SUMMARY
Long-standing peripheral monolateral facial paralysis in the adult has challenged otolaryngologists, neurologists and plastic surgeons for centuries. Notwithstanding, the ultimate goal of normality of the paralyzed hemi-face with symmetry at rest, and the achievement of a spontaneous symmetrical smile with corneal protection, has not been fully reached. At the beginning of the 20th century, the main options were neural reconstructions including accessory to facial nerve transfer and hypoglossal to facial nerve crossover. In the first half of the 20th century, various techniques for static correction with autologous temporalis muscle and fascia grafts were proposed as the techniques of Gillies (1934) and McLaughlin (1949). Cross-facial nerve grafts have been performed since the beginning of the 1970s often with the attempt to transplant free-muscle to restore active movements. However, these transplants were non-vascularized, and further evaluations revealed central fibrosis and minimal return of function. A major step was taken in the second half of the 1970s, with the introduction of microneurovascular muscle transfer in facial reanimation, which, often combined in two steps with a cross-facial nerve graft, has become the most popular option for the comprehensive treatment of long-standing facial paralysis. In the second half of the 1990s in France, a regional muscle transfer technique with the definite advantages of being one-step, technically easier and relatively fast, namely lengthening temporalis myoplasty, acquired popularity and consensus among surgeons treating facial paralysis. A total of 111 patients with facial paralysis were treated in Caen between 1997 and 2005 by a single surgeon who developed 2 variants of the technique (V1, V2), each with its advantages and disadvantages, but both based on the same anatomo-functional background and aim, which is transfer of the temporalis muscle tendon on the coronoid process to the lips. For a comprehensive treatment of the paralysis, the eyelids are usually managed by Paul Tessier's technique to lengthen the levator muscle of the upper eyelid by aponeurosis interposition, combined with external blepharorrhaphy with Krastinova-Lolov's technique. Facial reanimation using lengthening temporalis myoplasty is a dynamic procedure that has its roots in the techniques of Gillies and McLaughlin. This method is a true lengthening myoplasty procedure using no intermediate grafts. In general, the results with a 1-stage combination of lengthening temporalis myoplasty and static correction of the lagophthalmos appear comparable with the major series in the literature using free microneurovascular transfers combined with cross-facial nerve grafts for longstanding peripheral monolateral facial paralysis. The obvious advantages of temporalis elongation myoplasty consist in its technical ease, a single step, low incidence of complications and markedly reduced operating time.
KEY WORDS: Lenghtening temporalis myoplasty, Facial paralysis
RIASSUNTO
Le paralisi monolaterali periferiche di lunga data dell'adulto sono state per secoli un problema di difficile gestione per otorinolaringoiatri, neurologi e chirurghi plastici, e tuttora l'obiettivo finale della normalizzazione dell'emivolto paralizzato con simmetria a riposo ed un sorriso spontaneo e simmetrico, con al contempo la protezione della cornea non è stato ancora pienamente conseguito. All'inizio del ventesimo secolo le opzioni principali in questi casi erano le anastomosi nervose con altri rami, possibili solo quando il moncone distale del VII fosse stato recuperabile, ed eseguite con il nervo accessorio e l'ipoglosso. Nella prima metà del ventesimo secolo vennero proposte varie tecniche di correzione statica, tra queste quella di Gillies e quella di McLaughlin comportavano l'utilizzo del muscolo temporale e di innesti di fascia autologhi.Gli innesti nervosi incrociati sul nervo facciale sono stati eseguiti dall'inizio degli anni Settanta, talvolta combinati con il trapianto di tessuto muscolare nel tentativo di ripristinare dei movimenti attivi. Tuttavia nei primi trapianti il muscolo era non rivascolarizzato ed esitavano inevitabilmente in fibrosi e minimo recupero funzionale. Un importante passo avanti è venuto nella seconda metà degli anni Settanta con l'introduzione dei lembi liberi rivascolarizzati nella riabilitazione delle paralisi del facciale che, combinata, spesso come seconda procedura, con un innesto nervoso sul facciale funzionante controlaterale, è diventata l'opzione più popolare e praticata nel mondo dai cultori della materia. Nella seconda metà degli anni Novanta la plastica di allungamento del temporale, tecnica che prevede il trasferimento di un lembo muscolare regionale, con il vantaggio di essere monofasica, tecnicamente più semplice e relativamente rapida, si è progressivamente diffusa in Francia, guadagnando popolarità e consensi tra i chirurghi che si occupano di paralisi del facciale. A Caen un singolo chirurgo ha trattato 111 pazienti con paralisi del facciale tra il 1997 ed il 2005, sviluppando 2 diverse varianti della tecnica (V1 e V2) basate entrambe sugli stessi principi anatomofunzionali: il trasferimento del tendine del muscolo temporale dal processo coronoideo alla rima buccale. Ai fini di un trattamento completo della paralisi, la palpebra superiore viene solitamente gestita attraverso la tecnica di Paul-Tessier, che prevede un allungamento del tendine dell'elevatore tramite innesto di fascia, quella inferiore mediante una blefarrorrafia esterna secondo Krastinova-Lolov. La rianimazione del facciale attraverso la plastica di allungamento del temporale è una procedura dinamica che affonda le sue radici nelle tecniche descritte da Gillies e McLaughlin. A differenza di queste ultime, si tratta di una plastica di allungamento vera che non prevede l'utilizzo di innesti. Secondo la nostra opinione, complessivamente, la combinazione in un'unica procedura di suddetta plastica di allungamento con le sopracitate tecniche di correzione statica del lagoftalmo appare ottenere risultati comparabili con le maggiori casistiche descritte in letteratura di trasferimento di lembi muscolari microneurovascolari combinati con innesti incrociati sul facciale controlaterale, per il trattamento di paralisi facciali monolaterali periferiche di lunga data. Gli ovvi vantaggi della tecnica consistono nel fatto che prevede un unico tempo chirurgico, è tecnicamente relativamente semplice, sicuramente più rapida e quindi caratterizzata da un più basso tasso di complicanze perioperatorie.
