Abstract
Background:
Cerebral microdialysis (MD) provides valuable information about brain metabolism under normal and pathologic conditions. The CMA 600 microdialysis analyzer received US Food and Drug Administration (FDA) approval for clinical use in the United States in 2005. Since then, cerebral MD has been increasingly utilized nationally in the multimodal monitoring of traumatic brain injury (TBI), stroke, aneurysmal subarachnoid hemorrhage, and brain tumors. We describe a 5-year, single-institutional experience using cerebral MD at a community-based hospital, Legacy Emanuel Medical Center (LEMC). Implications for the adoption and utility of MD in medical centers with limited resources are discussed.
Methods:
This is a retrospective chart review and data analysis of 174 consecutive patients who had cerebral MD as part of multimodal brain monitoring. All cerebral MD catheters were placed by board-certified, attending neurosurgeons at LEMC. Clinical severity in the TBI patients was reported using initial Glasgow Coma Scale (GCS); radiologic severity was graded with the Marshall CT grading scale. Measures of the risks of MD placement included post-placement hemorrhage, cerebral infection, and dislodgement.
Results:
Between July 2005 and July 2010, 248 cerebral MD catheters were placed in 174 patients undergoing multimodal brain monitoring. One hundred and eighty-five catheters were placed at the time of open craniotomy. None were associated with cranial infection. Patients ranged in age from 5 months to 90 years, with a mean of 49 years. The male to female ratio was 1.4:1. The underlying pathologies were: TBI (126), cerebral vascular accident (24), aneurysmal subarachnoid hemorrhage (17), and tumor (7).
Conclusions:
Cerebral MD was readily implemented in a community-based hospital. No cerebral hemorrhages or infections were attributed to cerebral MD. Examples of how MD may be a useful adjunct in the clinical decision making of patients with brain injuries are presented.
Keywords: Brain glucose, microdialysis, multimodal brain monitoring, traumatic brain injury
INTRODUCTION
Cerebral microdialysis (MD) provides valuable information about brain metabolism under normal and pathologic conditions. Cerebral MD has been studied in the laboratory since the 1970s,[7,39] but it was not until the 1990s that techniques were developed to make its clinical use feasible.[24,39] The CMA 600 Analyzer gained US Food and Drug Administration (FDA) approval in 2005 for clinical use in the United States, and subsequently cerebral MD became more widely utilized in multimodal brain monitoring. MD had been used previously to assess relationships between the MD markers (glucose, lactate, pyruvate, glycerol) and intracranial pressure (ICP) and cerebral perfusion pressure (CPP).[19] Whether or not this information is useful and helpful in the management of patients with insults to the brain has been debated. Certainly, questions arise as to whether the measurements obtained may affect or predict outcome. The potential utility of MD has been enumerated in several recent reviews and studies;[4,10–12,20,25,38] nonetheless, it has yet to become standard in most medical institutions. Barriers to the widespread adoption of cerebral MD include the cost of such a program, the additional resources of time and labor required to implant the catheters, collect, and maintain the data. Furthermore, MD catheter implantation remains an invasive surgical procedure, with inherent risks. During its early stages, the technique of cerebral MD was reported to be readily implemented by the ICU staff.[30] Despite this, cerebral MD has largely been confined to research universities with the infrastructure to maintain a cerebral MD program. This study is the first report of the implementation of a cerebral MD program in a community-based hospital setting. This has far-reaching implications for hospitals and academic programs that on the surface do not have the infrastructure or the administrative support to establish and maintain an MD program. One of the key points is that this program was established at our hospital in the absence of neurosurgery house staff/fellows and in the absence of a dedicated neurosurgery/neuroscience ICU. The development of this program, the demographics of the patients, and potential utility as well as complications associated with MD placement are discussed.
MATERIALS AND METHODS
Nature of study
This study is a retrospective analysis of all patients who underwent cerebral MD during a 5-year period (from 07/01/05 to 07/01/10) at Legacy Emanuel Medical Center (LEMC). As this was a registry established for hospital operations, Institutional Review Board IRB approval was waived.
Facilities
LEMC is a community-based hospital with 554 licensed beds, and is one of the two Level I trauma centers in Oregon, verified by the American College of Surgeons. LEMC includes a pediatric hospital with the full complement of pediatric services including a pediatric intensive care unit. LEMC serves as a regional referral center for Oregon, northern California, and southwest Washington. The MD studies were conducted in a 10-bed shared adult ICU (neurosurgery, trauma, vascular, orthopedics, medicine) or a shared 16-bed multispecialty pediatric ICU.
Inclusion criteria
Patients undergoing open craniotomy for traumatic brain injury (TBI), cerebrovascular accident (CVA), aneurysmal subarachnoid hemorrhage (SAH), or brain tumor resection were considered as candidates for monitoring with cerebral MD. Patients selected included those with initial computed tomography (CT) scans demonstrating mass effect, edema, or midline shift. Patients with impaired neurological status who did not require open craniotomy, but who required ICP monitoring were considered for multimodal brain monitoring and cerebral MD via bolt or burr hole placement. Finally, patients with diabetes mellitus or impaired glucose metabolism were considered for cerebral MD monitoring to allow for better control of cerebral glucose.
Exclusion criteria
Patients with documented coagulopathy or those requiring anticoagulants (i.e., warfarin, Plavix, heparin) during the time of multimodal brain monitoring were not considered for placement of MD catheters.
Technique of cerebral MD
The CMA 600 and ISCUSflex cerebral MD Analyzers, CMA 70 catheters (molecular weight cutoff 20 kD), and CMA 106 pumps were obtained from CMA Microdialysis AB, Solna, Sweden. Sterile, artificial cerebrospinal fluid (CSF; P000151), obtained from CMA Microdialysis AB, was perfused at 0.3 μl/min. Samples were collected hourly by the bedside ICU nurse and analyzed immediately with the MD analyzers. MD was continued until neurological status and ICPs were stable, as determined by the attending neurosurgeon. According to FDA guidelines, the MD catheters were removed or replaced after 5 days of monitoring.
