Abstract
Neonatal diabetes mellitus (NDM) results from impaired insulin secretion. While rare, NDM presents complex challenges with regard to the management of glycemic control. NDM is classified as transient neonatal diabetes mellitus (TNDM) or permanent neonatal diabetes mellitus (PNDM). Determination of TNDM vs. PNDM is usually possible only after medical management has been initiated. Management of NDM begins with insulin; however, the correct dose, choice of formulation, and route of administration are complicated by the risk of neonatal hypoglycemia. For the first time, the successful management of TNDM in an extremely low birth weight (ELBW) neonate with the long-acting subcutaneous insulin analog, insulin glargine, is reported. In addition, potential pharmacokinetic barriers to treating ELBW neonates diagnosed with NDM with subcutaneous insulin products are discussed.
INDEX TERMS: hyperglycemia, insulin glargine, long-acting insulin, transient neonatal diabetes mellitus
INTRODUCTION
Defects in insulin secretion and beta-cell development comprise neonatal diabetes mellitus (NDM), a rare manifestation of insulin-dependent diabetes mellitus reported to occur at an incidence of 1:300,000 to 500,000 live births. NDM is broadly defined as persistent hyperglycemia within the first month of life lasting at least 2 weeks and requiring management with insulin. It typically presents with intrauterine growth retardation, volume depletion, profound hyperglycemia, glycosuria, polyuria, ketonuria, and ketoacidosis.1 NDM is subclassified into transient neonatal diabetes mellitus (TNDM) or permanent neonatal diabetes mellitus (PNDM). The diagnosis of TNDM vs. PNDM is not typically clear initially as a result of similar presenting symptoms and often requires further workup. PNDM accounts for approximately 50% of all cases of NDM and is most commonly caused by mutations in potassium channels on pancreatic β cells leading to decreased insulin secretion. TNDM accounts for the remaining half of NDM cases. Between 60% and 80% of patients with TNDM display genetic mutations, most commonly chromosome-6 abnormalities (uniparental disomy).2 The course of TNDM is highly variable, ranging from permanent resolution within the first several weeks or months of life to recurrence later in childhood. Long-term sequelae of either type of NDM include developmental delay, cardiac anomalies, seizures, poor weight gain, and recurrence of diabetes at an older age.3
CASE REPORT
A 28-week gestational age, 680-g female, monozygotic twin was born to a G1P1 mother (who had had 1 pregnancy and delivered once) via emergent caesarean section secondary to breech presentation. Oligohydramnios, abnormal placental sharing, preterm labor, and twin gestation complicated the pregnancy. The mother received 2 courses of betamethasone prior to delivery as a result of preterm labor and received enoxaparin throughout the pregnancy secondary to anti-phospholipid syndrome. Initial health issues during the immediate newborn period included intrauterine growth retardation, neonatal respiratory distress syndrome, patent ductus arteriosus, and right renal agenesis.
The patient was transferred to our institution from an outlying hospital on the sixth day of life (DOL) based on suspicion of coarctation of the aorta after abnormal echocardiogram findings. Prior to transfer, the patient was maintained on parenteral nutrition and had not received any enteral feeding. On DOL 7, trophic feeds of expressed breast milk at 6 mL/kg/day were initiated in addition to parenteral nutrition, with a total glucose infusion rate of 9 mg/kg/min (protein 2.5 g/kg/day and lipid 2.8 g/kg/day). On DOL 9, expressed breast milk was increased to 12 mL/kg/day, and parenteral dextrose calories were advanced to a total glucose infusion rate of 10 mg/kg/min.
