SUMMARY
At present, it is difficult to identify a gold standard for endoscopic staging of laryngeal cancer, especially considering the large number of endoscopic instruments available. We have coined the term multistep endoscopy to describe a method for staging laryngeal precancerous and neoplastic lesions that sequentially uses several endoscopic tools including high definition white light endoscopy (HDTV), stroboscopy and autofluorescence endoscopy. During the period from November 2007 to November 2009, 140 patients with a suspect laryngeal lesion underwent multistep endoscopy at the Department of Otorhinolaryngology at Martini Hospital in Turin. All patients were subjected to a series of endoscopic examinations in indirect laryngoscopy (white light endoscopy coupled to a HDTV camera, laryngostroboscopy, indirect autofluorescence) followed by white light endoscopy coupled to a HDTV camera and autofluorescence in direct microlaryngoscopy. The aim of the present prospective study was to evaluate the utility of multistep endoscopy in the diagnostic work-up of laryngeal lesions. Multistep endoscopy showed a higher sensitivity and "biological" predictive value in early cancer and precancerous lesions of the larynx (sensitivity, 97.9%; specificity, 90.5%) compared to individual endoscopic tools. It allows for better therapeutic planning of superficial lesions and more accurate orientation when performing mapping biopsies on diffuse lesions. In our opinion, more widespread use of indirect autofluorescence endoscopy during follow-up may be warranted to search for synchronous/metachronous second tumours of the upper aerodigestive tract.
KEY WORDS: Multistep endoscopy, Laryngeal endoscopy, Laryngeal cancer staging
RIASSUNTO
Oggi resta indubbiamente difficile identificare il gold standard procedurale per la stadiazione endoscopica del carcinoma laringeo, data l'ampia disponibilità di strumenti diagnostici endoscopici. Abbiamo coniato il termine di multistep endoscopy per definire un metodo di stadiazione endoscopica del carcinoma laringeo, utilizzando sequenzialmente diverse tecniche di endoscopia: endoscopia a luce bianca ad alta definizione (HDTV), stroboscopia ed endoscopia ad autofluorescenza). Nel periodo novembre 2007 - novembre 2009 presso la clinica di otorinolaringoiatria dell'Ospedale "Martini" di Torino, sono stati sottoposti a multistep endoscopy 140 pazienti, con diagnosi di sospetta neoformazione laringea. Tutti i pazienti sono stati sottoposti in successione ad una serie di esami endoscopici in laringoscopia indiretta (endoscopia a luce bianca con telecamera-HDTV, laringostroboscopia, endoscopia ad autofluorescenza) e successivamente in corso di microlaringoscopia diretta (MLD) ad endoscopia a luce bianca con telecamera HDTV ed endoscopia ad autofluorescenza. Lo scopo del presente studio prospettico è stato quello di valutare il guadagno diagnostico fornito dalla multistep endoscopy nel work-up diagnostico delle lesioni laringee. La multistep endoscopy ha mostrato una maggiore sensibilità e predittività "biologica" sulle lesioni iniziali e sui precursori delle neoplasie della laringe (sensibilità: 97,9%; specificità: 90,5%) rispetto alle singole metodiche endoscopiche, consentendo una migliore pianificazione terapeutica di lesioni superficiali ed un più accurato orientamento delle biopsie di mappaggio di lesioni diffuse. A nostro parere, in futuro, si potrà assistere ad un impiego più estensivo dell'endoscopia ad autofluorescenza indiretta anche nel follow-up, per la ricerca sistematica di secondi tumori sincroni/metacroni.
Introduction
Endoscopic tools for examination of the upper aerodigestive tract have undergone significant developments in recent years. Oncology in general, and in particular head and neck oncology, is increasingly oriented to a "custom-tailored" therapeutic choice to preserve both organ and function. In this regard, precise definition of the superficial extension of a neoplasm is a strategic and fundamental aspect, in addition to evaluation of larynx motility, in order to assess the indirect signs of deep extension of the lesion. Nonetheless, there are a large number of endoscopic instruments and techniques available, which render identification of a gold standard for endoscopic staging of laryngeal cancer difficult 1.
The term multistep endoscopy was coined to describe an endoscopic method for staging laryngeal cancer in which a series of sequential endoscopic tools are applied: high definition white light endoscopy (HDTV camera), stroboscopy and indirect autofluorescence, first with the patient awake and then under sedation, using telescopes with different angles.
The diagnostic sequence of multistep endoscopy is as follows:
Indirect laryngoscopy in HDTV with a rigid 5 mm, 70° and/or 90° telescopes, performed with xenon white light (WL) and a source of stroboscopic light (STRO BO-L).
Indirect flexible video-endoscopy only in cases that cannot be examined due to an intense gag reflex or to unfavorable anatomy, performed with WL and STRO BO-L.