At the time of the open craniotomy/craniectomy, catheters were placed directly into the brain via a 1 mm corticectomy. These were placed perpendicular to the surface of the brain and the tips were targeted to be 2–3 cm from the cortical surface. The catheters were tunneled out the skin via a stab incision, and secured to the skin with sutures. The flange on the CMA 70 catheter served as a plug or barrier at the exit site. If a single catheter was placed, we targeted an area near the area of injury (penumbra region). When two catheters or more were placed, one was placed in an area adjacent to the region of concern and another placed distant from this region. Additional multimodal monitoring probes were placed, and care was taken to have at least 1 cm between the tips of the MD catheter and the Hemedex probe to avoid potential interference between these two probes. We have found this interference to be likely due to currents created by MD that affect the thermodiffusion technology of the Hemedex. If an open craniotomy was not done, MD catheters were implanted via bolt technology. These were placed via a single lumen twist drill bolt (Codman and Shurtleff, Raynham, MA, USA) or a double or triple lumen bolt (Integra Neuroscience, Plainsboro, NJ, USA) with additional cerebral monitors.
Antibiotic prophylaxis
Routine prophylaxis with antibiotics was performed for the duration of the monitor placement. Cephalosporins were used primarily. In those with a documented penicillin or cephalosporin allergy, vancomycin or clindamycin was used.
MD and RN training
Neurosurgeons and ICU registered nurses (RNs) underwent specialized in-service training on the indications, implantation, and techniques for cerebral MD. Only attending, board-certified neurosurgeons placed the MD catheters. Nursing staff maintained and demonstrated competencies in MD by completing practical and written tests. Critical care nursing staff members were required to demonstrate established cerebral MD competency in order to be assigned to patients with these monitors. Our MD program at LEMC follows the guidelines set forth by Clinical Laboratory Improvement Amendments (CLIA). This ensures that all guidelines are followed within the established limits by keeping logs of all quality control test data and certifying user qualification.
Data collection and analysis
MD measurements collected by the CMA 600 and ISCUSflex were entered automatically into the ICU Pilot program (CMA Microdialysis AB). Concurrent physiologic parameters [CPP, ICP, brain temperature, partial pressure of brain tissue oxygenation (PbtO2), and cerebral blood flow (CBF)] were entered manually into the ICU pilot program. Significant neurological/physiological events or interventions were noted in the nursing annotations. All CT scans were reviewed by three of the authors (JC, ZG, SR) for each patient pre- and post-catheter placement to categorize primary pathology and Marshall classification.[9,16] Furthermore, catheter placement was reviewed with particular attention as to whether the catheter tip was in normal, edematous, or injured/hemorrhagic brain. Hemorrhage around the catheter tip or track (within 2 mm) and any neurologic sequelae from the hemorrhage were noted. Patient demographics [age, sex, Glasgow Coma Scale (GCS)], injury mechanism, and reports of cerebral infection (abscess, wound infection, meningitis) were obtained via chart review.
RESULTS
Number of MD catheters placed and data acquired
During the 5-year period between July 2005 and July 2010, 248 cerebral MD catheters were placed in 174 patients. The number of patients monitored with MD and the number of MD catheters used increased during the study period [Figure 1a and b].
Figure 1.
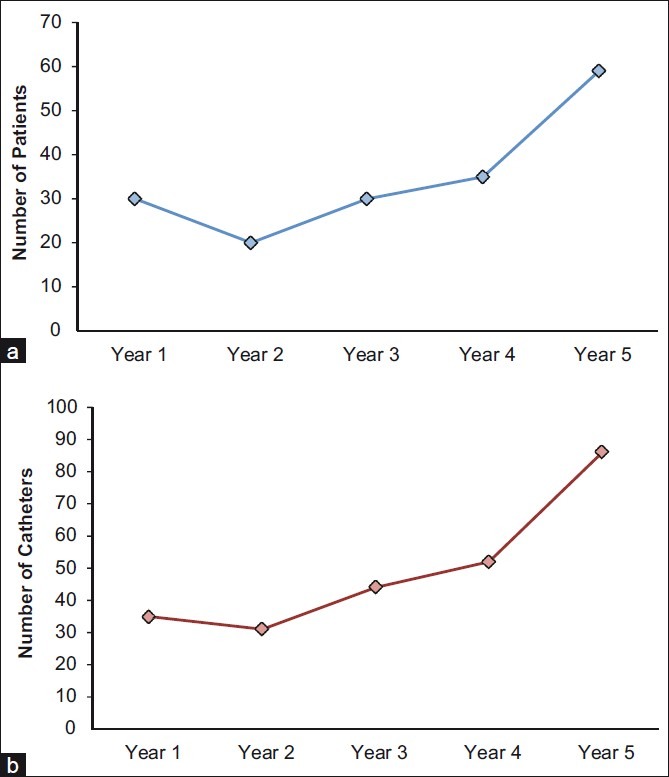
Characteristics of microdialysis monitoring at LEMC from 2005 to 2010. (a) Number of patients undergoing microdialysis monitoring each year from the inception of the program (07/01/05 until 07/01/10). (b) Number of microdialysis catheters placed each year from the inception of the program (07/01/05 until 07/01/10). These figures demonstrate the increase in the number of patients and catheters placed each year. This is likely the result of increased acceptance at our institution by both physicians and nursing staff. Most catheters remained in place during the first five days after insertion, and the majority of patients had one microdialysis catheter in place
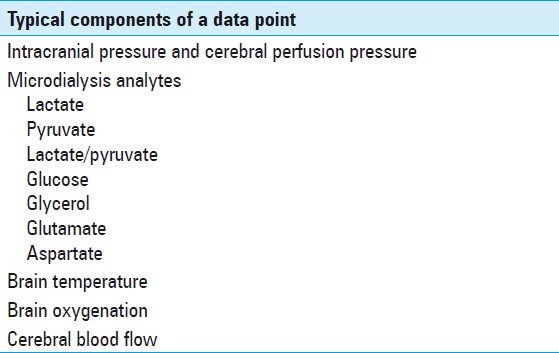
A data point was defined as the MD values acquired each hour in addition to the neurophysiological parameters from the multimodal brain monitoring. Table 1 lists some of the parameters that may be included in a typical data point. We estimated there were approximately 21,500 individual data points in 174 patients, for an average of 123.6 data points per patient. Patients were monitored from 1 to 8 days, the average number of days per catheter being 3.6.
Table 1.