On DOL 10, serum glucose was found to be 204 mg/dL. Glucose measurements prior to that time are depicted in the Table. Follow-up glucose measurements by point-of-care testing (Accu-chek, Roche, Indianapolis, IN) at the bedside ranged from 244 mg/dL to 500 mg/dL. At this time, trophic feeds as well as parenteral nutrition were stopped, and the patient's dextrose-containing intravenous fluids were switched to 0.2% saline at maintenance volume (total glucose infusion rate of 0 mg/kg/min). Bedside glucose readings were discontinued, and serum glucose measurements were evaluated at the central clinical laboratory. Repeat serum glucose measurements remained profoundly elevated, peaking at 1943 mg/dL (Figure 1). The patient's elevated serum β-hydroxybutyrate (0.3 mmol/L; reference range 0-0.29 mmol/L) was indicative of ketonemia. The patient's only other medication at this time was 10 mg/kg/day of caffeine (Cafcit, Bedford Labs, Bedford, OH) for apnea of prematurity, which continued throughout the hospitalization. Full septic workup, including blood cultures and C-reactive protein measurements, was ultimately negative. NDM was presumptively diagnosed in the absence of other causes for the profound hyperglycemia observed, such as infection, medication-induced glucose dysregulation, intravenous fluid preparation error, and pancreatic structural abnormalities (normal ultrasound).
Table.
Glucose Measurements (Serum or Point of Care) Prior to Day of Life (DOL) 10
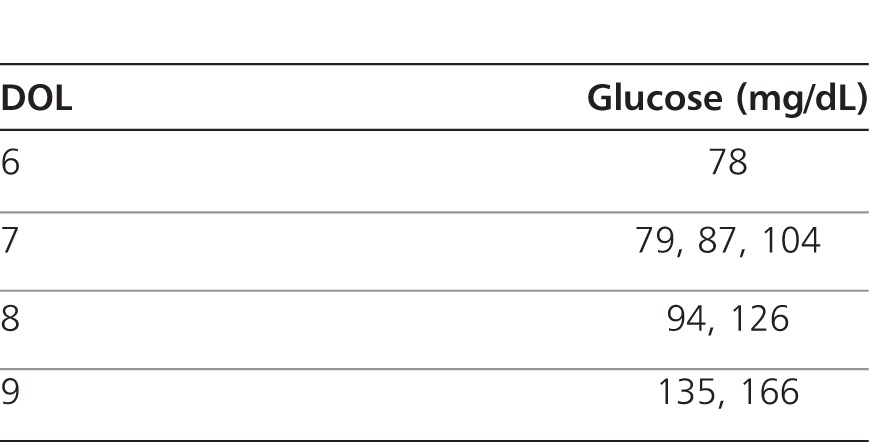
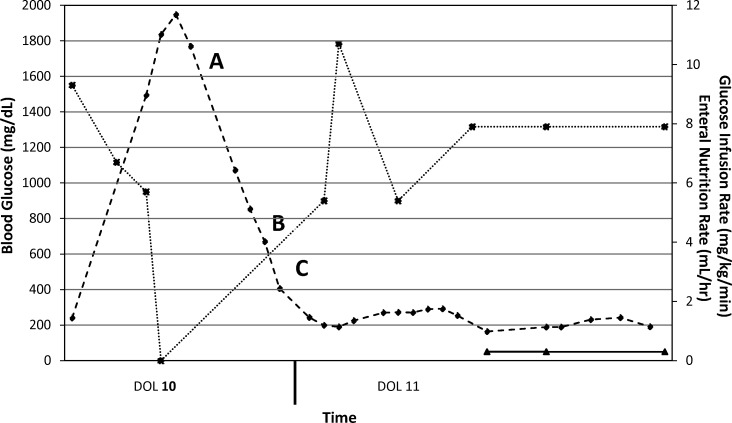
Figure 1.
Clinical course, days of life 10 and 11. KCl, potassium chloride; Kphos, potassium phosphate; IVF, intravenous fluids.
-♦- = Blood glucose (mg/dL); -*- = Glucose infusion rate (mg/kg/min); -▴- = Enteral nutrition (mL/hr).