Introduction
Facial nerve dysfunction can dramatically affect many attributes of a patient's quality of life as the human visage is a focal point for expression and interpersonal communication. Furthermore, facial motor movement contributes to eye protection, speech articulation, chewing and swallowing. The optimum management of facial nerve dysfunction is highly debated due to the lack of objective, quantitative measures of facial weakness and reliable prognostic indicators for spontaneous recovery. More importantly, rehabilitative outcomes are inconsistent in the existing literature.
An appropriate therapeutic approach requires thorough knowledge of the different aetiologies underlying facial dysfunction. There are numerous causes of facial dysfunction, and each is associated with a different clinical course. The first distinction must be made between central and peripheral facial dysfunction. Peripheral cases are often referred to an otolaryngologist, who has to accurately define each individual clinical picture. Peripheral facial paralysis refers to a condition in which all or portions of the facial nerves are paralyzed.
Spontaneous recovery is frequent in some peripheral deficits such as Bell's palsy, but impossible in others. In the literature, spontaneous recovery, independently of the aetiology, is considered possible in the absence of an anatomical interruption of the nerve within 6-8 months after clinical onset. In the present review, as in most of the literature 1, paralysis is considered long-standing after 18 months.
Herein, we focus on long-standing peripheral monolateral facial paralysis in the adult. This condition has challenged otolaryngologists, neurologists and plastic surgeons for centuries. Notwithstanding, the ultimate goal of normality of the paralysed hemi-face with symmetry, and achievement of a spontaneous symmetrical smile with corneal protection, has not been fully reached. Until the end of the 19th century, treatment involved non-surgical methods such as ointments, medicines and electrotherapy 2; nonsurgical measures are also employed at present, but when possible are supported by surgical procedures. In 1895, Sir Charles Balance, the founder and first president of the Society of British Neurological Surgeons, was the first to operate on the facial nerve and restore facial muscle function 1 3. Since then, many safe and innovative procedures have been suggested to treat facial paralysis sequelae, but none have been approved unanimously because the results are always not fully satisfactory. At the beginning of the 20th century, neural reconstructions included accessory to facial nerve transfer and hypoglossal to facial nerve crossover 4.
In the first half of the 20th century, various techniques for static correction with autologous muscle and fascia grafts were also proposed. Among such attempts, in 1934 Gillies 5 had the idea of using the middle third of the temporalis muscle, flipped over the zygomatic arch, to which he implanted a strip of the fascia lata. In 1949, McLaughlin 6 described a method that used the entire muscle after sectioning the coronoid process through an intraoral approach, using a strip of the fascia lata, as well.
The use of cross-facial nerve grafts to restore function of the paralyzed hemi-face had been attempted by Scaramella and Smith in 1971 and was popularized by Anderl in 1973 7. Thompson 8, in 1971, was the first to attempt free-muscle transplantation to restore active movements. However, these transplants were non-vascularized, and further evaluations revealed central fibrosis and minimal return of function.
In summary, the free tissue transfer techniques described above were unsuccessful because non-vascularised transplants 9 were used, although they did offer the possibility for transferred muscles to move with spontaneous emotion 1.
Starting from the 1970's, when microneurovascular muscle transfers were introduced, this approach has progressively become the most practiced for the reanimation of the paralyzed hemi-face, and at present is generally considered superior to static correction or regional muscle transposition 10.
In 1970, Tamai et al. 11 showed in dogs that muscle tissue could survive transplantation on microvascular transfer. In 1976, Chen Zhong Wei from Sixth People's Hospital in China and Ikuta et al. 12 from Japan reported on the transfer of the sternal portion of the pectoralis major muscle to obtain forearm flexors. The first case of facial reanimation exploiting the potential of microvascular free tissue transfer was carried out by Harii in 1976 using gracilis as the reanimating muscle, and the deep temporal motor branch of the trigeminal nerve as the donor nerve 13.
Increasing experience with the operating microscope soon saw vascularised muscle transfers being coupled with crossfacial nerve grafts, and with this the possibility of spontaneous emotion being restored to the paralyzed hemi-face seemed closer. In this perspective, Terzis and her group evaluated the relevant factors for functional restoration of the transplanted muscles first on an experimental model 14, and then described the technique coupling a cross-facial nerve graft and microneurovascular transfer of pectoralis minor muscle. Successively, Terzis published her early 4 and long-term 10 results in a clinical setting, describing a series of patients with longstanding or developmental facial paralysis who had undergo free-muscle transplantation (mainly gracilis or pectoralis minor). In the 1990s, one-stage reconstruction was introduced using a free-muscle transfer with a long supplying nerve to reach the contralateral facial nerve 15-17. At present, a two-step procedure involving cross facial nerve grafting followed by microneurovascolar muscle transfer as described by Terzis and many other authors 18 19 is probably the most popular approach in treatment of long-standing monolateral peripheral severe facial nerve weakness as it has been argued that it provides the important advantage of a coordinated, physiologic and potentially symmetric smile.