Indirect autofluorescence (AF) laryngoscopy with a rigid 5 mm 70° and/or 90° telescopes (Karl Storz, Tuttlingen, D-light AF system).
Endoscopy in HDTV with rigid 5 mm 0° and 70° telescopes in WL, during direct microlaryngoscopy (DML).
Direct autofluorescence with rigid 0° and 70° telescopes during direct microlaryngoscopy.
The aim of the present prospective study was to evaluate the utility of multistep endoscopy in the diagnostic workup of laryngeal lesions.
Materials and methods
The study was carried out between November 2007 and November 2009 at the Department of Otorhinolaryngology at Martini Hospital in Turin. A team of 3 endoscopists (G.S., E.C., D.M.) carried out independent evaluations on 146 patients with a diagnosis of suspect laryngeal neoplasm. All patients were first evaluated by endoscopic examination in HDTV (Karl Storz Image 1 Hub) using 70° and 90° telescopes (Karl Storz Hopkins II, 5 mm, 70° and 90°) with a Xenon 300 W light source (WL) to obtain an overview of the larynx in white light. Stroboscopic illumination was then used (STRO BO-L; Karl Storz Pulsar Stroboscope) to assess the rigidity of the cordal mucosa and mucosal wave. Sixteen patients presented an intense gag reflex during indirect laryngoscopy, and were administered a pre-anaesthetic with a benzodiazepine (midazolam HCl 0.07-0.1 mg/kg i.m.) and atropine to reduce the intense salivation and increase the possibility for examination. In 6 patients, who were not evaluable using indirect laryngoscopy using rigid, angled telescopes, the investigation was completed with transnasal flexible videoendoscopy in WL and STRO BO-L.
A total of 140 of the 146 patients were considered eligible on the basis of compliance to indirect laryngoscopy in WL and STRO BO-L, and were subjected to indirect autofluorescence laryngoscopy (indirect AF). At present, the examination cannot be associated with HDTV technology. For this reason, it was necessary to use a 3CCD camera equipped with a filter for AF (Karl Storz Tricam SLII Camera Control Unit) and a light source for AF endoscopy (Karl Storz D-Light C/AF Light System). The camera was set to an exposure time of 1/8 sec and was placed in AF(C) mode.
The lack of fluorescence of pre-neoplastic and neoplastic lesions, even in this phase, allowed for greater precision in the evaluation of margins, better resolution of multifocal lesions, and prediction of pathological grade.
After indirect laryngoscopy, a laryngeal lesion was detected in all 146 patients who were then subjected to DML with diagnostic/therapeutic intent.
DML consisted in the following sequential steps:
Positioning of the Lindholm laryngoscope (useful for endoscopic evaluation with rigid telescopes).
Evaluation with rigid 0° and 70° telescopes in HDTV using WL.
Evaluation with direct AF, using a 3-CCD camera equipped with a filter for AF and the same setting, and 5 mm, 0° and 70° telescopes with a filter for AF, thereby improving visualization of difficult-to-observe sites with the aid of dilator forceps for the false cords, together with micro-hooks and angled suction cannula.
After initial assessment, the operating microscope was positioned and either diagnostic (biopsy) or therapeutic procedures (excision with a CO2 laser) were carried out.
All surgical specimens were fixed to a solid support and sent for pathological analysis with marked margins; specimens were analyzed by two expert pathologists. In the case of discrepancy between AF and one of the exams carried out with WL (HDTV, operating microscope), preference was given to the exam that showed the greatest extension of the lesion. Accordingly, a wider excision was performed and the specimen was sent with a query about the discrepant area. The area of interest was also drawn by hand to aid the pathologist.
At the end of each multistep endoscopy investigation, the endoscopist filled out a diagnostic form for various laryngeal pathologies characterized by the following cut-off point: malignant/precancerous lesion vs. benign lesion.
All examinations were recorded digitally (Karl Storz AIDA System Control II). Successively, a fourth operator (F.P.) prepared 60-90 sec video-clips that were then appropriately catalogued. The multistep endoscopy of each patient was then re-evaluated by each of the three endoscopists. Next, the individual exams (HDTV endoscopy in WL, STRO BO-L, indirect AF) were again re-evaluated in a random sequence to assess the benefits of each endoscopic method in the diagnostic work-up of laryngeal lesions. The results were entered in a dedicated database; the results were considered definitive when agreement was reached between 2 of the 3 endoscopists.
Results
Six patients could not be evaluated by indirect laryngoscopy. Indeed, for accurate examination, good collaboration by the patient is necessary along with a reduced presence of the gag reflex, absence of hypersalivation and favourable anatomy.
The sensitivity, specificity, positive and negative predictive values for indirect AF, indirect HDTV in WL and STRO BO-L, and multistep endoscopy are shown in Tables I, II, III, respectively. Endoscopic evaluation in DML provided additional information in 36 patients, of which only 16 were detectable with HDTV in WL.
Table I.