(A) Serum glucose noted to be 1943 mg/dL. Parenteral nutrition and trophic feeds were discontinued, and dextrose was removed from subsequent intravenous fluids. (B) Continuous regular insulin infusion (0.02 unit/kg/hr) was begun. IVF contained 0.9% normal saline (NS) + 20 meq/L KCl + 20 meq/L Kphos at 1.5 times maintenance rate. The amount of dextrose in the IVF was titrated based on serum glucose. No dextrose was added if serum glucose was >300 mg/dL; D5W was added once serum glucose was 200 to 300 mg/dL; and D10W was added once serum glucose was below 200 mg/dL. (C) As a result of a rapid decline of serum glucose the insulin infusion was reduced to 0.01 unit/kg/hr.
On DOL 10, regular insulin (Humulin R, Eli Lilly, Indianapolis, IN) was initiated as a continuous infusion (0.02 unit/kg/hr) along with a protocol for intravenous fluids, as documented in Figure 1. Prior to initiation of regular insulin, the patient's C-peptide level was 8.6 ng/mL (reference range 0.4-2.2 ng/mL), which may have been indicative of endogenous insulin response in light of profoundly elevated serum glucose. The goal was to lower serum glucose by 100 mg/dL hourly until euglycemia (70-200 mg/dL) was attained. Excessive urinary output (>3 mL/kg/hr) was replaced at equal volumes with intravenous 0.9% saline every 4 hours. Continuous trophic feedings were restarted on DOL 11 and slowly advanced throughout the hospitalization. The regular insulin infusion was lowered to 0.01 unit/kg/hr as blood glucose rapidly fell and was continued until DOL 12, by which time serum glucose was well controlled under 200 mg/dL.
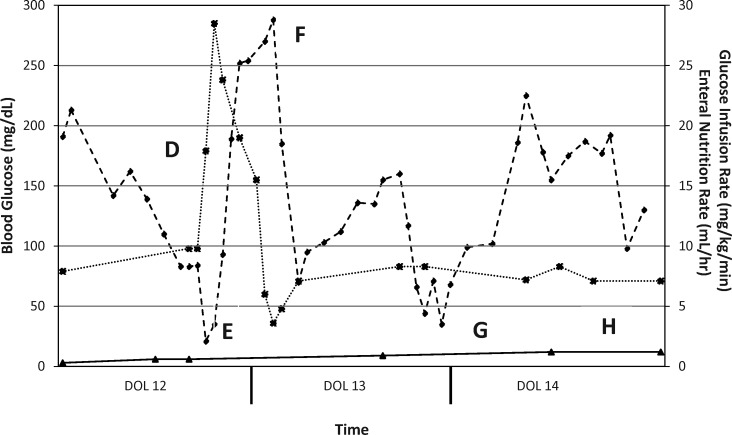
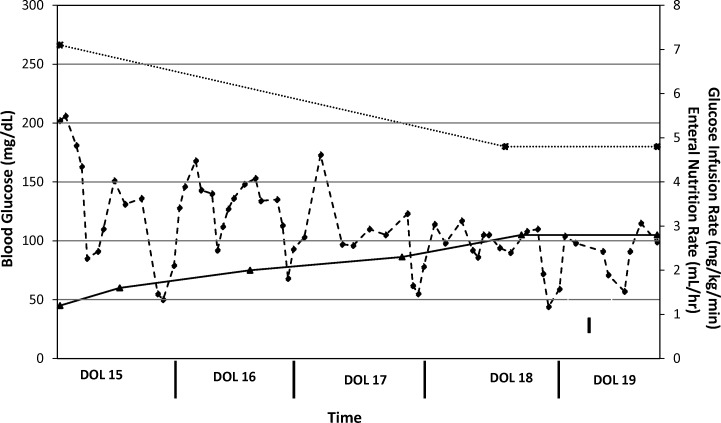
On DOL 12 (see Figure 2) the patient was transitioned to subcutaneous insulin detemir (Levemir, Novo Nordisk, Princeton, NJ) administered once every 24 hours (0.42 unit/kg/day) into the abdomen or buttocks. This conservative dose was selected based on the usual insulin requirements of a newly diagnosed type 1 diabetic (0.8-1 unit/kg/day) and the fact that neonates are likely to have highly variable responses to subcutaneous insulin. As a result of the minute dose that was to be administered (0.3 units/dose), a 1:10 dilution of insulin detemir in 0.9% saline was prepared using 0.1 mL of commercially available 100 unit/mL insulin detemir added to 0.9 mL of 0.9% saline to yield a 10-unit/mL diluted product. A volume of 0.03 mL of this dilution was drawn up in an insulin syringe and administered. The patient received insulin detemir doses on DOLs 12 and 13. However, the patient did not tolerate this regimen as a result of profound hypoglycemia, which occurred within a few hours post-dose, as well as hyperglycemia, which occurred after the time of peak