Typical components of each data point that are collected each hour
From March 2009 until October 2009, LEMC was the only US site allowed to beta test the ISCUSflex Microdialysis Analyzer, and the MD samples from 17 patients were analyzed using this machine. Though undergoing beta testing when used at LEMC, a recent paper states that data obtained from the ISCUSflex are valid, according to analytical evaluation.[35] The MD samples from the other 154 patients were analyzed on the CMA 600 Microdialysis Analyzer. In patients with a focal mass lesion [i.e., subdural hematoma (SDH)] or evacuated lesion [i.e., intracerebral hemorrhage (ICH)], we attempted to place two MD catheters in the brain, one in the tissue that was adjacent to the lesion and another in an area which was more distant and, in principle, less affected by the injury. Approximately 35% of our patients had two catheters implanted in this manner, while the majority (61%) had a single catheter placed. Six patients had data provided from three catheters.
Patient demographics
Age
Of the 174 patients in whom MD catheters were implanted, 102 were men and 72 were women (M:F = 1.4:1). The patients’ ages ranged from 5 months to 90 years, and included nine pediatric patients (age <18 years). The average patient age was 49 years, and the median age was 52 years. For the purpose of further analysis, ages were grouped. Three age groups were represented with equal frequency: 19–30, 41–50, and 51–60, each consisting of 33 patients. These data are presented in Figure 2a.
Figure 2.
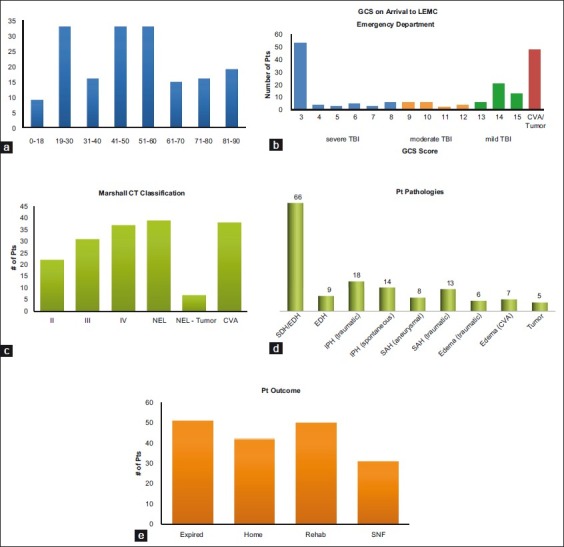
Patient demographics and outcome. (a) Age distribution of patients who had MD catheters placed. (b) Initial GCS of the patients upon arrival to LEMC ED. These patients ultimately had MD catheters placed. (c) Marshall classification of the patients in this study. (d) Primary patient pathology of those undergoing MD monitoring. Note: There were patients who may have had more than one underlying process, but these were scored based on the pathology that was dominant and believed to be the major contributing factor for the neurological dysfunction. (e) Patient disposition at the time of discharge from LEMC. Although we did not have true formal Glasgow Outcome Score evaluations, this provides some indication of the level of patient function at the time of discharge. Although the majority of patients was in the expired, SNF, or rehabilitation groups, this may be a reflection of the fact that the sickest patients were the candidates at our institute for MD monitoring
GCS (TBI)
GCS was obtained via chart reviews of the patients with TBI, and given that the other patients with MD had non-traumatic pathologies (i.e., CVA, tumor), they were not assigned a GCS.[33] The GCS which is reported is the presenting GCS on arrival to LEMC and was taken from the chart review. 58.7% of the patients with MD catheters implanted had an initial recorded GCS ≤8, and thus were placed in the severe TBI group. 13.5% of these patients had a GCS of 9–12 (moderate injury) and 27% of the patients had mild brain injury (GCS ≥ 13) [Figure 2b]. Forty-eight patients were not given a GCS score, as their mechanism was not that of TBI.
Pre-operative CT scan findings, the Marshall score
A pre-operative Marshall classification was assigned to each patient.[9,16] Those with CVA as their mechanism of injury were not classified according to the Marshall system of classification. Pre-operative Marshall classifications of our patients are outlined in Figure 2c, with the greatest number of patient CT scans receiving a Marshall classification of “non-evacuated mass lesion.”
The primary pathology
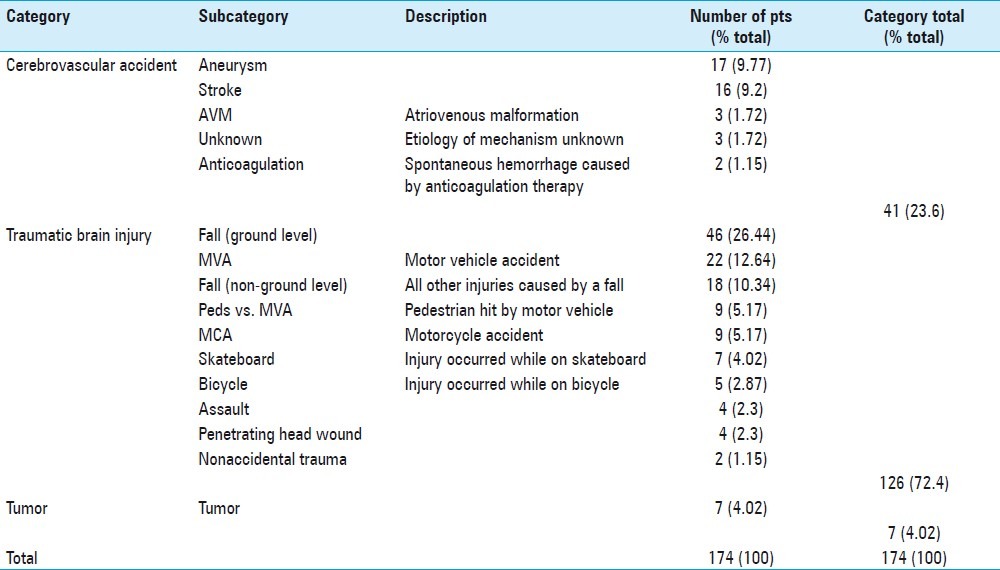
Patient charts and CT scans were reviewed retrospectively to determine the cause of potential cerebral stress and the primary pathology. The main categories were TBI, CVA, and tumor. TBI and CVA each had subcategories [Table 2]. TBI was the most common mechanism of injury, accounting for 72% of patient injuries. Primary pathology was defined as the reason for which surgery was done or for which multimodal monitoring was performed. Figure 2d highlights the different pathologies with the most prevalent being that of subdural hematoma(SDH), with 66 patients in this category. The five patients in the other pathology category included two who underwent large bone flap replacements, two with skull fractures, and one with diffuse axonal injury (DAI).