Sensitivity, specificity, positive and negative predictive values of indirect autofluorescence.
| Histology | |||
|---|---|---|---|
| + | - | ||
| Indirect AF | + | 89 | 24 |
| - | 10 | 17 | |
Sensitivity: 89.9%; specificity: 41.4%; positive predictive value: 78.8%; negative predictive value: 62.9%; 6 patients were non-evaluable.
Table II.
Sensitivity, specificity, positive and negative predictive values of indirect laryngoscopy in HDTV with white light and stroboscopic light.
| Histology | |||
|---|---|---|---|
| + | - | ||
| Indirect laryngoscopy in HDTV with white light and stroboscopic light | + | 85 | 24 |
| - | 15 | 16 | |
Sensitivity: 85%; specificity: 40%; positive predictive value: 77.9%; negative predictive value: 51.6% (6 patients were non-evaluable)
Table III.
Sensitivity, specificity, positive and negative predictive values of multistep endoscopy.
| Histology | |||
|---|---|---|---|
| + | - | ||
| Multistep endoscopy (Indirect laryngoscopy in HDTV with WL + STROBO-L + Indirect AF + Direct laryngoscopy in HDTV with WL and direct AF) | + | 96 | 4 |
| - | 2 | 38 | |
Sensitivity: 97.9%; specificity: 90.5%; positive predictive value: 96%; negative predictive value: 95%
The results of histological examination were: 102 (69.9%) patients had either precancerous lesions or invasive cancer. Mild/moderate dysplasia was seen in 26 cases (17.8%), severe dysplasia/carcinoma in situ was observed in 14 cases (9.6%) and microinvasive/invasive carcinoma in 62 cases (42.5%).
The systematic use of direct AF during DML permitted a more detailed analysis of the superficial extension of the lesion, and in particular of the margins of the lesion, even in the presence of abundant superficial keratosis. This led to up-staging in 18 precancerous/invasive lesions (17.6% of histologically positive lesions), with safer definition of the superficial margins of excision, which always resulted negative. Lastly, the sensitivity, specificity, positive and negative predictive values of indirect AF, indirect laryngoscopy in HDTV with WL and STRO BO-L, and multistep endoscopy are shown in Table IV.
Table IV.
Sensitivity, specificity, positive and negative predictive values of indirect autofluorescence, indirect laryngoscopy in HDTV with WL + stroboscopic light for multistep endoscopy in the entire patient cohort.
| Indirect AF (%) | Indirect HDTV WL + STROBO-L (%) | Multistep endoscopy (%) | |
|---|---|---|---|
| Sensitivity | 89.9 | 85 | 97.9 |
| Specificity | 41.4 | 40 | 90.5 |
| Positive predictive value | 78.8 | 77.9 | 96 |
| Negative predictive value | 62.9 | 51.6 | 95 |
Discussion
It is clear that any additional endoscopic examination will provide further information, and that each investigation is useful in reducing the proportion of patients with laryngeal lesions that are under- and over-staged during the pre-treatment phase. In the present study, we assessed the benefits of multistep endoscopy in the diagnostic work-up of laryngeal lesions. With respect to the individual endoscopic techniques, multistep endoscopy showed a greater sensitivity and biological predictive value (sensitivity, 97.9%; specificity, 90.5%) on early lesions and neoplastic precursors in the upper aerodigestive tract. Multistep endoscopy allowed for better therapeutic planning of superficial lesions, and more accurate orientation of mapping biopsies in diffuse lesions.
The increased sensitivity provided by multistep endoscopy is related to the application of both HDTV and AF in direct and indirect modes. Direct endoscopy is characterized by the absence of movement of the larynx and the possibility to aspirate secretions and move anatomic structures in order to visualize the entire lesion. Accordingly, in this series only three false negative cases were observed (one carcinoma and two precancerous lesions); all three false negative lesions were characterized by an abundant keratin layer that did not allow visualization of the underlying epithelium.
HDTV can provide very detailed images that are highly realistic with respect to both the colour and contour of the mucosal surface and vascular network. The combination of HDTV and STRO BO-L optimized assessment of both cordal motility and the mucosal wave. The absence or reduction of the latter can provide information, to an expert eye, concerning suspicion of a mucosal lesion with infiltrative or microinfiltrative character 2-4. However, HDTV has the tendency to over-stage chronic hyperplastic laryngitis (irregularities of the mucosa, reduction of mucosal wave), seen as areas of hyperplasia ± parakeratosis considering irregularities of the superficial profile that render it clinically similar to dysplastic lesions and papillomatosis without dysplasia. The four false negative cases seen with HDTV alone (one carcinoma, three precancerous lesions) were related to superficial lesions with slight mucosal alterations. Of these four cases, one had undergone prior radiotherapy, and two had undergone previous laser surgery. At the same time, intraoperative HDTV allowed improvement of the specificity, eliminating doubt between lesions characterized by either dyskeratosis or mucosal hyperplasia. In contrast, AF depends on differences in cellular metabolism and histomorphology between healthy and pathological cells.
