Abstract
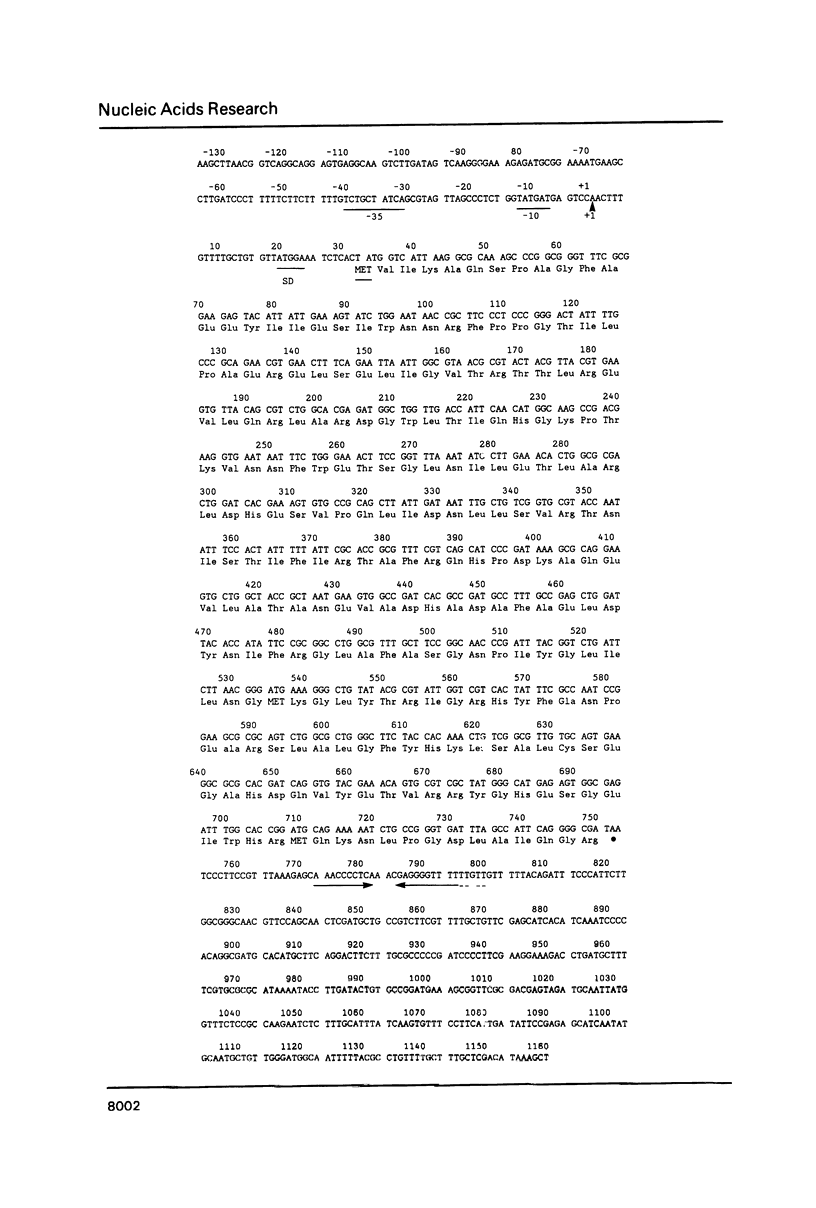
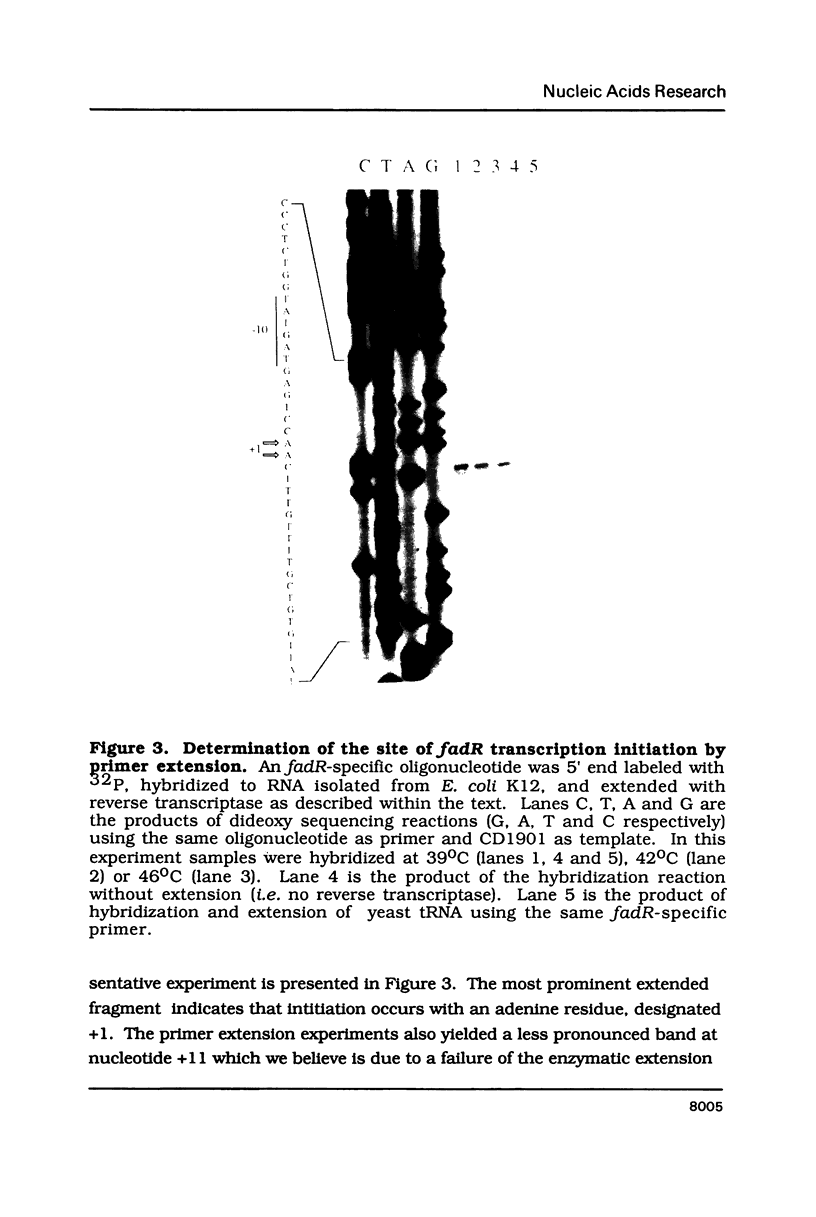
The Escherichia coli fadR gene is a multifunctional regulator of fatty acid and acetate metabolism. In the present work the nucleotide sequence of the 1.3 kb DNA fragment which encodes FadR has been determined. The coding sequence of the fadR gene is 714 nucleotides long and is preceded by a typical E. coli ribosome binding site and is followed by a sequence predicted to be sufficient for factor-independent chain termination. Primer extension experiments demonstrated that the transcription of the fadR gene initiates with an adenine nucleotide 33 nucleotides upstream from the predicted start of translation. The derived fadR peptide has a calculated molecular weight of 26,972. This is in reasonable agreement with the apparent molecular weight of 29,000 previously estimated on the basis of maxi-cell analysis of plasmid encoded proteins. There is a segment of twenty amino acids within the predicted peptide which resembles the DNA recognition and binding site of many transcriptional regulatory proteins.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. N., Kianian S. F., DiRusso C. C., Nunn W. D. Long-chain fatty acid transport in Escherichia coli. Cloning, mapping, and expression of the fadL gene. J Biol Chem. 1985 Feb 10;260(3):1780–1789. [PubMed] [Google Scholar]
- Black P. N., Said B., Ghosn C. R., Beach J. V., Nunn W. D. Purification and characterization of an outer membrane-bound protein involved in long-chain fatty acid transport in Escherichia coli. J Biol Chem. 1987 Jan 25;262(3):1412–1419. [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clark D. Regulation of fatty acid degradation in Escherichia coli: analysis by operon fusion. J Bacteriol. 1981 Nov;148(2):521–526. doi: 10.1128/jb.148.2.521-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli. J Biol Chem. 1973 Feb 25;248(4):1188–1195. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- DiRusso C. C., Nunn W. D. Cloning and characterization of a gene (fadR) involved in regulation of fatty acid metabolism in Escherichia coli. J Bacteriol. 1985 Feb;161(2):583–588. doi: 10.1128/jb.161.2.583-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Frerman F. E., Bennett W. Studies on the uptake of fatty acids by Escherichia coli. Arch Biochem Biophys. 1973 Nov;159(1):434–443. doi: 10.1016/0003-9861(73)90471-2. [DOI] [PubMed] [Google Scholar]
