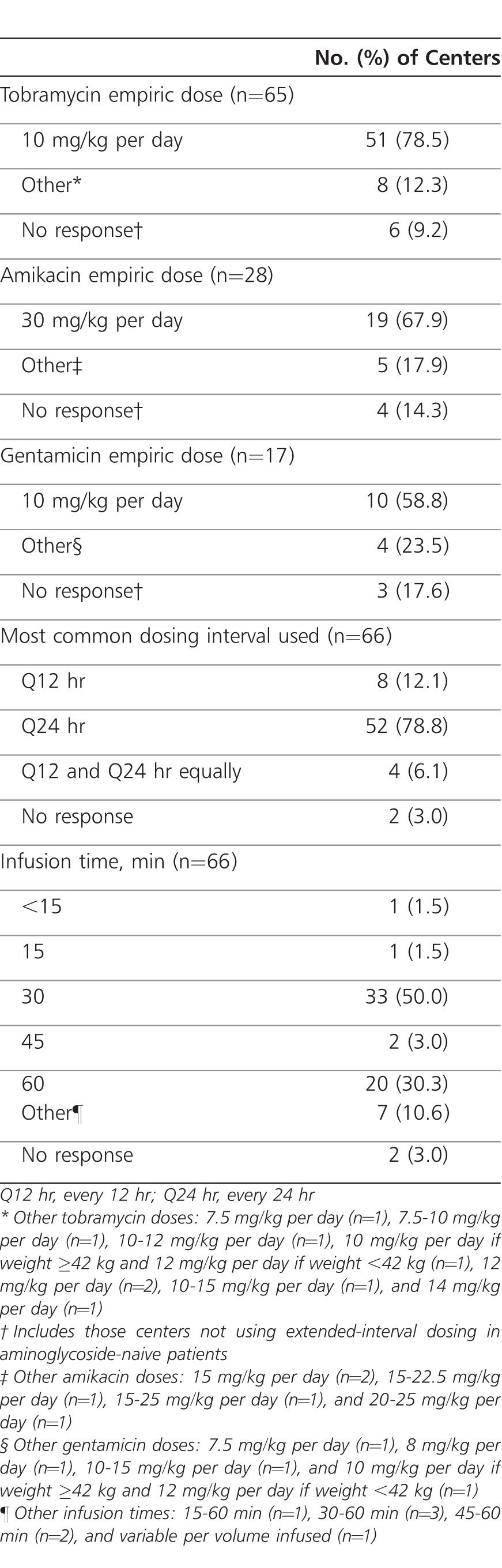
EID intervals of 12 and 24 hours were used by 20 of 66 (30.3%) and 59 of 66 (89.4%) pediatric CF programs, respectively. An interval of 24 hours was identified as most common by 78.8% of pediatric CF programs (Table 2). When tobramycin, the most commonly used aminoglycoside, was dosed twice daily, 13 of 20 pediatric CF programs (65.0%) used an empiric dosage of 10 mg/kg per day, whereas the remaining programs used dosages ranging from 7.5 to 14 mg/kg per day. Regardless of dosage, an infusion time of 30 or 60 minutes was reported by 53 of 66 pediatric CF programs (80.3%; Table 2).