Table 2.
Mechanisms of injury and type of cerebrovascular accident in the patients monitored with cerebral microdialysis at Legacy Emanuel Medical Center
Patient outcomes
Figure 2e summarizes the outcomes of the patients in this study. Patient outcomes at the time of discharge from LEMC included expiration (n = 51, 29%), discharge to a rehabilitation center (n = 50, 28.7%), a skilled nursing facility (n = 31, 17.8%), or to home (n = 42, 24.1%).
Monitors used
In our ICU at LEMC, six different brain monitors have been used as part of our multimodal brain monitoring program. These monitors measure and record ICP and CPP (ventriculostomy, ICP Express, Camino), PbtO2 (Licox), CBF (Hemedex), brain temperature (Camino, Licox), and brain metabolites (cerebral MD). Table 3 provides additional information about the monitors used. Figure 3a demonstrates the use of additional monitors along with MD. Of our 174 patients, 158 had additional cerebral monitoring [Figure 3b]. The most common monitor used simultaneously with MD was the ventriculostomy. This was used in accordance with the guidelines for the management of severe TBI.[34]
Table 3.
Multimodal monitors used in conjunction with cerebral microdialysis at Legacy Emanuel Medical Center
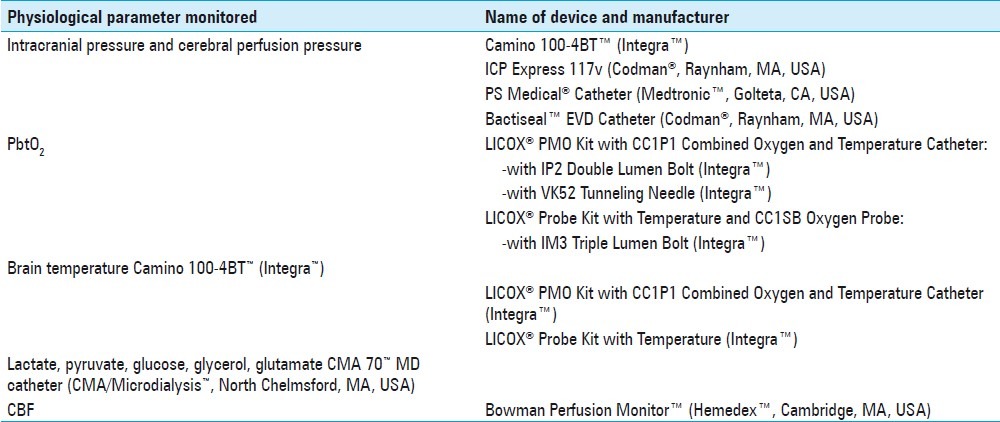
Figure 3.
Multimodal brain monitoring used in conjunction with microdialysis. (a) The number of patients with the indicated multimodal brain monitor in addition to microdialysis. (b) The number of patients who had from zero to four concurrent additional brain monitors with the microdialysis catheter
Evaluation of MD catheter placement
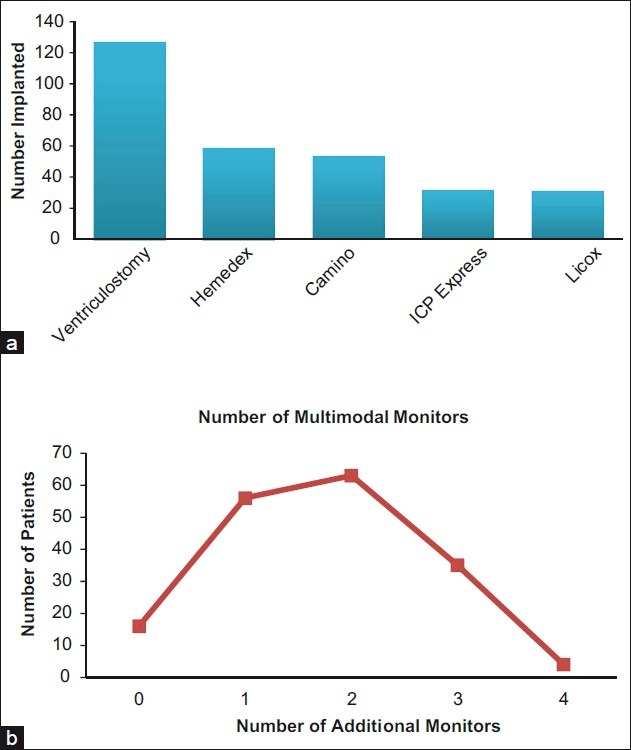
As a part of this study, we conducted postoperative reviews of the brain CT scans of each patient to verify MD catheter placement, as well as that of the other multimodal monitors. Catheter placement was categorized as follows: (1) normal brain, defined as tissue that radiographically appears normal; (2) impaired brain, defined as edematous (low attenuation) or hemorrhagic; or (3) in the ventricle. Figure 4a illustrates various catheter placements, and Figure 4b and c shows CT scans that demonstrate placement in both normal brain and impaired brain, respectively. Sixty-two percent were placed in normal brain, 28% in abnormal brain, and 6% were placed in a ventricle. Ten catheters (4%) were not seen on the CT or did not have a postoperative CT scan due to the early expiration of the patient or mechanical failure and possible early dislodgement of the MD catheters. No hemorrhages were identified that were attributable to MD catheter placement.
Figure 4.
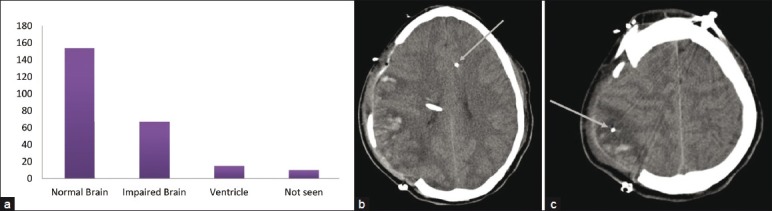
Microdialysis catheter placement. (a) Number of catheters that were determined by postoperative CT scans to be in normal or abnormal appearing brain. Abnormal appearing brain on CT scans included areas of low attenuation (edema) or areas of hemorrhage (from the injury). Some of the catheters were also in the ventricles or could not be seen. Presumably, the latter had been pulled out during the closure or transport and were not in the brain. (b) Example of a patient with a right frontal–parietal cranial defect after deompressive craniectomy. The arrow points to a left frontal microdialysis catheter inserted via a bolt. The tip appears to be in normal appearing brain by CT criteria. (c) The same patient in b demonstrating another catheter (denoted by the arrow) in an area of low attenuation suggestive of edema or ischemia
Infection
No infections of the brain attributable to MD catheter placement were detected as determined by chart review. An infection was defined as a cranial wound infection, bone flap infection, or positive CSF culture. As part of the routine fever work-up in the ICU, CSF was cultured in patients with an indwelling ventriculostomy. If an intracranial infection was suspected after the patient had left the ICU, magnetic resonance imaging (MRI) was done to evaluate for brain abscess and CSF was analyzed with a spinal tap.