effect of insulin detemir (Figure 2). On DOL 14, the patient was transitioned to subcutaneous insulin glargine (Lantus, Sanofi, Bridgewater, NJ) administered every 12 hours (0.27 unit/kg/day) into the abdomen or buttocks. The daily insulin dose was lowered as a result of the severe hypoglycemia encountered with insulin detemir. As with the insulin detemir dose, a minute dose of insulin glargine (0.1 units/dose) was required. In this instance, a 1:100 dilution of insulin glargine in 0.9% saline was prepared using 0.1 mL of commercially available 100-unit/mL insulin glargine added to 9.9 mL of 0.9% normal saline to yield a 1-unit/mL diluted product. A volume of 0.1 mL of this dilution was drawn up in an insulin syringe and administered. The patient received the aforementioned dose of insulin glargine from DOL 14 through DOL 19 (see Figure 3) with excellent response, since only several glucose measurements were outside the euglycemic range of 70 to 200 mg/dL. On DOL 19, persistent euglycemia prompted discontinuation of insulin glargine. To date, the patient has displayed no further episodes of hyperglycemia or hypoglycemia and has not required further treatment with any insulin products. Genetic studies to evaluate for mutations related to PNDM and TNDM were deferred, as this patient's condition fully resolved rapidly. The patient was discharged from the neonatal intensive care unit after a lengthy hospitalization secondary to poor weight gain, nosocomial infections, cholestasis, and chronic lung disease.
Figure 2.
Clinical course, days of life 12, 13, and 14.
-♦- = Blood glucose (mg/dL); -*- = Glucose infusion rate (mg/kg/min); -▴- = Enteral nutrition (mL/h).
(D) Regular insulin infusion discontinued. Initiation of subcutaneous insulin detemir given every 24 hours. Enteral nutrition restarted slowly. Parenteral nutrition restarted to provide more caloric intake. (E) Serum glucose was 21 mg/dL, and the patient was given several D10W boluses (1 mL/kg/dose). Glucose infusion rate was increased as a result of profoundhypoglycemia and reduced following sufficient rise in serum glucose. (F) Hyperglycemia noted as serum glucose trends above 200 mg/dL approximately 12 to 15 hours after insulin detemir dose. (G) Insulin detemir dose given and followed by another episode of profound hypoglycemia (<50 mg/dL) shortly after administration. (H) Discontinued insulin detemir as a result of inconsistent response pattern. Initiation of subcutaneous insulin glargine administered every 12 hours.
Figure 3.
Clinical course, days of life 15 to 19.
-♦- = Blood glucose (mg/dL); -*- = Glucose infusion rate (mg/kg/min); -▴- = Enteral nutrition (mL/hr). I. Adequate euglycemia (70-200 mg/dL) obtained. Discontinued insulin glargine.