Therapeutic Drug Monitoring of EID in CF
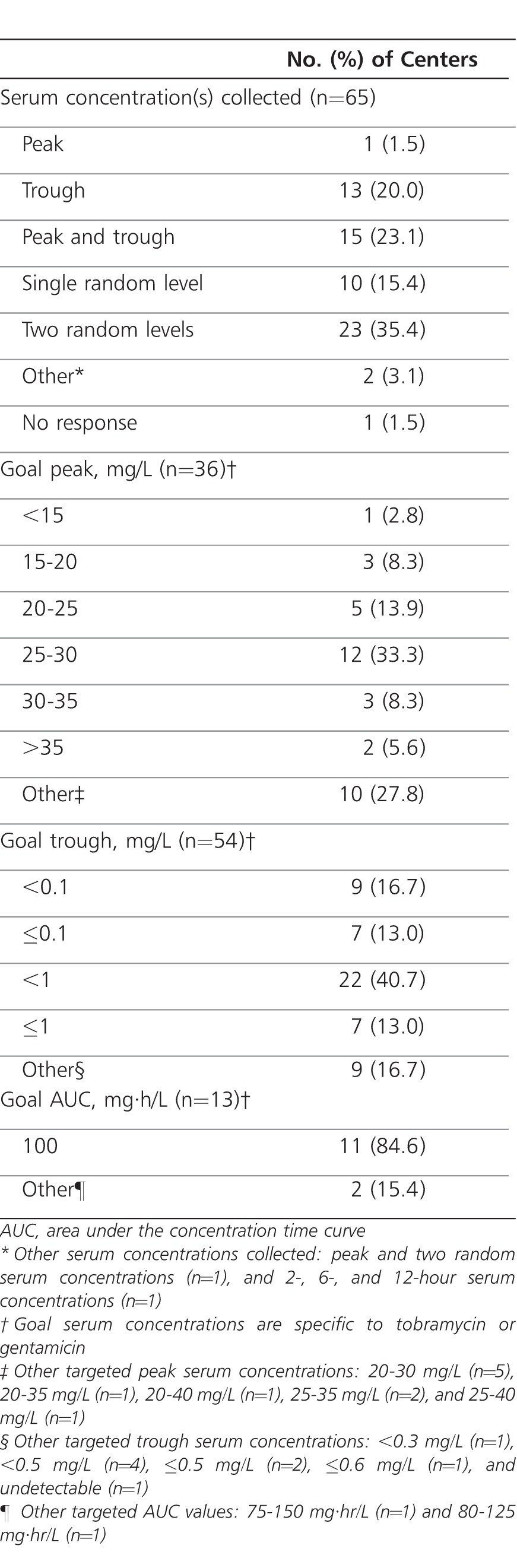
Therapeutic drug monitoring of aminoglycoside serum concentrations for patients receiving EID was reported by 65 of 66 pediatric CF programs (98.5%; one program did not respond and was assumed to not monitor serum concentrations). The methods of monitoring varied widely among pediatric CF programs (Table 3). Obtaining two random aminoglycoside serum concentrations was the most frequently reported method, with the range of the first and second serum concentrations being 2 to 5 hours after dose and 6 to 18 hours after dose, respectively. The most frequent target serum concentrations (for tobramycin or gentamicin) were a peak of 25 to 30 mg/L and a trough of <1 mg/L, respectively. Area under the concentration time curve (AUC) was infrequently used for therapeutic drug monitoring (13 of 65 [20.0%]) (Table 3). A dosing nomogram was employed to guide therapeutic drug monitoring by 13 of 65 pediatric CF programs (20.0%). Details regarding the specific nomogram were not specified by 11 of 13 pediatric CF programs (84.6%). Among the two programs that provided details, an “institution-specific nomogram” and a “published nomogram” validated for tobramycin 12 mg/kg per day were used.22 Aminoglycoside serum concentrations were repeated by 58 of 65 pediatric CF programs (89.2%), with monitoring occurring every 3 days in 3 of 58 (5.2%), every 7 days in 31 of 58 (53.4%), and every 14 days in 1 of 58 (1.7%) programs. Some programs monitored on an as-needed basis using clinical judgment (9 of 58 [15.5%]); others monitored every 7 days or per clinical judgment (3 of 58 [5.2%]), or by another manner (11 of 58 [19.0%]).
Table 3.
Therapeutic Drug Monitoring Practices With Extended-Interval Aminoglycoside Dosing Across Cystic Fibrosis Foundation Accredited Pediatric Care Centers and Affiliate Programs
Safety Monitoring Parameters with EID in CF
Monitoring for nephrotoxicity was performed using serum creatinine, as a marker of renal function, in 64 of 66 pediatric CF programs (97.0%), whereas serum electrolytes, urine output, and urinalysis were monitored by 26 of 66 (39.4%), 21 of 66 (31.8%) and 15 of 66 (22.7%) programs, respectively. Among pediatric CF programs measuring serum creatinine, 8 of 64 (12.5%), 32 of 64 (50.0%), and 2 of 64 (3.1%) indicated monitoring this parameter every 3, 7, and 14 days, respectively, whereas 8 of 64 (12.5%) monitored serum creatinine based on clinical judgment alone. Among pediatric CF programs monitoring for ototoxicity, 30 of 66 programs (45.5%) and 21 of 66 (31.8%) programs reported using high- and low-frequency audiometry, respectively, whereas 6 of 66 programs (9.1%) reported monitoring for vestibular toxicity. Ototoxicity was not monitored for by 26 of 66 pediatric CF programs (39.4%).