AF endoscopy enhances the pre- and intraoperative work-up of multistep endoscopy, providing information about the biological characteristics of the lesion. In fact, following an overview in WL of the larynx, performing the same exam in AF allows identification of superficial pathological areas only on the basis of chromatic differences with respect to healthy tissue. Nonetheless, AF can only reveal pathologies that involve the mucosal surface of the larynx, but not those involving the submucosal or deep layers. The aspect and degree of fluorescence in each tissue depends on three factors: the presence of endogenous fluorophores, morphological aspects and the wavelength of excitation (Figs. 1, 2, 3a, 3b, 4a, 4b) 5-7.
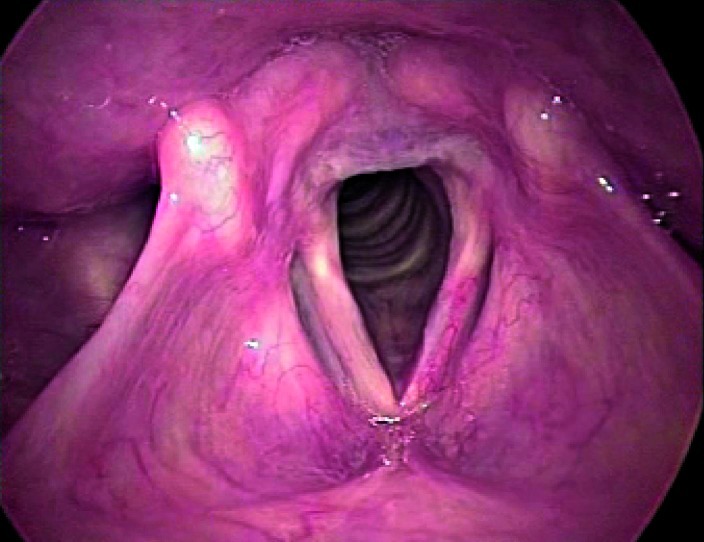
Fig. 1.
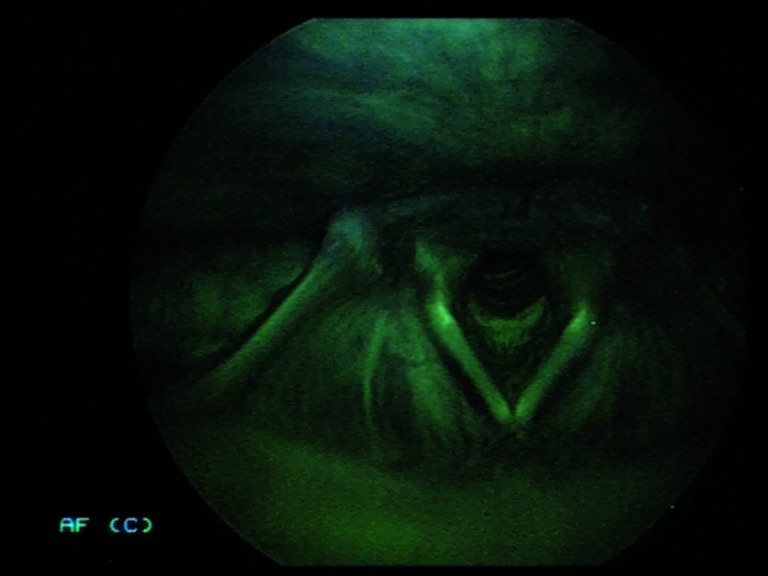
Indirect autofluorescence endoscopy with 70° rigid telescope: the normal mucosa has a classic green colour.
Fig. 2.
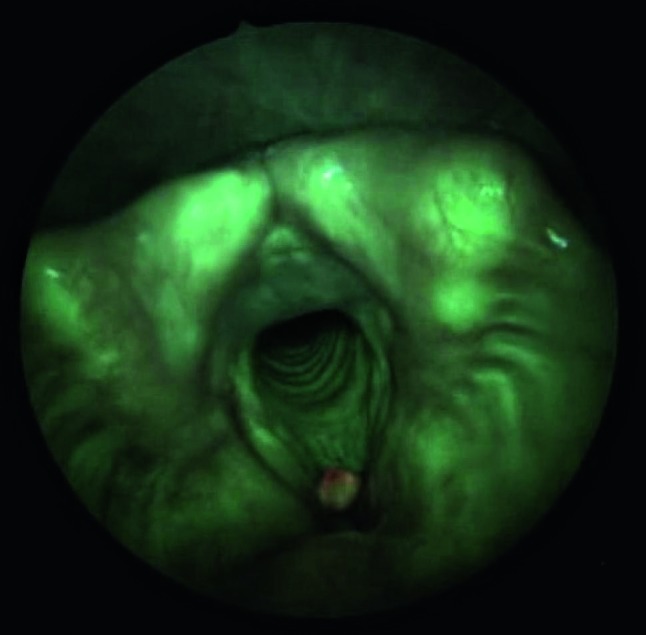
Indirect autofluorescence endoscopy with 70° rigid telescope: right vocal cord polyp. The lesion does not show any colour differences other than normal green colour. Of interest is the intense red fluorescence caused by the keratotic component.