DISCUSSION
Common causes of hyperglycemia in neonates include metabolic stress, infection, medications, and hepatic immaturity. In very low birth weight (<1500-g) neonates, the incidence of hyperglycemia is as high as 20% to 86%.4 Hyperglycemia in extremely low birth weight (ELBW; <1000-g) neonates has been associated with the development of intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, infection, and late mortality.5 Although the patient in this case was premature and of ELBW, her profound hyperglycemia was atypical for a neonate.
The management of a hyperglycemic neonate presents multiple clinically significant challenges.6 These issues include 1) compromise of caloric provision if glucose is withheld; 2) lack of a pharmacokinetic profile for subcutaneously administered insulin in neonates; 3) use of small doses that are highly error-prone; 4) limited data for dilution of commercially available insulin formulations; and 5) lack of subcutaneous fat deposits in a premature neonate through which to administer subcutaneous insulin.
Absorption of drugs from subcutaneous injection sites is affected by several interpatient variables, such as blood flow to the injection site, muscle mass, and quantity of adipose tissue and muscle. Absorption may also be affected by physiochemical characteristics of drugs, such as pH, ease of diffusion through capillary membranes, and surface area over which the volume of injection spreads.7 With regard to developmental pharmacology, subcutaneous drug absorption is usually reduced in preterm infants as a result of lower regional perfusion and reservoir mass, making this administration route problematic.8
Insulin detemir is a long-acting, basal insulin analogue manufactured by recombinant DNA expression in Saccharomyces cerevisiae, with removal of an amino acid residue at position B30 and placement of a 14-carbon fatty acid attached to the amino acid residue at B29. The prolonged action of insulin detemir is the result of reversible binding of the fatty acid residue to serum albumin, which slows release of active insulin monomers into systemic circulation following subcutaneous injection.9 From a pharmacokinetic perspective, the duration of action of insulin detemir is dose-dependent, ranging from 5.7 to 23.2 hours, with onset of action at approximately 1 to 3 hours and with peak effect occurring between 3 and 10 hours post-administration. The pharmacokinetics of insulin detemir have been evaluated in patients as young as 6 years of age and do not differ significantly when compared to pharmacokinetics in adolescents and adults.10 There have been no pharmacokinetic studies evaluating insulin detemir in neonates. As mentioned previously, lack of subcutaneous fat could have profound effects on the pharmacokinetics of insulin detemir. Ample amounts of subcutaneous fat are essential to achieve sustained release of free insulin. In the absence of fatty acid residues, insulin detemir will not maintain the same pharmacokinetic profile. Another possible contributing factor to response to insulin detemir is serum albumin. Neonates, especially those who are premature, are often found to be hypoalbuminemic. In addition, neonatal albumin has diminished binding affinity compared with albumin of an older child. In our patient, an ELBW neonate with minimal subcutaneous fat, we observed a peak effect of insulin detemir resulting in hypoglycemia followed by hyperglycemia. We hypothesize that these issues resulted from too-rapid release of free insulin. Also, at the time of insulin detemir initiation, the patient's serum albumin measurement was 1.7 g/dL (reference range 2.6-4.4 g/dL), which may have contributed to the lack of response. The manufacturer's information states that this product should not be diluted or mixed with other types of insulin as a result of the potential for alteration of the product's pharmacokinetic profile. No studies have evaluated the efficacy in terms of release pattern or stability of diluted long-acting insulin products. However, given the volume of commercially available solution that would have been required for the dose (0.003 mL), it was felt that preparing a dilution would have been the best way to administer subcutaneous insulin detemir in this patient. The dilution of detemir product may have adversely affected its pharmacokinetic profile in this ELBW neonate.