DISCUSSION
The current survey of aminoglycoside EID practices across pediatric CF programs in the US is the first to investigate the use of this regimen specifically in the pediatric CF population, and it is the first to assess EID practices (both once- and twice-daily dosing) with tobramycin, gentamicin, and amikacin in those with CF. Furthermore, the current study was conducted after the publication of the CF Foundation pulmonary exacerbation guideline, and therefore reflects current practices of EID across US pediatric CF programs. Demographic data indicate that two-thirds of the responding CF programs were geographically located in the Eastern US, which is similar to the distribution of programs across the country. More than 75% of respondents were CF center medical directors, CF center physicians, or CF center pharmacists (those practitioners most directly involved in medication therapy management of patients with CF).
The data presented indicate that EID is now commonplace in the US pediatric CF population. Most pediatric CF programs (94%) use EID (as once-daily or twice-daily dosing) in the treatment of pulmonary exacerbations, and 84% of pediatric CF programs prescribe once-daily EID. That noted, the percent of pediatric CF programs reporting the use of once-daily EID may better reflect current EID practices because a twice-daily dosing approach may not have been considered to be EID by all respondents. Comparing these data with US adult/pediatric CF program data collected November 2007 to January 2008 (prior to the release of the CF Foundation pulmonary exacerbation guideline) suggests the use of EID (dosed once daily) in those with CF is becoming increasingly common, likely reflecting the recent US CF Foundation endorsement of this regimen.11 The use of EID in pediatric patients with CF appears to be age dependent, with its use being more common among the older age groups; EID use in adolescents appeared to be commonplace, whereas its use was less frequent among children and was least common among neonates and infants (despite potential overreporting in the neonate population as a result of the common misconception that standard doses given once daily are EID). This is not unexpected, given the age groups (mean age, 11.4 to 23 years; age range, 5.1 to 50.4 years) in which safety and efficacy studies have been conducted to date and the fact that P aeruginosa colonization is more frequent among older children and adolescents.13,14,16,17,19,21,23
Tobramycin is the most commonly used aminoglycoside for EID in pediatric patients with CF, whereas amikacin and gentamicin are used less frequently. This likely reflects the availability of well-designed safety and efficacy studies and meta-analyses evaluating tobramycin EID in this population, and the lack of published research studies assessing the use of gentamicin and amikacin EID in those with CF.13,14,16–21 That almost all CF programs used EID in combination with an antipseudomonal beta-lactam is expected because combination antibiotic treatment is considered to be the standard of care for pulmonary exacerbations in those with CF.12 Empiric dosing of tobramycin was most commonly prescribed at 10 mg/kg per day. This practice is consistent with the dosage used in a large, randomized, controlled, noninferiority study published in 2005.17 However, among patients treated with tobramycin previously, many pediatric CF programs individualize the dose based on pharmacokinetic parameters that were available from previous therapeutic drug monitoring. An interval of 24 hours is used by most CF programs, and was identified as the most commonly used regimen. However, as has been previously reported, some CF programs are using a twice-daily dosing regimen.6 This suggests that despite well-designed short-term studies reporting noninferiority with once-daily EID in CF,17,18 there may be concerns that a 24-hour dosing interval is inadequate. To ensure the postantibiotic effect of aminoglycosides is not exceeded, efficacy is not compromised, and resistance is not increased, some CF programs may prefer a twice-daily regimen to a once-daily regimen, even though published safety and efficacy data are lacking.13
Therapeutic drug monitoring of aminoglycoside serum concentrations is common, which is expected, given the narrow therapeutic index of this class of antibiotics. However, the methods of monitoring and the targeted serum concentrations associated with EID vary widely across US pediatric CF programs. This survey indicates that pediatric CF programs most commonly obtain two random serum concentrations, with the first random concentration obtained at least 2 hours after the dose. A tobramycin peak serum concentration of 20 to 30 mg/L and a trough value of ≤1 mg/L are targeted by approximately half of pediatric CF programs. Although this is similar to those goals used by Smyth et al17 a tobramycin peak concentration of 25 to 30 mg/L and an extrapolated trough value of ≤0.1 mg/L, each used by approximately a third of programs, may actually be more appropriate targets because they better reflect the mean peak serum concentration of 28.4 mg/L and the mean trough value of 0.1 mg/L reported by Smyth et al17 Furthermore, targeting a trough value of ≤0.1 mg/L (instead of ≤1 mg/L) may minimize the risk of toxicity that may be associated with elevated AUCs over an extended treatment duration.24 It should be noted that these goals relate to once-daily EID with tobramycin or gentamicin, and would not be appropriate target concentrations for EID with amikacin or twice-daily EID with tobramycin or gentamicin. This survey indicates a dosing nomogram is used to guide therapeutic drug monitoring in 20% of pediatric CF programs. Although a once-daily EID nomogram for tobramycin 12 mg/kg per day has been validated in the pediatric CF population and was used by one responding program, this dose is infrequently used by pediatric CF programs, thus limiting its usefulness.22 Approximately 90% of pediatric CF programs repeat serum concentrations for follow-up, with most programs repeating serum concentrations once weekly and/or based on clinical judgment.