Figs. 3a, 3b.
Indirect endoscopy with 70° rigid telescope (white light + autofluorescence): left vocal cord neoplastic lesion (carcinoma in situ).
Figs. 4a, 4b.
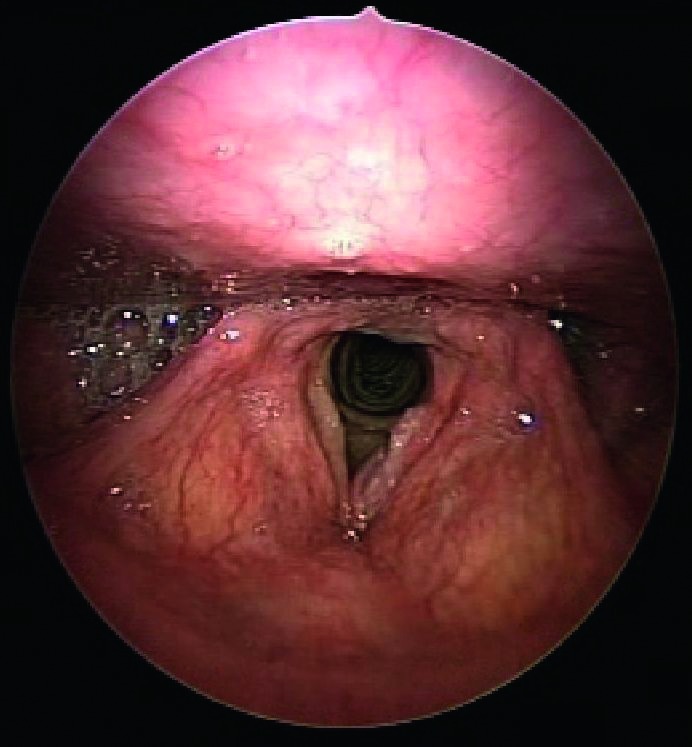
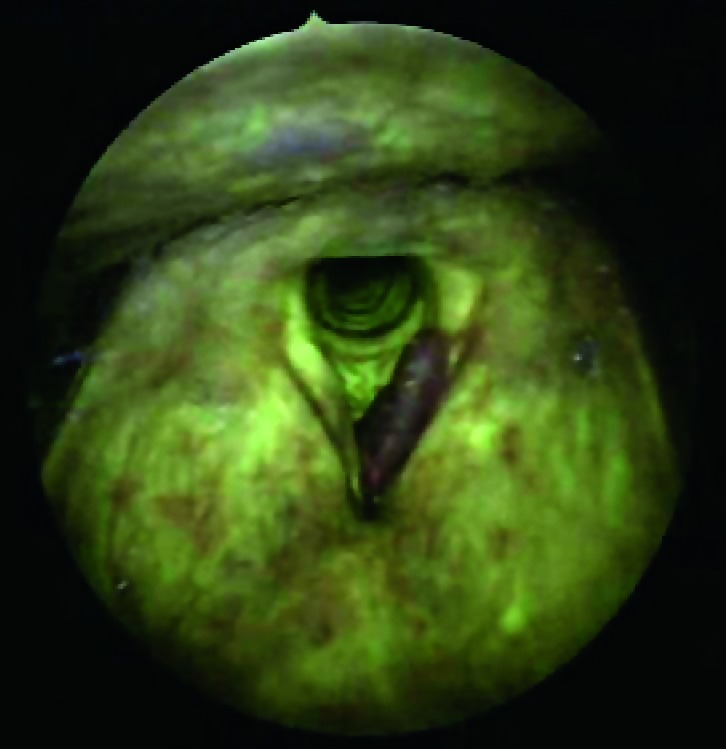
Indirect endoscopy with 70° rigid telescope (white light + autofluorescence): left vocal cord neoplastic lesion.
The following features can negatively impact the sensitivity and specificity of AF relative to its ability to distinguish between benign lesions and dysplastic/neoplastic lesions 8-10:
Hyperkeratosis-leukoplakia: shows a field of intense autofluorescence that is very bright white/red (Fig. 5). The presence of underlying dysplasia/cancer can lead to a slight change in the underlying colour to dark red/ light brown.
Lesions characterized by abnormal hyperplasia: leads to a reduction in normal fluorescence, with varying degrees of colour from bright green to blue/violet.
Hypervascularized lesions, chronic laryngitis and lesions with bacterial infection: can lead to a reduction in AF compared to normal tissues.