DISCUSSION
This paper describes, to our knowledge, the first and longest implementation of a cerebral MD program in a community-based hospital setting in the US. Although we present the experience over a 5-year time period, this MD program continues currently into its seventh year. Retrospective, observational studies of MD have been conducted on large patient cohorts,[2,36,37] but these studies have been performed at large research, university-based hospitals. The demographics of the 174 patients who received cerebral MD monitoring during a 5-year period at LEMC in Portland, Oregon, USA, are outlined. We give details on the specifics of MD monitoring, and how it is integrated as a part of multimodal brain monitoring at LEMC.
Multimodal monitoring, which often includes cerebral MD as an integral piece in the neuroscience ICU, provides information that may affect individualized, patient-specific care and therapy.[32,46] According to the American Hospital Association, about 86% of US hospitals are defined as community hospitals.[1] Community hospitals represent a large majority of hospitals in the US, and thus it is reasonable to consider the implementation of cerebral MD and multimodal monitoring at some of these hospitals. However, it is often difficult to gather the resources, which include neurosurgeons to place the MD catheters and ICU staff to manage the MD monitoring. At our institute, the MD studies are not done in a dedicated neuroscience ICU, but rather in a 10-bed shared trauma, surgical, and medical ICU. Therefore, this study demonstrates that with proper training and motivation of physicians and nurses, it is possible to have a successful MD brain monitoring effort in a community hospital setting. Additionally, there are many academic centers that may be concerned that they do not have the resources, infrastructure, and administrative support to develop and maintain an MD monitoring program. Our studies demonstrate the feasibility for both community-based hospitals and such academic centers.
The MD program began in 2005 at LEMC, and each year the number of patients monitored by cerebral MD and the number of total catheters placed increased [Figure 1a and b]. This was likely due to the acceptance of these monitoring techniques by the neurosurgeons, nursing staff, trauma surgeons, critical care intensivists, and neurologists. This adoption may be due to increasing information that MD studies have potential to improve patient care. For example, LEMC developed and implemented a new insulin protocol when our MD data consistently showed abnormally low brain glucose levels when patients were treated according to a tight glycemic control insulin protocol.[4] This was consistent with independent MD studies done at other institutes.[21,22,45] The specifics of the use of MD at our institution for cerebral glucose management will be discussed subsequently.
Patient selection and demographics
Our 5-year study contains a tremendous amount of data from a wide range of ages, from 5 months to 90 years [Figure 2a]. There were no exclusion criteria based on age for MD monitoring, thus the extremes in age are represented. These data are valuable, as they allow us to compare how brain injury affects the cerebral metabolism in a wide variety of age groups including the very young and very old. Each age group has a sufficient number of patients to allow for meaningful studies. For example, there are few published studies of MD in the pediatric brain,[5] and further analysis of our data is in progress.
Our selection of prospective MD patients was based primarily on the severity of brain injury and is biased toward those patients who underwent craniotomy. Figure 2b demonstrates the GCS recorded on arrival to the LEMC emergency department (ED). Although there were a disproportionate number of patients with GCS 3, these numbers may be skewed as this was a retrospective analysis. In most instances, the GCS recorded was the GCS on arrival to LEMC ED and patients routinely had received paralytics and sedation on transport. This does not reflect the first responders’ reports in the field. The retrospective analysis did not capture those patients who improved with resuscitation or re-examination after the medications dissipated. GCS alone was not the determining factor for subsequent surgery/monitoring. The decision to place MD catheters and other multimodal monitors was at the discretion of the attending neurosurgeon. In general, preference was given to those patients who underwent an open craniotomy. These patients had clear mass lesions and were thus most likely to benefit from multimodal monitoring in order to detect potential secondary brain insults. Additionally, as the brain was exposed at the time of surgery, the catheters were placed under direct visualization. This may account for absence of hemorrhages associated with catheter placement in our series. Figure 2b shows a significant number of patients (n = 40) presenting as mild TBIs. We found that these patients included those who demonstrated subsequent neurological deterioration as well as the elderly patients who were able to compensate initially for large SDHs.
The elderly population represents a particularly vulnerable subgroup. Despite their presenting favorable neurological status (GCS 13–15), often despite a very large SDH or ICH, these elderly patients were considered at greater risk for secondary brain injury and were included as candidates for MD monitoring. The leading cause of death among people aged 65 years or older is falls.[27,29] In this same age group, falls contribute to 61% of all TBIs. Elderly persons who fall are typically admitted to the hospital with a mild brain injury. A disproportionately higher number of these patients are discharged from the hospital with moderate or severe disability.[6]
Figure 2c demonstrates that CT classification of the patients using the Marshall criteria[9,16] revealed that all of the patients in the TBI subgroup had demonstrable pathology that necessitated surgery and monitoring. A number of patients monitored with cerebral MD did not have a TBI. This group included patients with CVA (aneurysmal SAH, ICHs) and brain tumors. The rationale for monitoring the patients with aneurysmal SAH was as an adjunct in the surveillance for vasospasm.[2,28] The rationale for the monitoring of patients with the brain tumors was directed at patients with significant edema on pre-operative scans or who had known problems with glucose metabolism.
Patient outcome and complications
The patient outcome at the time of discharge from LEMC is delineated in Figure 2e. A large number of patients expired or went to a skilled nursing facility. This is not surprising given that the patients who were selected for cerebral MD monitoring were the most neurologically impaired and most vulnerable patients. Additionally, what is not reflected in Figure 2e is that many of the patients expired from co-morbidities or because the families elected to withdraw care. The overall mortality in our study is 29% which is similar to the 26% in a recent report by Stuart et al. in 2010.[32] If one factors in the additional 18% in their study that expired because of withdrawal of care/DNR, their overall mortality was 44%. Because of the design of our study, there is no control group. It is difficult to compare the mortality from other published studies because of the differences in the criteria used in the selection of patients to monitor with MD. From our study, we cannot determine specifically whether or not MD has any effect on mortality or morbidity; however, it does not appear to have an adverse effect.