The risk of acute nephrotoxicity and ototoxicity with aminoglycoside therapy is well known. The occurrence of these toxicities does not appear to differ significantly among patients with CF who are treated with once-daily EID and traditional every-8-hour dosing, and the acute risk of nephrotoxicity may actually be lower in pediatric patients receiving once-daily EID.17,18 Surveyed pediatric CF programs most commonly monitor serum creatinine concentrations every 7 days. Monitoring for ototoxicity using diagnostic techniques such as audiometry and vestibular function testing is less common. Among pediatric CF programs monitoring for ototoxicity, the methods of doing so vary widely, the reasons for which are unclear.
Several limitations of the current study must be acknowledged. Although the response rate of 42.2% is acceptable, and should be reflective of the study population, nonresponse bias should be considered when evaluating the results. A second limitation is that although the survey was validated by a pediatric pulmonologist with experience using EID in the treatment of pediatric CF pulmonary exacerbations, and most survey responses were provided by the CF center medical director or a CF physician, the validation process did not include reviewers representative of all potential survey respondents (CF center clinical coordinator, CF center nurse or nurse practitioner, CF center physician assistant, and CF center pharmacist). Questions might have been answered differently among different members at the same program based on their role in therapeutic drug monitoring and interpretation of the questions, potentially introducing bias in data reporting. A third limitation is that EID was not specifically defined in the survey, which may have led to an interpretation that EID referred only to once-daily dosing, instead of to both once- and twice-daily dosing using the same daily doses (ie, 10 mg/kg per day divided into one or two daily doses). Therefore, the latter may not have been considered to be EID by all respondents.
In conclusion, the use of EID aminoglycoside therapy is commonplace among pediatric CF programs in the US, with its use being most common in the adolescent population. Tobramycin, dosed 10 mg/kg per day every 24 hours, infused over 30 minutes, in combination with an antipseudomonal beta-lactam, is the most commonly used regimen. Monitoring this therapeutic regimen with two random serum concentrations targeting a peak of 25 to 30 mg/L and a trough of <1 mg/L is most common, although calculating an AUC of 100 mg·hr/L is also used. Pediatric CF programs in the US currently using EID should use these data to compare and contrast their practices with those of pediatric CF programs across the country, whereas those pediatric CF programs considering the use of EID should use these data for reference when implementing EID protocols at their institution.
ACKNOWLEDGMENTS
I would like to thank Dr Bruce Marshall and his staff at the Cystic Fibrosis Foundation for distributing this survey to pediatric CF programs in the US. I would also like to thank Dr Michelle Khait for her contributions throughout this project, Dr Jack Sharp for his assistance in validating our survey tool, and Dr Alan Forrest for his assistance with statistical analysis.