Scarring from prior surgical treatment or radiotherapy: leads to a reduction in AF similar to that seen in pre-neoplastic and neoplastic lesions.
Fig. 5.
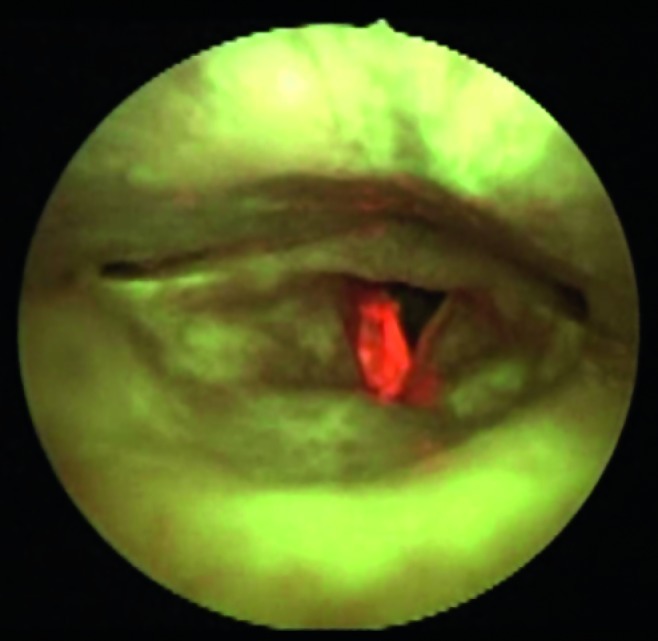
Indirect autofluorescence endoscopy with 70° rigid telescope: right vocal cord hyperkeratosis (without dysplasia). The intense red fluorescence should be noted.
Direct AF showed a greater sensitivity than indirect AF, as it identified areas with reduced AF at the periphery/ angles of neoplastic lesions characterized by intense overlying keratosis. The proportion of false positive lesions seen was still high, even after HDTV and direct AF in cases of chronic hyperplastic laryngitis; this is due to the reduction of AF observed, even during the course of DML.
Multistep endoscopy, similar to HDTV-NBI 11-14, undoubtedly provides a wealth of endoscopic data that leads to better clinical definition in terms of prediction of pre-neoplastic or neoplastic lesions and their superficial extension. The sensitivity and specificity of the two techniques are similar. However, with respect to NBI, multistep endoscopy is limited by the extensive examination times and high level of organization required, including:
The need for two different light sources (stroboscopy, autofluorescence), and at least four endoscopes (5 mm 0-70° with AF filter + 120° telescope + long 5 mm 0° telescope for tracheo-broncho-oesophagoscopy).
The need for two cameras, one for HDTV and another for 3CCD with a filter for AF.
Impossibility to perform part of the procedure (indirect laryngoscopy) in flexible, transnasal videoendoscopy (some patients cannot be subjected to indirect evaluation; 6 cases in the present study).
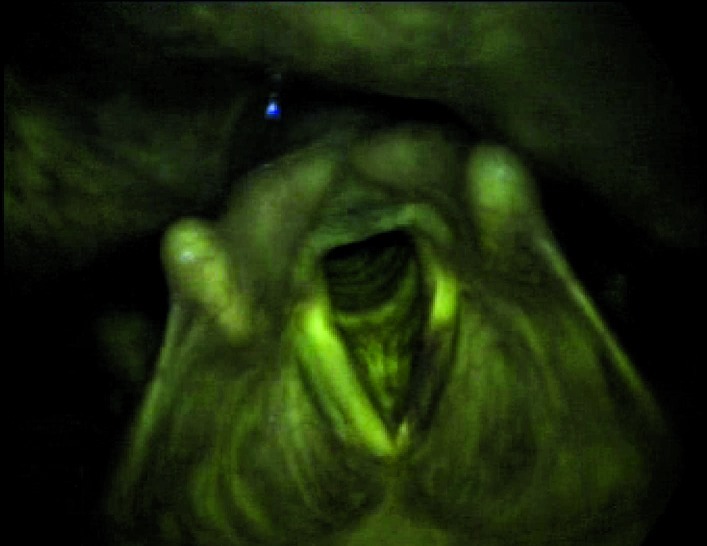
Possibility that anatomical narrowing of the oropharynx will have a negative impact on the procedure (especially during indirect laryngoscopy) (Fig. 6a, 6b), with laryngeal images that are dark with little value.
Examinations are carried out while awake and under sedation.
Figs. 6a, 6b.