No cerebral infections were attributed to MD catheter implantation. This is lower than the 5% reported in other studies with MD.[32] This may be in part because different studies use different criteria to define an infection. In our study, an infection was considered to be present if there was a positive CSF culture, cranial wound, or bone flap infection. An elevated protein, CSF pleocytosis, or decreased glucose in the absence of a positive CSF culture was not included in our criteria. Additionally, with a 29% mortality, many patients expired or care was withdrawn before an infection could manifest itself. Brain abscesses and bone flap infections/osteomyelitis may be delayed and may not be found in our analysis if the patient does not re-present to our hospital.
No hemorrhages were seen from MD catheter placement as determined by evaluations of postoperative CT scans. This hemorrhage rate is lower than the 3% rate reported at a large university hospital with an established neuroscience ICU.[32] This is likely because the majority of the MD catheters in our study were placed via an open craniotomy technique and only by attending board-certified neurosurgeons with many years of experience in neurotrauma. The technique of making a focal 1 mm cortical incision facilitates the passage of the fine MD catheter into the brain to the specified depth. The placement of an MD catheter via a bolt or twist drill hole does not allow the visualization afforded by the craniotomy. Furthermore, in cases in which the arachnoid is thickened, one must make a rather blind perforation with a sharp blade or spinal needle to allow passage of the fine MD catheter. This may lead to inadvertent damage to cortical vessels with an inability to coagulate them. Because of these concerns, some of the 63 MD catheters that were placed via twist drill or bolt techniques were placed at the time of the open craniotomy adjacent to the edge. This allowed for better control of the arachnoid perforation and passage of the catheters. These were done early in our experience in particular to allow for the fixation of the double or triple lumen Licox bolts for simultaneous brain oxygenation and MD monitoring. With the advent of the tunneled Licox monitor (2009, 2010), the use of these bolt placements for MD has decreased.
Figure 4a addresses the question of the placement of the catheters. Those catheters that were in the ventricle are clearly the ones that were misplaced. Even though all of these catheters were placed by experienced board-certified neurosurgeons, this speaks of the difficulty of placing these catheters precisely in a pre-conceived area. The placement of the catheters via the open craniotomy technique allows the surgeon to select more precisely where the catheter is placed. However, one must be cognizant of the potential shifts in brain anatomy that occur with surgery. With the bolt technique, the MD catheter is placed at a fixed angle and depth from the skull. This provides little flexibility in targeting the catheter. This is best used for diffuse injuries. MD provides regional information about the brain. Thus, if the catheter is in injured brain (edematous or hemorrhagic), the chemistry is understandably abnormal. It has been debated whether one should direct therapy to salvage an injured area or to maintain the normal brain.[8,19] In our study, the majority of the catheters were placed ipsilateral to the pathology, as these were done at the time of open surgery. However, in the subsequent analysis, the majority appeared to be in normal appearing brain by CT criteria. Those areas that were considered to be “impaired brain” were clearly the areas that demonstrated hemorrhage (not attributable to catheter placement) or low attenuation (edema).
Those catheters that were not seen were attributed to dislodgement or termination of monitoring prior to the postoperative CT scan. The technique of securing the catheters evolved over time, and simply using 4-0 braided nylon (Nurolon, Ethicon, U.S.A.) sutures decreased the number of dislodged catheters. Glucose metabolism in the brain has come to the forefront as an important determinant of outcome. Previous research has demonstrated that maintaining tight control of systemic blood glucose, and thus administering a higher dosage of insulin reduced mortality.[40–44] Through careful monitoring of glucose data at LEMC, we have observed that a tight glycemic control, and thus a lower level of brain glucose as measured by MD, resulted in higher lactate/pyruvate ratios (LPRs), which have historically been associated with poor neurologic outcomes.[3,13,14,17,26] Upon observing this, the insulin drip therapy protocol at LEMC was altered, allowing for higher levels of systemic blood glucose. Figure 5 is an example of the changes in our protocol from one of tight glycemic control to one of loose glycemic control. Figure 6 is an example of ICU pilot data from a patient with TBI who had MD monitoring. Initially, the patient was treated with the tight glycemic control. We noted the persistent decreased brain glucose in the abnormal range (<0.8 mM) and a correspondingly increased LPR (arrows). With the transition to the loose glycemic control parameters, the brain glucose increased to the normal range and the LPR dramatically decreased to the normal range. The neurocritical care teams at Columbia University and the University of California, Los Angeles (UCLA) have similar findings and have published their results.[15,18,21,22,40–43,45] The maintenance of cerebral glucose in the normal range may help to maintain the LPR in the normal range. This is a marker for cerebral stress and has been correlated with poor outcome.[17,21,22,45]
Figure 5.
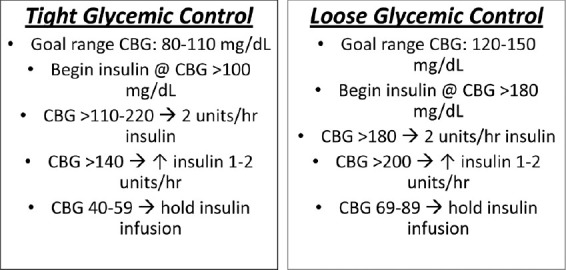
Tight and loose glycemic control. The protocols and definitions of tight and loose glycemic control which have been implemented at LEMC. These protocols have been defined based on our observations with brain MD glucose and LPR data and are similar to what have been reported
Figure 6.