ABBREVIATIONS
- AUC
area under the concentration time curve
- CF
cystic fibrosis
- EID
extended-interval dosing
- US
United States
Footnotes
DISCLOSURE The author declares no conflicts or financial interest in any products or services mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Prescott WA, Jr, Nagel JL. Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis. Pharmacotherapy. 2010;;30(1):95–108. doi: 10.1592/phco.30.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Barza M, Ioannidis JP, Cappelleri JC, et al. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;;312(7027):338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, et al. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;;114(1):e111–e118. doi: 10.1542/peds.114.1.e111. [DOI] [PubMed] [Google Scholar]
- 4.Chuck SK, Raber SR, Rodvold KA, et al. National survey of extended-interval aminoglycoside dosing. Clin Infect Dis. 2000;;30(3):433–439. doi: 10.1086/313692. [DOI] [PubMed] [Google Scholar]
- 5.Schumock GT, Raber SR, Crawford SY, et al. National survey of once-daily dosing of aminoglycoside antibiotics. Pharmacotherapy. 1995;;15(2):201–209. [PubMed] [Google Scholar]
- 6.Condren M, Luedtke SA. Prescribing patterns for extended interval aminoglycoside dosing in pediatric patients. J Pediatr Pharmacol Ther. 2001;;6(5):385–391. [Google Scholar]
- 7.Touw DJ, Vinks AATMM, Mouton JW, et al. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis: current practice and suggestions for future directions. Clin Pharmacokinet. 1998;;35(6):437–459. doi: 10.2165/00003088-199835060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Phillips JA, Bell SC. Aminoglycosides in cystic fibrosis: a descriptive study of current practice in Australia. Intern Med J. 2001;;31(1):23–26. doi: 10.1046/j.1445-5994.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 9.Tan KH, Hyman-Tylor P, Mulheran M, et al. Lack of concordance in the use and monitoring of intravenous aminoglycosides in UK cystic fibrosis centers. Pediatr Pulmonol. 2002;;33(2):165. doi: 10.1002/ppul.10036. [DOI] [PubMed] [Google Scholar]
- 10.Cheong J, Jacklin A, Hodson M. The influence of the TOPIC study on the prescribing guidelines of aminoglycosides in cystic fibrosis units. Pediatr Pulmonol. 2008;;43((S31)):363. [Google Scholar]
- 11.Van Meter DJ, Corriveau M, Ahern JW, et al. A survey of once-daily dosage tobramycin therapy in patients with cystic fibrosis. Pediatr Pulmonol. 2009;;44(4):325–329. doi: 10.1002/ppul.20985. [DOI] [PubMed] [Google Scholar]
- 12.Flume PA, Mogayzel PJ, Jr, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;;180(9):802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt O, Lehmann C, Madabushi R, et al. Once-daily tobramycin in cystic fibrosis: better for clinical outcome than thrice-daily tobramycin but more resistance development? J Antimicrob Chemother. 2006;;58(4):822–829. doi: 10.1093/jac/dkl328. [DOI] [PubMed] [Google Scholar]
- 14.Master V, Roberts GW, Coulthard KP, et al. Efficacy of once-daily tobramycin monotherapy for acute pulmonary exacerbations of cystic fibrosis: a preliminary study. Pediatr Pulmonol. 2001;;31(5):367–376. doi: 10.1002/ppul.1060. [DOI] [PubMed] [Google Scholar]
- 15.Powell SH, Thompson WL, Luthe MA, et al. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983;;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- 16.Riethmueller J, Franke P, Schroeter TW, et al. Optimized intravenous antibiotic treatment with Ceftazidime (thrice daily vs. continuous) and tobramycin (thrice vs. once daily) in CF patients. Abstracts of the 24th European Cystic Fibrosis Conference. 2001. p. P192.
- 17.Smyth A, Tan KH, Hyman-Taylor P, et al. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis--the TOPIC study: a randomised controlled trial. Lancet. 2005;;365(9459):573–578. doi: 10.1016/S0140-6736(05)17906-9. [DOI] [PubMed] [Google Scholar]
- 18.Smyth AR, Bhatt J. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2010. CD002009. [DOI] [PubMed]
- 19.Vic P, Ategbo S, Turck D, et al. Efficacy, tolerance, and pharmacokinetics of once daily tobramycin for pseudomonas exacerbations in cystic fibrosis. Arch Dis Child. 1998;;78(6):536–539. doi: 10.1136/adc.78.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vic P, Ategbo S, Turck D, et al. Tolerance, pharmacokinetics and efficacy of once daily amikacin for treatment of Pseudomonas aeruginosa pulmonary exacerbations in cystic fibrosis patients. Eur J Pediatr. 1996;;155(11):948–953. doi: 10.1007/BF02282885. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead A, Conway SP, Etherington C, et al. Once-daily tobramycin in the treatment of adult patients with cystic fibrosis. Eur Respir J. 2002;;19(2):303–309. doi: 10.1183/09031936.02.00221602. [DOI] [PubMed] [Google Scholar]
- 22.Massie J, Cranswick N. Pharmacokinetic profile of once daily intravenous tobramycin in children with cystic fibrosis. J Paediatr Child Health. 2006;;42(10):601–605. doi: 10.1111/j.1440-1754.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry, 2008 Annual Data Report to the Center Directors. Bethesda, MD: The Cystic Fibrosis Foundation; 2009. [Google Scholar]
- 24.Drusano GL, Ambrose PG, Bhavnani SM, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;;45(6):753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]