In the second half of the 1990's we proposed a regional muscle transfer technique, namely lengthening temporalis myoplasty, with the definite advantages of being one-step, technically easier and relatively fast 20. This technique, in our opinion, provides outstanding results in terms of smile restoration, but does not rehabilitate the eye. Accordingly, it should be combined with another procedure for correction of lagophthalmos. The technique has been practiced by Labbé for the last 15 years with satisfactory results, and was recently implemented at the Carmel Medical Center, Haifa, at Policlinico "Agostino Gemelli", Rome and in several other centres worldwide 21. In the present review, the technique, physiological and anatomical mainstays, and arguments supporting the use of this technique, in comparison with the more popular microneurovascular free transfers, in monolateral long-standing peripheral facial paralysis, are summarized.
Lengthening temporalis myoplasty
Preoperative Evaluation
Preoperative evaluation of the patient is mandatory for the determination of smile classification. We suggest the Rubin 22 classification. If the patient has a "Mona Lisa" type smile, the temporalis tendon will be preferably fixed on the mobile part of the zygomaticus major muscle in the labial commissure area. If the patient has a "canine" type of smile, the temporalis tendon will be preferably fixed on the upper lip levator muscles (musculus levator labii superioris alaeque nasi) and the posterior part of the nasal alae. If the patient has a "full teeth" smile, fixation will be preferably made, as far as possible, on the zygomatic major muscle, on the upper lip levator muscles.
Surgical steps for smile restoration
We developed two variants of the technique (V1, V2) each one with its advantages and disadvantages, but both with the same anatomofunctional background and aim, namely transfer of the temporalis muscle tendon on the coronoid process to the lips. Therefore, a mobile point, the temporalis muscle, is transferred toward other mobile points, the lips, with respect to a fixed temporal point. Considering both the V1 and V2 approaches, 111 patients with facial paralysis have been treated in Caen between 1997 and 2005 by a single surgeon (LD) (Table I).
The patient is placed in supine position, with slight proclivity. Nasotracheal intubation is preferred to allow a better appreciation of the symmetry of the lips during and at the end of the procedure.
Classic coronal approach (V1)
A strip 2 cm wide is shaved starting behind the level of the helix insertion, from one ear to the other. The anterior limit of the strip is first vertical for approximately 5 cm in the temporal area. It then runs 4 to 5 cm behind the hair line and parallel to it. The hair is braided on each side 20 23.
The incision line starts right above the insertion of the helix and runs at the posterior limit of the shaved area. Three reference marks are made for closure lining. The patient is meticulously prepared and draped under general anaesthesia in the classic manner, leaving the scalp and the face exposed. Subcutaneous injection with 250 cc of normal saline and 0.25 mg of epinephrine is made along the incision line. In the zygomatic area, the injection is made subperiosteally. The incision is made all the way to the periosteum with the scalpel tilted to protect the hair follicles that will later grow into the scar. Clips such as Cologne staples are placed on the edges to minimize bleeding, and electric cautery is avoided to prevent alopecia.
From the coronal incision, the forehead flap is elevated in the subgaleal plane until 1 or 2 cm above the orbital rim. In the temporal area, elevation is pursued posteriorly along the temporalis muscle insertion. A sharp scalpel is used with its blade always in contact with the superficial temporal aponeurosis. While the assistant gently pulls the flap down, the dissection progresses in a plane between the superficial temporal fascia and the temporal aponeurosis until 15 to 20 mm above the zygomatic arch.
An incision is then made parallel to the zygomatic arch on the superficial temporal fascia that joins the frontal periosteal incision medially and superiorly. The incision continues inferiorly between the two layers of the temporalis aponeurosis to the zygomatic arch. When the dissection is made medially on the arch, bone contact should always be kept in a safe plane. Subperiosteal dissection is carried out by an elevator on the zygomatic arch laterally to medially. By sweeping movements of the elevator, both zygomatic and external orbital rim dissections are joined together in the zygomatic area.
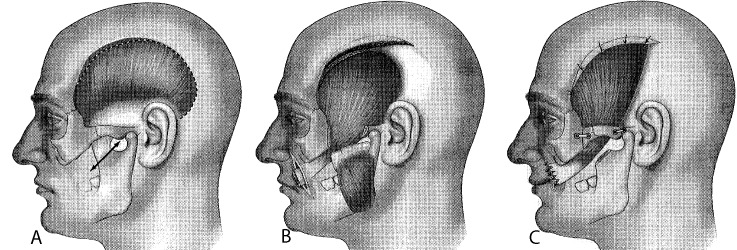
The zygomatic arch is then clearly exposed and can be sectioned, using a saw, and shifted inferiorly, still attached to the masseter muscle. The zygomatic arch should be sectioned far anteriorly to obtain the best access to the coronoid process, which can be better visualized by mobilizing the mandible. When the temporalis tendon is correctly isolated, the coronoid process will be osteotomized. The temporalis tendon is still attached to the bone, which allows easier transfer towards the lips through a cheek tunnel. Temporalis aponeurosis is incised 1 cm below the temporal crest and on its anterior half to leave a strip of aponeurosis attached to the crest for the final suturing of the muscle (Fig. 1a).
Fig. 1.
Surgical steps of lengthening temporalis myoplasty through a classic coronal approach (V1): osteotomy of the coronoid process after flipping the zygomatic arch incision of the temporal aponeurosis, preserving a 1-cm strip anteriorly (a); elevation of the muscle in the temporal fossa passing through the synsarcosis of the corpus adiposum buccae (b); insertion to the lips and reinsertion to the aponeurotic strip (c).
Once the aponeurosis is incised, the entire muscle is dissected off the bone by an elevator. The dissection is carried out in the temporal fossa, on the lateral orbital rim, down to the infratemporal crest. This preserves the deep temporal nerve and vessels, which are often clearly visible on the lower part of the deep muscle surface at the level of the zygomatic arch.