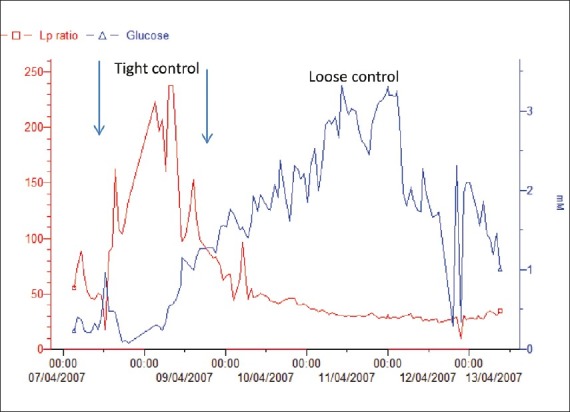
Example of a patient undergoing tight and loose glycemic control, the effect on LPR. ICU pilot data demonstrating MD sample analysis under conditions of tight glycemic (arrows) and loose glycemic control. Note the low brain glucose and corresponding high lactate/pyruvate ratios
Optimization of cerebral glucose is one example of how cerebral MD may be used to decrease the LPR. An argument may be made as to whether or not MD used in a non-prospective fashion in a community hospital setting has any meaningful effect on outcomes or clinical decision making. Indeed, we found as a component of multimodal brain monitoring, MD data were extremely useful in clinical decision making. However, we emphasize that this varied on a case by case basis and was dependent on variables such as the pathology, the patient age, and the catheter location. LPR was an extremely useful marker of cerebral stress and we used the LPR trend to guide the subsequent therapy. For example, an upward trend in LPR could guide the team to earlier imaging studies or laboratory studies. Figure 7 shows the actual ICU pilot MD data from a patient with severe TBI with ICPs in the 20–30 range, but with LPRs in the 60–70 range. There was no focal mass lesion, but small frontal petechiae and edema. The mesencephalic cisterns were open. The upward trend of the LPR was quite evident, and given the higher than normal ICPs, the MD data helped in the decision for early intervention with a decompressive craniectomy (arrow) rather than continuing with an attempt at maximal medical management. Note the rapid decrease in the LPR to the normal range postoperatively.
Figure 7.
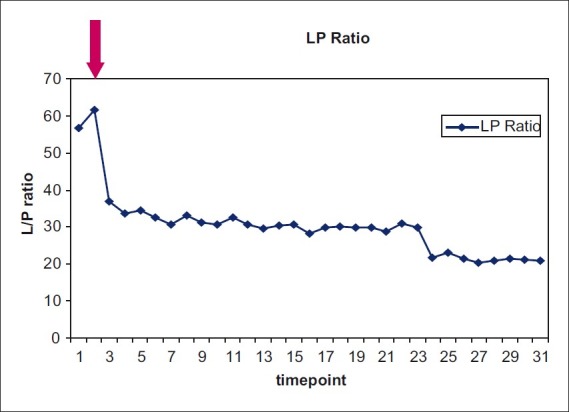
MD ICU pilot data from a patient with a severe bifrontal TBI and ICPs in the 20–30 range and CPPs >60. Note the initial highly elevated LPRs with an upward trend. The MD data led us to perform an early bifrontal decompressive craniectomy (arrow). The LPRs immediately decreased to the normal range. The patient did extremely well
These examples and our entire experience with cerebral MD monitoring raise the questions about the funding for such an endeavor. The initial expenditure for the equipment came from hospital capital equipment funds and Legacy Foundation (charitable) grants. The actual MD catheters and implants are patient charge items just as a ventriculostomy catheter or bolt is charged. The CPT code for placement of an MD catheter via a bolt or twist drill is 61107. For catheters placed directly at the time of open craniotomy, there is no surgeon fee. The funding for the database management was via Legacy Foundation grants. Physician and nursing education specifically for MD was done by continue medical education (C.M.E) programs at the hospital with volunteer speakers.
CONCLUSION
The 5-year experience at LEMC demonstrates that cerebral MD can be successfully and safely implemented in a community-based hospital setting with intervention related risks (infection and hemorrhage) that are comparable to that reported at a major university with residents/fellows and the infrastructure for a cerebral MD initiative.[32] The risk to benefit ratio is difficult to assess. We found that the additional information about cerebral metabolism was useful in helping with the decision paradigms for treatment. Figures 6 and 7 provide some examples of how MD data helped in our treatment paradigm in these specific cases. However, because the information and implications from cerebral MD and multimodal brain monitoring are rapidly evolving,[23,31] it is difficult to recommend a protocolized approach to the use of cerebral MD data at this time. All of the information including clinical status and radiographic findings must be taken into consideration.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/57/96868
Contributor Information
Jeff W. Chen, Email: jchen@lhs.org.
Shana L. Rogers, Email: slrogers@lhs.org.
Zoe J. Gombart, Email: gombartz@gmail.com.
David E. Adler, Email: dadler@columbianeurosurgical.net.
Sandy Cecil, Email: scecil@lhs.org.
REFERENCES
- 1.American Hospital Association: Fast Facts on U.S. Hospitals. [cited in 2010]. Available from: http://www.aha.org/research/rc/stat-studies/fast-facts.shtml .