- Ginsburgh C. L., Black P. N., Nunn W. D. Transport of long chain fatty acids in Escherichia coli. Identification of a membrane protein associated with the fadL gene. J Biol Chem. 1984 Jul 10;259(13):8437–8443. [PubMed] [Google Scholar]
- Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Kolb A., Spassky A., Chapon C., Blazy B., Buc H. On the different binding affinities of CRP at the lac, gal and malT promoter regions. Nucleic Acids Res. 1983 Nov 25;11(22):7833–7852. doi: 10.1093/nar/11.22.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lakshmi T. M., Helling R. B. Acetate metabolism in Escherichia coli. Can J Microbiol. 1978 Feb;24(2):149–153. doi: 10.1139/m78-027. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Bohlander M., Nunn W. D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J Bacteriol. 1980 Aug;143(2):720–725. doi: 10.1128/jb.143.2.720-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham B. E., Harper J. E., Mount D. W., Sancar G. B., Sancar A., Rupp W. D., Kenyon C. J., Walker G. C. Analysis of mRNA synthesis following induction of the Escherichia coli SOS system. J Mol Biol. 1984 Sep 15;178(2):237–248. doi: 10.1016/0022-2836(84)90142-6. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Ohlendorf D. H., Anderson W. F., Takeda Y. Structure of the DNA-binding region of lac repressor inferred from its homology with cro repressor. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1428–1432. doi: 10.1073/pnas.79.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Simons R. W. Transport of long-chain fatty acids by Escherichia coli: mapping and characterization of mutants in the fadL gene. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3377–3381. doi: 10.1073/pnas.75.7.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Sallus L., Haselbeck R. J., Nunn W. D. Regulation of fatty acid transport in Escherichia coli: analysis by operon fusion. J Bacteriol. 1983 Sep;155(3):1450–1454. doi: 10.1128/jb.155.3.1450-1454.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Hughes K. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: dominance studies with strains merodiploid in gene fadR. J Bacteriol. 1980 Aug;143(2):726–730. doi: 10.1128/jb.143.2.726-730.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vanderwinkel E., De Vlieghere M., Fontaine M., Charles D., Denamur F., Vandevoorde D., De Kegel D. Septation deficiency and phosphilipid perturbation in Escherichia coli genetically constitutive for the beta oxidation pathway. J Bacteriol. 1976 Sep;127(3):1389–1399. doi: 10.1128/jb.127.3.1389-1399.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]