A 4-cm incision is made in the nasolabial crease. In elderly patients, skin resection is performed. The cheek is tunnelized by scissors in the plane of the corpus adiposum buccae synsarcosis, medial to the masseter muscle. The coronoid process is grabbed by forceps and pulled with the temporalis tendon into the cheek to the labial commissure through the cheek tunnel. This plane follows the temporal extension of the corpus adiposum buccae in the masticatory synsarcosis.
Once in the labial commissure, the temporalis tendon is detached from its coronoid bone attachments, spread 3 to 4 cm wide, and sutured to the perioral muscles. Suturing sites are selected based on the patient's smile type and go up to the nasal alae (Fig. 1b, 1c). The temporalis muscle body is then stretched and sutured to the aponeurotic strip left on the anterior portion of the crest. The temporalis muscle is lengthened at the expense of the posterior third. The traction of the muscle before its fixation creates an overcorrection of the paralyzed side. The zygomatic arch is fixed, and closing and dressing of the coronal approach are done in the classic manner (Fig. 1c).
Modified hemicoronal approach (V2)
The V2 version is a technical simplification 24 of the lengthening temporalis myoplasty V1, based on the temporalis muscle anatomical structure, which is featherlike and supported by the observation of muscle lengthening in more than 100 lengthening temporalis myoplasties.
This technique can be considered also in cases in which the main pedicles of the muscle (anterior and posterior deep temporal artery) are compromised or are not reliable, and the muscle may be vascularized by a contralateral flow.
The surgical approach is hemicoronal (not coronal as in V1) and in the nasolabial fold. The undermining of scalp in subgaleal plane is limited to the posterior half of muscle, and the anterior limit is an imaginary line traced from the radix of helix upward to respect the perforator arteries from the contralateral superficial temporal artery.
The temporalis aponeurosis is incised only on the posterior half of temporal crest leaving a strip of aponeurosis attached for final suturing. The undermining of the posterior part of muscle is performed in the retrozygomatic region under direct vision.
The muscle is undermined in sub-periosteal plane under the all surface, but the insertions on superior margin of zygoma, on lateral orbital rim are left. The dissection is carried out from the temporal fossa to the infratemporal fossa until reaching the infratemporal crest blindly (without direct vision). The coronoid process is reached through the nasolabial fold approach. After incision in the nasolabial crease and after tunnelizing in the corpus adiposum buccae synsarcosis by scissor, as in the V1, the coronoid process is visualized and sectioned. The remaining steps are as in the V1 approach.
Thus, the major modification from version 1 (V1) consists in avoiding superficial undermining of the anterior part of the temporal muscle and in performing osteotomy of the coronoid process with a nasolabial approach avoiding the osteotomy of the zygoma.
The surgery is quicker and less invasive in V2, and usually the postoperative hospital stay is shorter. However, with V2 the main pedicles are damaged. Nevertheless, as in some steps exposure of the structures is less comfortable and some maneuvers are almost blind (as the final part of the dissection of the deep surface of the muscle towards the infratemporal crest), V2 requires a higher degree of confidence with the regional anatomy. We suggest to practice V1, at least on cadavers, before performing a lengthening temporalis myoplasty with a V2 approach.
Correction of lagophthalmos
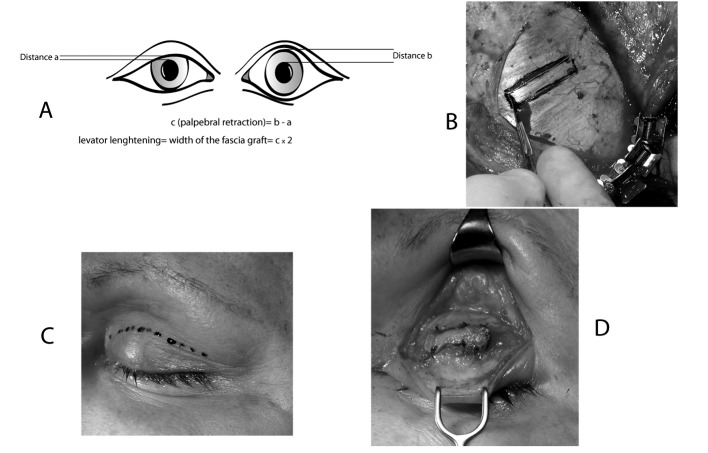
There are several surgical techniques for the treatment of lagophthalmos, and all give satisfactory functional results. We usually prefer Paul Tessier's technique for lengthening the levator muscle of the upper eyelid by aponeurosis interposition, combined with the external blepharorrhaphy by Krastinova-Lolov's technique. These operations are performed, under either general anaesthesia (if combined with temporalis lengthening myoplasty during the same operations) or under local anaesthetic with intravenous sedation. Paul Tessier's technique for lengthening the levator of the upper eyelid by aponeurosis 25 26 is reliable, simple and reproducible, allowing the weakening of the levator, which, in contrast is reinforced by the gold plate in the standard technique. The aponeurotic graft (Fig. 3b) is removed via coronal (V1) or temporal (V2) incision to expose the temporal aponeurosis. The aponeurotic graft is rectangular, and its width is twice that of the palpebral retraction (c) observed before the operation (Fig. 3a). This retraction corresponds to the distance observed between the superior edge of the contracted pupil and the free edge of the upper eyelid on the paralyzed side subtracted from the equivalent distance measured in the healthy eye. The length of the graft is about 2 cm, and is adapted to the patient's eye morphology. First, the scalp and upper eyelid are infiltrated with adrenaline in serum (1/1000). Palpebral incision is made in the superior palpebral fold (Fig. 3c). The upper edge of the tarsal plate is identified and dissected to separate the aponeurosis from the eyelid levator. With its inferior end free, the internal surface of the levator is then released by incision of the Muller muscle. During the procedure, the conjunctiva can be infiltrated with adrenaline in serum to prevent damage. The inferior edge of the upper eyelid levator is pulled (by the assistant) towards the lower section, and its internal and external horn is sectioned high up to release the tissue fully. Next, the temporal muscle is exposed taking care not to perforate the fascia temporalis. The contours of the graft are marked in ink, using either ruler or calipers to ensure accurate measurements. The aponeurosis is infiltrated with adrenaline in saline solution to facilitate graft removal. The aponeurosis is sectioned with a cold scalpel and removed from the muscle. The graft is positioned between the superior edge of the tarsal plate and the inferior edge of the levator and sutured with a few separate stitches using resorbable suture material 5-0 (Fig. 3d). The cutaneous area is closed with a few separate stitches using nonresorbable suture 5-0. This technique is simple, reliable, and easily reproduced, and involves a mathematical formula, which can be applied to all patients, to calculate lengthening (Fig. 3a).