Insulin glargine is another long-acting analogue produced by recombinant DNA technology using nonpathogenic strains of Escherichia coli for the production medium. Insulin glargine differs from human insulin in that glycine is substituted for asparagine at position A21, and 2 arginine residues are added at the C-terminus of the B-chain. This formulation is dissolved in solution at acidic pH. The prolonged duration of action after subcutaneous injection results from formation of microprecipitates (hexamers) at neutral physiologic pH, which gradually release active insulin monomers over a 24-hour period without the pronounced peak typically observed with insulin detemir. With regard to pharmacokinetics, the onset of action for insulin glargine resembles that of insulin detemir. Studies in patients as young as 6 years of age have not found any significant difference with regard to safety and efficacy for glargine when compared with findings in adults.11 As is the case with insulin detemir, no pharmacokinetic studies have evaluated insulin glargine in neonates. As with insulin detemir, insulin glargine should be administered subcutaneously, but it should not be diluted or mixed with other insulin formulations. In the case of insulin glargine, if one were to use the commercially available product undiluted, the volume required for this patient would have been 0.001 mL. As with insulin detemir, we felt it was necessary to dilute insulin glargine to facilitate drug administration in this patient. Our patient experienced a more favorable response to insulin glargine than to insulin detemir. It was felt that dosing of insulin glargine every 12 hours was safer for this patient since the newborn experienced poor glycemic control with once-daily dosing of insulin detemir as a result of the apparent lack of a consistent insulin release pattern. Dosing insulin glargine every 12 hours allows for a short window of time to evaluate the pharmacokinetics of insulin glargine and to better make dose adjustments were permitted. The delayed and sustained release of insulin glargine depends on the rise in ambient pH upon administration and not upon the availability of fatty acid residues, as in the case of insulin detemir.
As a result of the rarity of NDM, no universal clinical guidelines exist for TNDM or PNDM. Though genetic etiologies of TNDM have been identified, there has been no consensus on its management.12–15 Various modalities reported as effective include oral sulfonylureas, intravenous regular insulin, continuous subcutaneous insulin infusion pump therapy, neutral protamine hegadorn (NPH) insulin, and subcutaneous insulin glargine1,6,16,17 Loomba-Albrecht et al17 described a neonate who developed TNDM on DOL 3 and subsequently failed subcutaneous insulin therapy as a result of glucose excursions. This infant required a prolonged continuous insulin infusion and was weaned off insulin after initiation of glyburide, and the TNDM resolved by DOL 49.17 Bharucha.6 reported 2 infants who failed to maintain glycemic control once transitioned from intravenous to subcutaneous insulin but who attained euglycemia when transitioned to subcutaneous insulin infusion pump therapy. They suggested a protocol to determine the proper insulin pump basal rate and correctional scale.6 Jeha et al1 described 3 cases of successful management of TNDM with insulin glargine. Two of 3 cases involved term neonates (one with uniparental disomy on chromosome 6) who developed disease at DOL 7 and 8, respectively, who failed transition from intravenously administered regular insulin to subcutaneous NPH insulin. After NPH insulin failure, the 2 patients were switched to subcutaneous insulin glargine and exhibited lower mean serum glucose measurements (251 mg/dL to 162 mg/dL, 302 mg/dL to 225 mg/dL, respectively) and fewer hypoglycemic episodes (1.5 to 0.6 episodes per 24 hours, 0.9 to 0.3 episodes per 24 hours) than while on NPH insulin. Neither of these patients required insulin by 1 year of age. The third case involved a term neonate with intrauterine growth retardation who developed TNDM on DOL 1 and was transitioned from intravenous regular insulin to subcutaneous insulin glargine. Throughout the hospitalization, the patient's mean serum glucose was 189 mg/dL, and the infant exhibited 0.3 episodes of hypoglycemia per 24 hours. This patient remained insulin dependent at 1 year of follow-up. In these 3 cases, insulin glargine was initiated at 0.5 to 1 unit/day and titrated by 0.5 unit/day to a goal glucose of 150 to 220 mg/dL. The final insulin dose upon discharge ranged between 0.7 and 1 unit/kg/day. The authors did not report how insulin glargine was prepared or where it was administered.