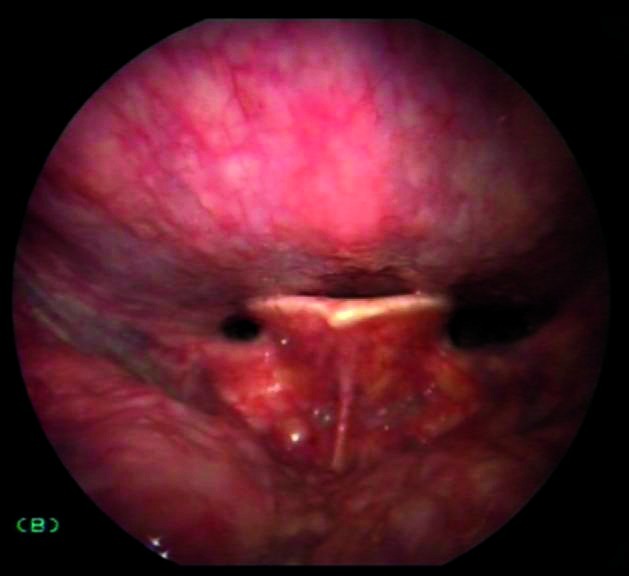
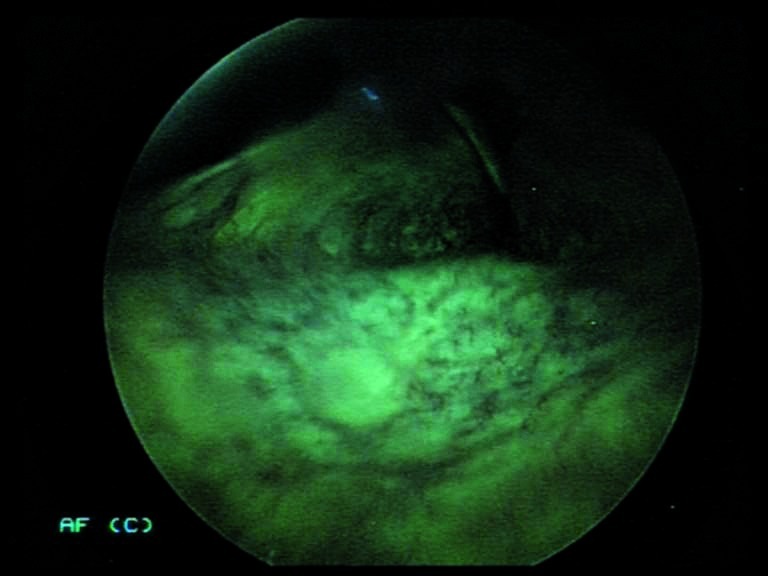
Indirect endoscopy with 70° rigid telescope (white light + autofluorescence): the exam is negatively influenced by narrow oropharyngeal anatomy.
Conclusions.
The extensive and combined utilization of new endoscopic tools, such as those used in multistep endoscopy, provide at least three distinct advantages with respect to traditional diagnostic methodology:
Greater sensitivity and biological predictive value for early lesions and neoplastic precursors of the upper aerodigestive tract. This is useful when lesions are first detected for better planning of excision/biopsy of superficial lesions, in counselling patients with glottic lesions, and when performing mapping biopsies in the case of diffuse lesions.
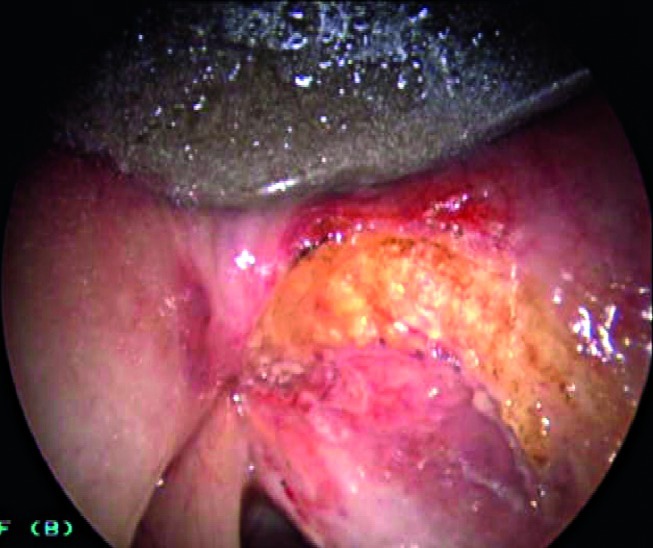
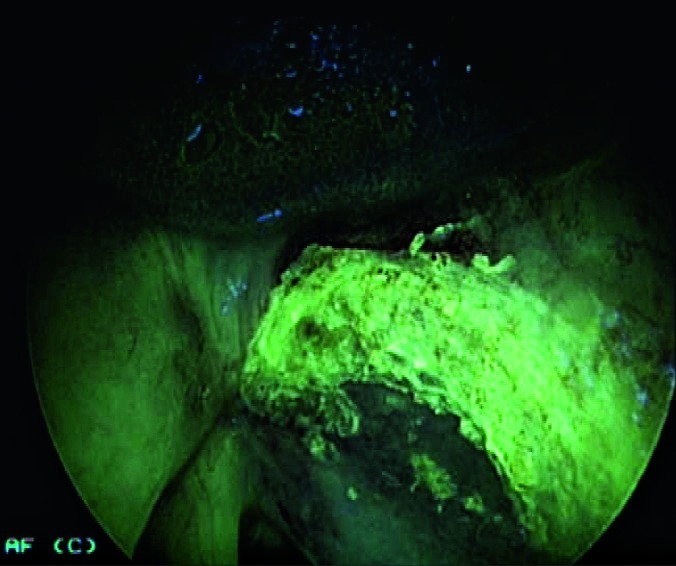
Improved definition in direct microlaryngoscopy of the superficial extension of a neoplastic lesion, which is useful to widen superficial laser resections (Fig. 7a, 7b).