Fig. 3.
Paul Tessier's technique for lengthening the levator muscle of the upper eyelid. This technique is simple, reliable and easily reproduced, and involves a mathematical formula, which can be applied to all patients, to calculate lengthening. The aponeurotic graft is rectangular, and its width is double the palpebral retraction (c) observed before the operation (a). The aponeurotic graft is removed via a coronal (V1) or a temporal (V2) incision to expose the temporal aponeurosis (b). The palpebral incision is made in the superior palpebral fold (c). The upper edge of the tarsal plate is identified and dissected to separate the aponeurosis from the eyelid levator. The aponeurosis is sectioned with a cold scalpel and removed from the muscle. The graft is positioned between the superior edge of the tarsal plate and the inferior edge of the levator and sutured (d).
Fig. 2.
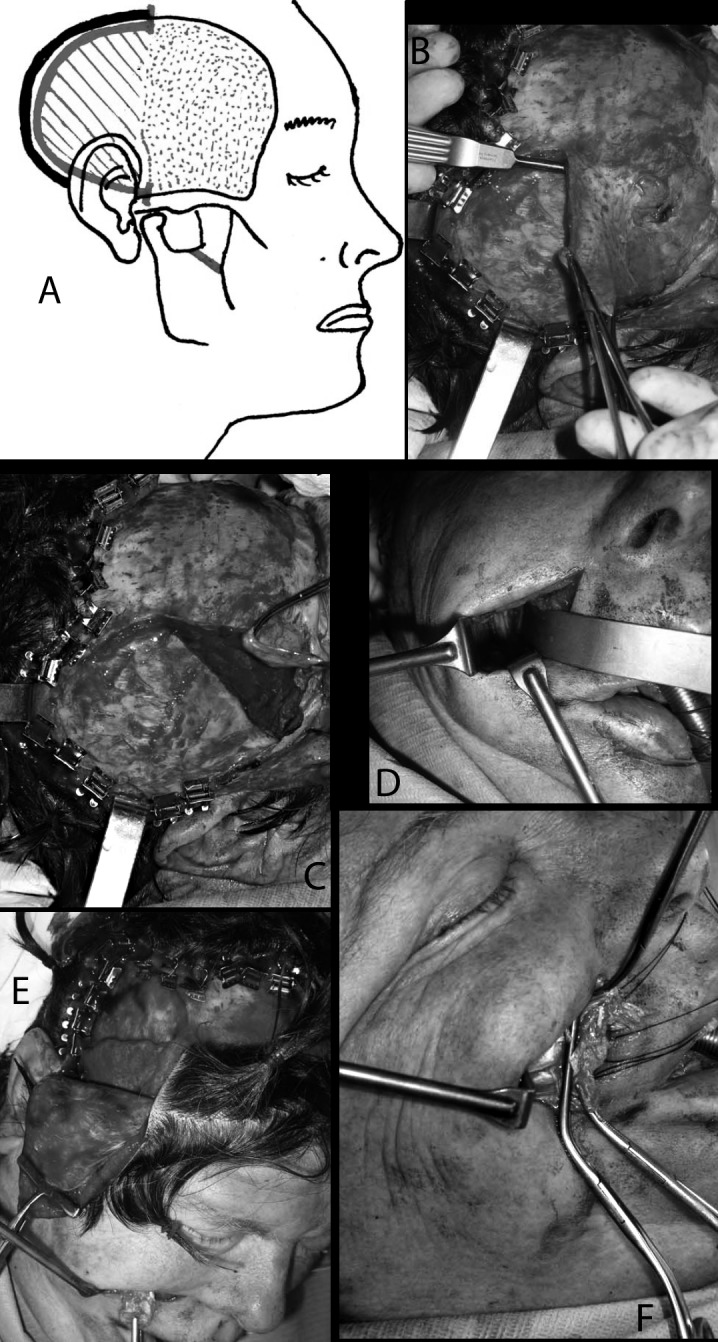
The continuous line shows incision of the temporal aponeurosis on the posterior part of the insertion of temporalis muscle, which in the hatched area is completely dissected and in the dotted area (anteriorly) is dissected only on its deeper surface (a). In the following pictures, the steps on the surgical bed are shown more in detail. After posterior detachment of the temporal aponeurosis (b), dissection of the posterior part of the muscle is carried out starting from the retrozygomatic region. The deep surface of the temporalis is dissected along a subperiostal plane down to the sphenotemporal crest below and to the temporal crest above (c). The coronoid process is reached through the nasolabial fold approach (d). After incision in the nasolabial crease and after tunnelizing in the corpus adiposum buccae synsarcosis by scissors, as in V1, the coronoid process is visualized and sectioned, preserving the attachments of the temporalis muscle. The coronoid process is grabbed and pulled towards the labial rim through the tunnel, with the muscle, whose dissection from masseterine fibres is completed in this phase, and the lengthening at the expense of the posterior part becomes evident (e). Finally, the coronoid process can be detached from the tendon which is sutured to the lips as in V1.