CONCLUSION
The rarity of TNDM has limited the evidence available with which to validate the use of subcutaneous insulin in neonates. Pharmaceutical properties and mechanisms of sustained activity support our conclusion that insulin glargine may be more efficacious than insulin detemir in the treatment of an ELBW neonate with limited subcutaneous fat deposits. The experience with our patient indicates that the release pattern of glargine, as a truly “peak-less” insulin, may be most ideal for TNDM management during the neonatal period and early infancy, when patients are frequently or continuously fed. Our findings were similar to those of Jeha et al1 It may be prudent in the future to perform studies evaluating the stability and pharmacokinetics of different dilutions of long-acting insulin products, which may be used in this patient population. Although unanswered questions remain regarding the use of long-acting insulin products in neonates, we hope that our recent experience may help to guide other clinicians managing TNDM.
ABBREVIATIONS
- DOL
day of life
- ELBW
extremely low birth weight
- NDM
neonatal diabetes mellitus
- PNDM
permanent neonatal diabetes mellitus
- TNDM
transient neonatal diabetes mellitus
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Jeha GS, Venkatesh MP, Edelen RC, et al. Neonatal diabetes mellitus: patient reports and review of current knowledge and clinical practice. J Pediatr Endocrinol Metab. 2005;;18(11):1095–1102. doi: 10.1515/jpem.2005.18.11.1095. [DOI] [PubMed] [Google Scholar]
- 2.Polak M, Cave H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J Rare Dis. 2007;;2(1):12–23. doi: 10.1186/1750-1172-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;;359(18):1873–1884. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 4.Von Muhlendahl KE, Herkenhoff H. Long-term course of neonatal diabetes. N Engl J Med. 1995;;333(11):704–708. doi: 10.1056/NEJM199509143331105. [DOI] [PubMed] [Google Scholar]
- 5.Kao LS, Morris BH, Lally KP, et al. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;;26(12):730–736. doi: 10.1038/sj.jp.7211593. [DOI] [PubMed] [Google Scholar]
- 6.Bharucha T, Brown J, McDonnell C, et al. Neonatal diabetes mellitus: Insulin pump as an alternative management strategy. J Paediatr Child Health. 2005;;41((9-10)):522–526. doi: 10.1111/j.1440-1754.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe SJ, Aranda JA. Pediatric Pharmacology: Therapeutic Principles in Practice (2/e) Philadelphia, PA: WB Saunders Co;; 1992. [Google Scholar]
- 8.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 9.NovoNordisk. Levemir [package insert] NovoNordisk;; 2009. [Google Scholar]
- 10.Danne T, Lupke K, Walte K, et al. Insulin detemir is characterized by a consistent pharmacokinetic profile across age groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care. 2003;;26(11):3087–3092. doi: 10.2337/diacare.26.11.3087. [DOI] [PubMed] [Google Scholar]
- 11.Sanofi Aventis. Lantus [package insert] Sanofi Aventis;; 2007. [Google Scholar]
- 12.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;;350(18):1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 13.Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;;355(5):467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 14.Bababenko AP, Polak M, Cave H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;;355(5):456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 15.Zwaveling-Soonawala N, Hagebeuk EE, Slingerland AS, et al. Successful transfer to sulfonylurea therapy in an infant with developmental delay, epilepsy and neonatal diabetes (DEND) syndrome and a novel ABCC8 gene mutation. Diabetologia. 2011;;54(2):469–471. doi: 10.1007/s00125-010-1981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beardsall K, Pesterfield CL, Acerini CL. Neonatal diabetes and insulin pump therapy. Arch Dis Child Fetal Neonatal Ed. 2011;;96(3):F223–F224. doi: 10.1136/adc.2010.196709. [DOI] [PubMed] [Google Scholar]
- 17.Loomba-Albrecht LA, Glaser NS, Styne DM, et al. An oral sulfonylurea in the treatment of transient neonatal diabetes mellitus. Clin Ther. 2009;;31(4):816–820. doi: 10.1016/j.clinthera.2009.04.003. [DOI] [PubMed] [Google Scholar]