Extensive use in follow-up in searching for synchronous/ metachronous second tumours.
Figs. 7a, 7b.
Direct autofluorescence endoscopy with 70° rigid telescope (white light + autofluorescence) after CO2 laser vestibulectomy. The neoplastic lesion on the right vocal cord extends to the homolateral ventricle.
At present, one limitation is the inability to combine AF with HDTV. Considering specificity, a substantial number of false positives are observed in patients that have been previously treated, which is associated with inflammation/ superinfection and modification of the mucosal surface. The learning curve is rather rapid, but there is, especially in indirect laryngoscopy, the need to avoid any condition that will obstruct visualization of AF.
References
- 1.Remacle M, Lawson G. Exploration du larynx. Encycl Méd Chir (Elsevier, Paris), Oto-rhino-laryngologie. 1997;20:635-A-10–635-A-10. [Google Scholar]
- 2.Casolino D, Ricci Maccarini A, Magnani M. La laringostroboscopia. :134–149. Rel. Uff. LXXXIX Congresso Nazionale. [Google Scholar]
- 3.Piazza C, Dessouky O, Peretti G, et al. Narrow band imaging: a new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol Ital. 2008;28:49–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Piazza C, Cocco D, Benedetto L, et al. Narrow band imaging and high definition television in the assessment of laryngeal cancer: a prospective study on 279 patients. Eur Arch Otorhinolaryngol. 2010;267:409–414. doi: 10.1007/s00405-009-1121-6. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe A, Tsujie H, Taniguchi M, et al. Laryngoscopic detection of pharyngeal carcinoma in situ with narrow band imaging. Laryngoscope. 2006;116:650–654. doi: 10.1097/01.mlg.0000204304.38797.34. [DOI] [PubMed] [Google Scholar]
- 6.Muto M, Nakane M, Katade C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–1381. doi: 10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 7.D'Hallewin M, Bezdetnaye L, Guillemin F. Fluorescence detection of bladder cancer: a review. Eur Urol. 2002;42:417–425. doi: 10.1016/s0302-2838(02)00402-5. [DOI] [PubMed] [Google Scholar]
- 8.Freyen A, Glanz H, Lohmann W, et al. Significance of autofluorescence fort the optical demarcation of field cancerization in the upper aerodigestive tract. Acta Otolaryngol. 1997;117:316–319. doi: 10.3109/00016489709117795. [DOI] [PubMed] [Google Scholar]
- 9.Alfano R, Tata D, Cordero J. Laser induced fluorescence spectroscopy for native cancerous and normal tissue. IEEE J Quant Electron. 1984;20:1507–1511. [Google Scholar]
- 10.Saetti R, Derosas F, Silvestrini M, et al. Efficacy of autofluorescence videoendoscopy in the diagnosis of laryngeal lesions. Acta Otorhinolaryngol Ital. 2007;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- 11.Malzahn K, Dreyer T, Glanz H, et al. Autofluorescence endoscopy in the diagnosis of early laryngeal cancer and its precursor lesions. Laryngoscope. 2002;112:488–493. doi: 10.1097/00005537-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Arens C, Dreyer T, Glanz H, et al. Indirect autofluorescence laryngoscopy in the diagnosis of laryngeal cancer and its precursor lesions. Eur Arch Otorhinolaryngol. 2004;261:71–76. doi: 10.1007/s00405-003-0653-4. [DOI] [PubMed] [Google Scholar]
- 13.Otto KJ, Hapner ER, Baker M, et al. Blinded evaluation of the effects of high definition and magnification on perceived image quality in laryngeal imaging. Ann Otol Rhinol Laryngol. 2006;115:110–113. doi: 10.1177/000348940611500205. [DOI] [PubMed] [Google Scholar]
- 14.Hagiike M, Phillips EH, Berci G. Performance differences in laparoscopic surgical skills between true high-definition and three-chip CCD video systems. Surg Endoscop. 2007;21:1849–1854. doi: 10.1007/s00464-007-9541-0. [DOI] [PubMed] [Google Scholar]