There are a number of methods by which the lower eyelid can be elevated, but Krastinova-Lolov's external blepharorraphy is our choice. It is a simple procedure that is quickly performed and gives a high degree of aesthetic quality and function 27. The technique consists in making an incision approximately 1 mm deep in the grey line of the upper and lower lid from lateral canthus as far medially as the length of the intended tarsorraphy. Normally, one-fourth of length in the upper lid is used and the double of length in the inferior lid, with a ratio of 1:2. The incongruity of length is well tolerated and allows tightening the inferior lid without shortening the palpebral fissure. The incisions just anterior to the tarsal plates are deepened to split the lids into anterior and posterior lamellae as far as the proximal borders of the tarsal plates. Double-armed 4/0 non-absorbable mattress suture is then inserted which passes through the cut edges and through the tarsorrhaphy tubing. The sutures are left in place for 12-15 days.
Physical therapy
Physical therapy is fundamental to achieve the best functional and cosmetic results after any surgery for facial reanimation, and especially after lengthening temporalis myoplasty, as it can help to obtain a fully spontaneous smile.
Specific physical therapy will work on labial mobility, facial and smiling symmetry, swallowing and speech articulation. It starts on the 20th postoperative day, once a week. The patient will be asked to pursue the exercises four times a day at home. Booklets are given to the patient. First is muscular workout. Regarding the lips, commissure elevation is obtained by soliciting mandibular movements that correspond to the original function of the temporalis muscle (e.g., biting, retropulsion, and lateral movement to the operated side); opposite labial movements are also used for plasticity of the temporalis muscle and lip mobility (pursing and contraction of the orbicularis muscle). Labial function needs to be improved in its new position. The patient has to contract the cheek between the teeth. Secondarily, the following functional therapy will start.
A smile is first obtained by mobilizing the mandible. After a few sessions, it appears without mobilization simply by independent contraction of the temporalis muscle. It is then integrated with exercises on concrete and natural unpredictable daily situations to obtain a temporal smile.
Swallowing is combined with labial function to ensure salivary continence, cheek strengthening for salivary emptying and mastication. At the beginning, this work should be done first on the operated side (e.g., by pushing the food with the tongue to the cheek laterally), and then on the normal side when the contraction of the temporalis muscle is independent so that the muscle is not used during mandibular mobilization. Special attention is given to commissure traction during the stretched phonemes I, E, S, and Z; to lip function for the bilabial phonemes, P, B, M; to cheek strength to prevent cheek puffing by pronouncing the bilabial phonemes and labiodental phonemes P, B, M, F, and V.
Physical therapy allows spontaneous elevation of lip commissure during the appropriate moments. The temporalis muscle should be trained for different functions, both for mobility and for command.
Discussion
Patients with unilateral facial paralysis suffer from facial asymmetry, which can cause severe problems in social interactions, and from dysfunctions in eye protection, speech articulation, chewing and swallowing. The challenging goal for reconstructive surgeons is to re-establish facial symmetry, tone and coordinated animation of the paralyzed face.
Various static procedures, from gold weight to elongation of the tendon of the elevator palpebrae, various partial tarsorrafies and cartilage graft implantation in the lower lid are aimed, with overall satisfying results, at obtaining closure and protection of the eye on the paralyzed side and a certain symmetry of the upper third of the face at rest 28. Dynamic procedures offer notable improvements, but are burdened by a higher degree of complexity 29 30. In management of the upper third of the face eye protection is critical: the proposed static operations are not technically demanding or particularly invasive and are often performed under local anaesthesia. For these reasons, surgical static correction of eye closure may always be attempted in paralytic lagophthalmos and some authors 1 propose performing only these types of procedures in patients over 55 years of age or in poor general condition.
In fact, the middle and lower third of the face can be reanimated only with more complex, technically demanding, time consuming, often invasive and requiring general anaesthesia. In these cases, the goal is restoring the smile, and therefore static procedures cannot be completely satisfying. At present, a two-step procedure involving cross facial nerve grafting (CFNG) followed by microneurovascolar muscle transfer as described by Terzis and several other authors 1 4 18 19 31-33 is probably the most popular approach for treatment of middle and lower third of the face in long-standing monolateral peripheral severe facial nerve weakness. The gracilis, latissimus dorsi and pectoralis minor are the most commonly-used muscles in that approach. In the first stage, the contralateral facial nerve is usually chosen as the donor nerve, and a redundant zygomaticus branch is selected for grafting. Terzis and other surgeons in the first stage also perform a "baby sitter" procedure, which is a coaptation of ipsilateral 40% hypoglossal to facial nerve on the affected side to improve trophism of the facial muscles while nerve regeneration proceeds through the CFNG 34. In the CFNG, the sural nerve is anastomosed to the contralateral facial nerve and tunneled subcutaneously from the donor nerve to the planned site of free-muscle transfer and the distal segment of the graft is tagged. The ideal time for the muscle transfer occurs when a Tinel sign is detected in the distal nerve end, indicating completion of axon growth, usually 9-12 months later. In the second step, the neuromuscular flaps (usually gracilis, latissimus dorsi or pectoralis minor) are harvested and transferred to the paralyzed hemiface where arterial, venous and neural microanastomosis are performed. It has been argued that this technique offers several advantages as it can provide an adequate extent of muscular contraction and, most of all, a coordinated spontaneous smile under control of a functioning facial nerve. Nevertheless, the results are partial, as with any other corrective method, and sometimes unpredictable even at the end of a long learning curve, requiring further surgical revisions even after the two planned costly and time consuming procedures 35. Furthermore, this surgery is very demanding for the surgeon and anaesthesiologist, and most of all for the patient: such complexity substantially increases both the rate of early complications and costs.