- 2.Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–9. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- 3.Belli A, Sen J, Petzold A, Russo S, Kitchen N, Smith M. Metabolic failure precedes intracranial pressure rises in traumatic brain injury: A microdialysis study. Acta Neuroch (Wien) 2008;150:461–9. doi: 10.1007/s00701-008-1580-3. discussion 470. [DOI] [PubMed] [Google Scholar]
- 4.Cecil S, Chen PM, Callaway SE, Rowland SM, Adler DE, Chen JW. Traumatic brain injury: Advanced multimodal neuromonitoring from theory to clinical practice. Crit Care Nurse. 2011;31:25–36. doi: 10.4037/ccn2010226. quiz 37. [DOI] [PubMed] [Google Scholar]
- 5.Charalambides C, Sgouros S, Sakas D. Intracerebral microdialysis in children. Child's Nerv Syst. 2010;26:215–20. doi: 10.1007/s00381-009-1031-3. [DOI] [PubMed] [Google Scholar]
- 6.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: Characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20:215–28. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Delgado JM, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch Int Pharmacodyn Ther. 1972;198:9–21. [PubMed] [Google Scholar]
- 8.Engstrom M, Polito A, Reinstrup P, Romner B, Ryding E, Ungerstedt U, et al. Intracerebral microdialysis in severe brain trauma: The importance of catheter location. J Neurosurg. 2005;102:460–9. doi: 10.3171/jns.2005.102.3.0460. [DOI] [PubMed] [Google Scholar]
- 9.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 10.Goodman JC, Robertson CS. Microdialysis: Is it ready for prime time? Curr Opin Crit Care. 2009;15:110–7. doi: 10.1097/MCC.0b013e328325d142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: A focus on cerebral microdialysis. Curr Opin Crit Care. 2006;12:112–8. doi: 10.1097/01.ccx.0000216576.11439.df. [DOI] [PubMed] [Google Scholar]
- 12.Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: The current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 13.Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- 14.Hlatky R, Valadka AB, Goodman JC, Robertson CS. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery. 2004;55:1318–23. doi: 10.1227/01.neu.0000143029.42638.2c. discussion 1324. [DOI] [PubMed] [Google Scholar]
- 15.Langouche L, Vanhorebeek I, Van den Berghe G. Therapy insight: The effect of tight glycemic control in acute illness. Nat Clin Pract Endocrinol Metab. 2007;3:270–8. doi: 10.1038/ncpendmet0426. [DOI] [PubMed] [Google Scholar]
- 16.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. discussion 1173-82. [DOI] [PubMed] [Google Scholar]
- 17.Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36:2871–7. doi: 10.1097/CCM.0b013e318186a4a0. [DOI] [PubMed] [Google Scholar]
- 18.Mebis L, Gunst J, Langouche L, Vanhorebeek I, Van den Berghe G. Indication and practical use of intensive insulin therapy in the critically ill. Curr Opin Crit Care. 2007;13:392–8. doi: 10.1097/MCC.0b013e3281c1c9c8. [DOI] [PubMed] [Google Scholar]
- 19.Nelson DW, Thornquist B, MacCallum RM, Nystrom H, Holst A, Rudehill A, et al. Analyses of cerebral microdialysis in patients with traumatic brain injury: Relations to intracranial pressure, cerebral perfusion pressure and catheter placement. BMC Med. 2011;9:21. doi: 10.1186/1741-7015-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordstrom CH. Cerebral energy metabolism and microdialysis in neurocritical care. Childs Nerv Syst. 2010;26:465–72. doi: 10.1007/s00381-009-1035-z. [DOI] [PubMed] [Google Scholar]
- 21.Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Critical Care Med. 2008;36:3233–8. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- 22.Oddo M, Schmidt JM, Mayer SA, Chiolero RL. Glucose control after severe brain injury. Curr Opin Clin Nutr Metab Care. 2008;11:134–9. doi: 10.1097/MCO.0b013e3282f37b43. [DOI] [PubMed] [Google Scholar]
- 23.Oddo M, Villa F, Citerio G. Brain multimodality monitoring: An update. Curr Opin Crit Care. 2012;18:111–8. doi: 10.1097/MCC.0b013e32835132a5. [DOI] [PubMed] [Google Scholar]
- 24.Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- 25.Presciutti M, Schmidt JM, Alexander S. Neuromonitoring in intensive care: Focus on microdialysis and its nursing implications. J Neurosci Nurs. 2009;41:131–9. [PubMed] [Google Scholar]
- 26.Sarrafzadeh AS, Kiening KL, Unterberg AW. Neuromonitoring: Brain oxygenation and microdialysis. Curr Neurol Neurosci Rep. 2003;3:517–23. doi: 10.1007/s11910-003-0057-2. [DOI] [PubMed] [Google Scholar]
- 27.Sattin RW. Falls among older persons: A public health perspective. Annu Rev Public Health. 1992;13:489–508. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- 28.Skjoth-Rasmussen J, Schulz M, Kristensen SR, Bjerre P. Delayed neurological deficits detected by an ischemic pattern in the extracellular cerebral metabolites in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;100:8–15. doi: 10.3171/jns.2004.100.1.0008. [DOI] [PubMed] [Google Scholar]
- 29.Sosin DM, Sacks JJ, Sattin RW. Causes of nonfatal injuries in the United States, 1986. Accid Anal Prev. 1992;24:685–7. doi: 10.1016/0001-4575(92)90022-b. [DOI] [PubMed] [Google Scholar]
- 30.Stahl N, Mellergard P, Hallstrom A, Ungerstedt U, Nordstrom CH. Intracerebral microdialysis and bedside biochemical analysis in patients with fatal traumatic brain lesions. Acta Anaesthesiol Scand. 2001;45:977–85. doi: 10.1034/j.1399-6576.2001.450810.x. [DOI] [PubMed] [Google Scholar]
- 31.Stover JF. Actual evidence for neuromonitoring-guided intensive care following severe traumatic brain injury. Swiss Med Wkly. 2011;141:w13245. doi: 10.4414/smw.2011.13245. [DOI] [PubMed] [Google Scholar]
- 32.Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, et al. Intracranial multimodal monitoring for acute brain injury: A single institution review of current practices. Neurocrit Care. 2010;12:188–98. doi: 10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 33.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 34.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Recommendations for intracranial pressure monitoring technology. J Neurotrauma. 2000;17:497–506. doi: 10.1089/neu.2000.17.497. [DOI] [PubMed] [Google Scholar]
- 35.Tholance Y, Barcelos G, Quadrio I, Renaud B, Dailler F, Perret-Liaudet A. Analytical validation of microdialysis analyzer for monitoring glucose, lactate and pyruvate in cerebral microdialysates. Clin Chim Acta. 2011;412:647–54. doi: 10.1016/j.cca.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: A microdialysis study of 223 patients. Brain. 2011;134:484–94. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- 37.Timofeev I, Czosnyka M, Carpenter KL, Nortje J, Kirkpatrick PJ, Al-Rawi PG, et al. Interaction between brain chemistry and physiology after traumatic brain injury: Impact of autoregulation and microdialysis catheter location. J Neurotrauma. 2011;28:849–60. doi: 10.1089/neu.2010.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tisdall MM, Smith M. Cerebral microdialysis: Research technique or clinical tool. Br J Anaesth. 2006;97:18–25. doi: 10.1093/bja/ael109. [DOI] [PubMed] [Google Scholar]
- 39.Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Curr Pharm Des. 2004;10:2145–52. doi: 10.2174/1381612043384105. [DOI] [PubMed] [Google Scholar]
- 40.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: Benefit versus harm. Diabetes. 2006;55:3151–9. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 42.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 43.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: Facts and controversies. Chest. 2007;132:268–78. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 44.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control: What is the evidence? Crit Care Med. 2007;35(Suppl 9):S496–502. doi: 10.1097/01.CCM.0000278051.48643.91. [DOI] [PubMed] [Google Scholar]
- 45.Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–6. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 46.Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507–38. doi: 10.1016/j.ccc.2007.06.002. [DOI] [PubMed] [Google Scholar]