Facial reanimation using lengthening temporalis myoplasty is a dynamic procedure which has its roots in the techniques of Gillies and McLaughlin 5 6, described in 2000 by Labbé and Huault 20. The technique is a true lengthening myoplasty procedure using no intermediate grafts. The temporalis muscle is elongated and the released coronoid tendon of the muscle is transferred to the nasolabial fold and lips, thus preserving a fixed temporal point. This is a dynamic correction since the motor innervation to the temporalis muscle coming from the third branch of the trigeminus is preserved (which is, of course, a fundamental requirement for success), and the muscle normally contracts at the end of the operation.
The advocates of one-step or two-step cross facial nerve grafting combined with microneurovascolar muscle transfers assert that symmetric and spontaneous smile in facial reanimation is a typical feature of reinnervation through the VIIth nerve. Nevertheless, several authors reported very good results using trigeminal motor branches as donor nerves; in particular, Bae, when evaluating labial commissure excursion following gracilis microneurovascular transfer, found significantly better muscle function when the donor nerve was the masseteric nerve versus cross facial nerve graft 36. Spontaneous, physiologic smile, on the other hand, does not rely mainly on muscular strength but on neural motor patterns. Nonetheless, it appears to be an infrequent occurrence in patients after both "facial" and "trigeminal" reanimation, but, according to some authors, in the latter group the overall quality of the smile seems to be better 37, leading to hypothesize a wider employment of the masseteric branch as the donor nerve even in early cases 38. Using the Labbè technique, always based on trigeminal branches, we observed a significant portion of patients with a spontaneous smile, which is probably attributable to cortical plasticity as also documented by functional imaging data (data not shown).
On the whole, the results of a 1-stage lengthening temporalis myoplasty 20 23 24 39 combined with static correction of lagophthalmos appear to be, in our opinion, comparable with the main series in the literature involving free microneurovascular transfers for long-standing peripheral monolateral facial paralysis (Fig. 4). The advantages of temporalis elongation myoplasty obviously consist in its technical ease, only 1 step, a low incidence of complications and markedly reduced operating time.
Fig. 4.

Lengthening temporalis myoplasty in two patients. On the left side, the preoperative static (a, e) and dynamic (c, g) pictures are shown. On the right side, static (b, f) and dynamic (d, h) outcomes.
References
- 1.Ghali S, MacQuillan A, Grobbelaar AO. Reanimation of the middle and lower face in facial paralysis: review of the literature and personal approach. J Plast Reconstr Aesthet Surg. 2011;64:423–431. doi: 10.1016/j.bjps.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Graaf RC, Nicolai JP. Bell's palsy before Bell: Cornelis Stalpart van der Wiel's observation of Bell's palsy in 1683. Otol Neurotol. 2005;26:1235–1238. doi: 10.1097/01.mao.0000194892.33721.f0. [DOI] [PubMed] [Google Scholar]
- 3.Graaf RC, Nicolai JP. Was Thomasz Drobnik really the first to operate on the facial nerve? Otol Neurotol. 2003;24:686–690. doi: 10.1097/00129492-200307000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Terzis JK, Noah ME. Analysis of 100 cases of free-muscle transplantation for facial paralysis. Plast Reconstr Surg. 1997;99:1905–1921. doi: 10.1097/00006534-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Gillies H. Experiences with fascia lata grafts in the operative treatment of facial paralysis. Proc R Soc Med. 1934;27:1372–1382. [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin CR. Surgical support in permanent facial paralysis. Plast Reconstr Surg. 1953;11:302–314. doi: 10.1097/00006534-195304000-00007. (1946) [DOI] [PubMed] [Google Scholar]
- 7.Anderl H. Cross-face nerve transplantation in facial palsy. Ann Chir Gynaecol. 1982;71:70–76. [PubMed] [Google Scholar]
- 8.Thompson N. Autogenous free grafts of skeletal muscle. A preliminary experimental and clinical study. Plast Reconstr Surg. 1971;48:11–27. doi: 10.1097/00006534-197107000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Freilinger G. A new technique to correct facial paralysis. Plast Reconstr Surg. 1975;56:44–48. doi: 10.1097/00006534-197507000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Terzis JK, Olivares FS. Long-term outcomes of free-muscle transfer for smile restoration in adults. Plast Reconstr Surg. 2009;123:877–888. doi: 10.1097/PRS.0b013e31819ba316. [DOI] [PubMed] [Google Scholar]
- 11.Tamai S, Komatsu S, Sakamoto H, et al. Free muscle transplants in dogs, with microsurgical neurovascular anastomoses. Plast Reconstr Surg. 1970;46:219–225. doi: 10.1097/00006534-197009000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ikuta Y, Kubo T, Tsuge K. Free muscle transplantation by microsurgical technique to treat severe Volkmann's contracture. Plast Reconstr Surg. 1976;58:407–411. doi: 10.1097/00006534-197610000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis. A preliminary report. Plast Reconstr Surg. 1976;57:133–143. doi: 10.1097/00006534-197602000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Terzis JK, Sweet RC, Dykes RW, et al. Recovery of function in free muscle transplants using microneurovascular anastomoses. J Hand Surg Am. 1978;3:37–59. doi: 10.1016/s0363-5023(78)80116-6. [DOI] [PubMed] [Google Scholar]
- 15.Koshima I, Moriguchi T, Soeda S, et al. Free rectus femoris muscle transfer for one-stage reconstruction of established facial paralysis. Plast Reconstr Surg. 1994;94:421–430. doi: 10.1097/00006534-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Harii K, Asato H, Yoshimura K, et al. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sajjadian A, Song AY, Khorsandi CA, et al. One-stage reanimation of the paralyzed face using the rectus abdominis neurovascular free flap. Plast Reconstr Surg. 2006;117:1553–1559. doi: 10.1097/01.prs.0000206378.90174.6b. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien BM, Franklin JD, Morrison WA. Cross-facial nerve grafts and microneurovascular free muscle transfer for long established facial palsy. Br J Plast Surg. 1980;33:202–15. doi: 10.1016/0007-1226(80)90013-2. [DOI] [PubMed] [Google Scholar]
- 19.Manktelow RT. Free muscle transplantation for facial paralysis. Clin Plast Surg. 1984;11:215–220. [PubMed] [Google Scholar]
- 20.Labbe D, Huault M. Lengthening temporalis myoplasty and lip reanimation. Plast Reconstr Surg. 2000;105:1289–1297. [PubMed] [Google Scholar]
- 21.Byrne PJ, Kim M, Boahene K, et al. Temporalis tendon transfer as part of a comprehensive approach to facial reanimation. Arch Facial Plast Surg. 2007;9:234–241. doi: 10.1001/archfaci.9.4.234. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LR. The anatomy of a smile: its importance in the treatment of facial paralysis. Plast Reconstr Surg. 1974;53:384–387. doi: 10.1097/00006534-197404000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Labbe D. Lengthening of temporalis myoplasty and reanimation of lips. Technical notes. Ann Chir Plast Esthet. 1997;42:44–47. [PubMed] [Google Scholar]
- 24.Labbe D. Lenghtening temporalis myoplasty V.2. and lip reanimation. Ann Chir Plast Esthet. 2009;54:571–576. doi: 10.1016/j.anplas.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Guillou-Jamard MR, Labbe D, Bardot J, et al. Paul Tessier's technique in the treatment of paralytic lagophthalmos by lengthening of the levator muscle: evaluation of 29 cases. Ann Plast Surg. 2011;67:S31–S35. doi: 10.1097/SAP.0b013e318218360b. [DOI] [PubMed] [Google Scholar]
- 26.Tessier P, Delbet JP, Pastoriza J, et al. Paralized eyelids. Ann Chir Plast. 1969;14:215–223. [PubMed] [Google Scholar]
- 27.Krastinova-Lolov D, Seknadje P, Franchi G, et al. Aesthetic blepharoplasty. Ann Chir Plast Esthet. 2003;48:350–363. doi: 10.1016/j.anplas.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Pirrello R, D'Arpa S, Moschella F. Static treatment of paralytic lagophthalmos with autogenous tissues. Aesthetic Plast Surg. 2007;31:725–731. doi: 10.1007/s00266-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 29.Terzis JK, Bruno W. Outcomes with eye reanimation microsurgery. Facial Plast Surg. 2002;18:101–112. doi: 10.1055/s-2002-32200. [DOI] [PubMed] [Google Scholar]
- 30.Terzis JK, Karypidis D. Blink restoration in adult facial paralysis. Plast Reconstr Surg. 2010;126:126–139. doi: 10.1097/PRS.0b013e3181dbbf34. [DOI] [PubMed] [Google Scholar]
- 31.Chuang DC. Technique evolution for facial paralysis reconstruction using functioning free muscle transplantation - experience of Chang Gung Memorial Hospital. Clin Plast Surg. 2002;29:449–459. doi: 10.1016/s0094-1298(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 32.Kumar PA, Hassan KM. Cross-face nerve graft with freemuscle transfer for reanimation of the paralyzed face: a comparative study of the single-stage and two-stage procedures. Plast Reconstr Surg. 2002;109:451–462. doi: 10.1097/00006534-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Harrison DH. The treatment of unilateral and bilateral facial palsy using free muscle transfers. Clin Plast Surg. 2002;29:539–549. doi: 10.1016/s0094-1298(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 34.Terzis JK, Tzafetta K. "Babysitter" procedure with concomitant muscle transfer in facial paralysis. Plast Reconstr Surg. 2009;124:1142–1156. doi: 10.1097/PRS.0b013e3181b2b8bc. [DOI] [PubMed] [Google Scholar]
- 35.Terzis JK, Olivares FS. Secondary surgery in adult facial paralysis reanimation. Plast Reconstr Surg. 2009;124:1916–1931. doi: 10.1097/PRS.0b013e3181bcee62. [DOI] [PubMed] [Google Scholar]
- 36.Bae YC, Zuker RM, Manktelow RT, et al. A comparison of commissure excursion following gracilis muscle transplantation for facial paralysis using a cross-face nerve graft versus the motor nerve to the masseter nerve. Plast Reconstr Surg. 2006;117:2407–2413. doi: 10.1097/01.prs.0000218798.95027.21. [DOI] [PubMed] [Google Scholar]
- 37.Faria JC, Scopel GP, Ferreira MC. Facial reanimation with masseteric nerve: babysitter or permanent procedure? Preliminary results. Ann Plast Surg. 2010;64:31–34. doi: 10.1097/SAP.0b013e3181999ea9. [DOI] [PubMed] [Google Scholar]
- 38.Faria JC, Scopel GP, Busnardo FF, et al. Nerve sources for facial reanimation with muscle transplant in patients with unilateral facial palsy: clinical analysis of 3 techniques. Ann Plast Surg. 2007;59:87–91. doi: 10.1097/01.sap.0000252042.58200.c3. [DOI] [PubMed] [Google Scholar]
- 39.Labbe D. Lengthening temporalis myoplasty. Rev Stomatol Chir Maxillofac. 2002;103:79–83. [PubMed] [Google Scholar]