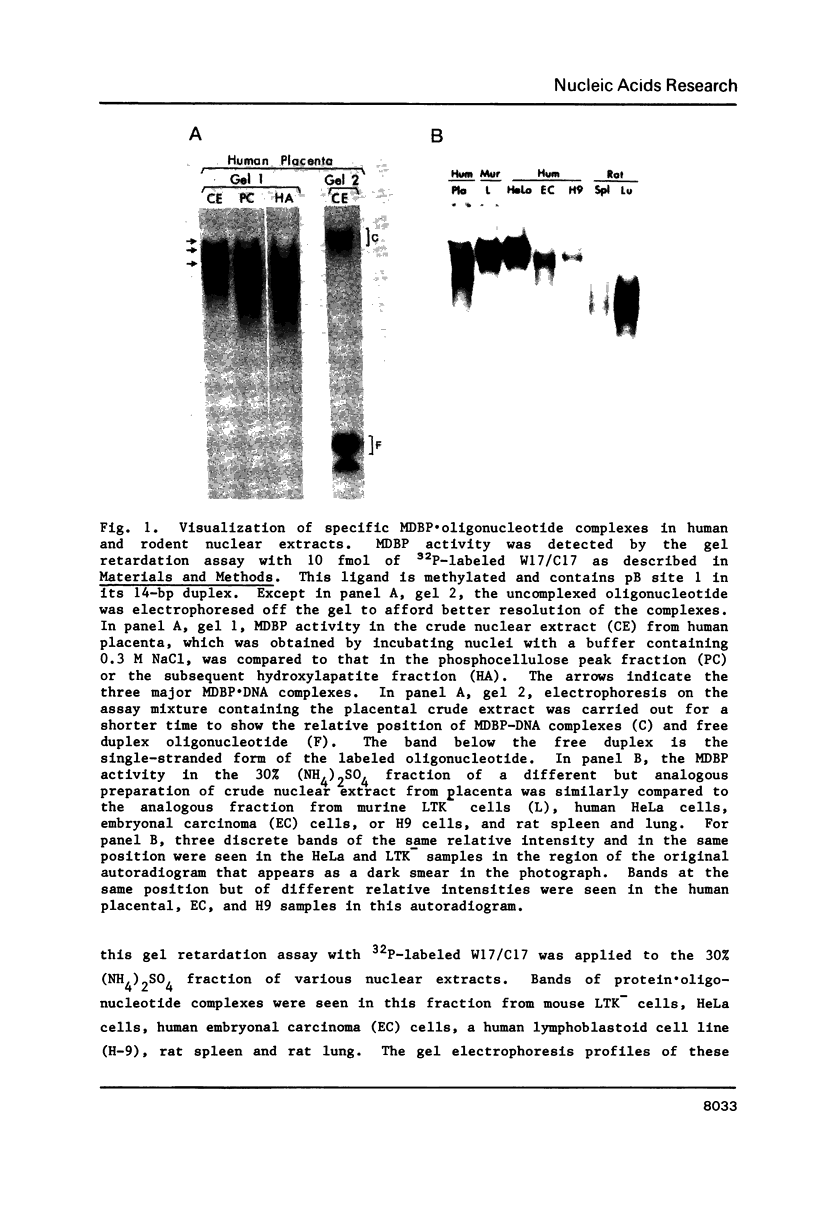
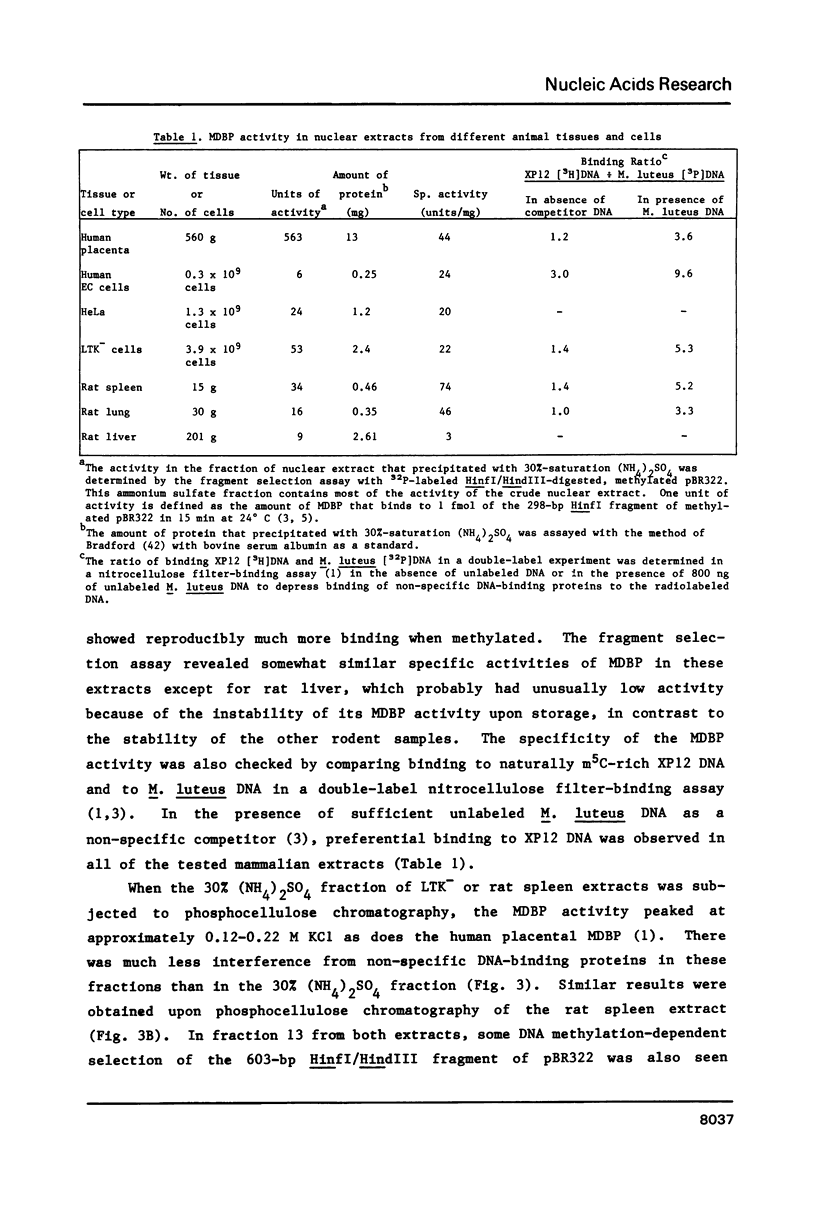
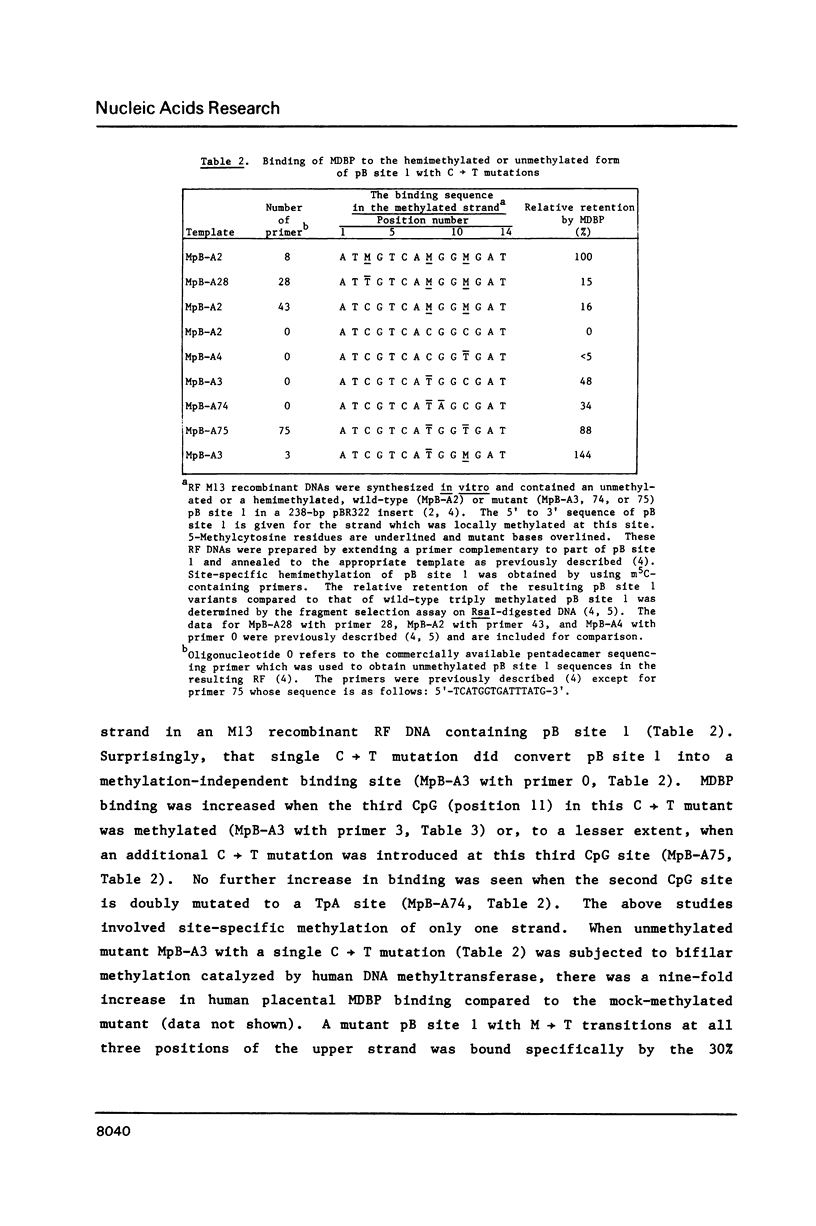
Abstract
A DNA-binding protein from human placenta, methylated DNA-binding protein (MDBP), binds to certain DNA sequences only when they contain 5-methylcytosine (m5C) residues at specific positions. We found a very similar DNA-binding activity in nuclear extracts of rat tissues, calf thymus, human embryonal carcinoma cells, HeLa cells, and mouse LTK cells. Like human placental MDBP, the analogous DNA-binding proteins from the above mammalian cell lines formed a number of different low-electrophoretic-mobility complexes with a 14-bp MDBP-specific oligonucleotide duplex. All of these complexes exhibited the same DNA methylation specificity and DNA sequence specificity. From the extracts of rat and calf tissues, oligonucleotide protein complexes formed that also had the same specificity as human placental MDBP although they had a higher electrophoretic mobility probably due to digestion by proteases in the nuclear extracts. Although MDBP activity was found in various mammalian cell types, it was not detected in extracts of cultured mosquito cells and so may be associated only with cells with vertebrate-type DNA methylation.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., McKay E. L., Craig L. M., Burdon R. H. Methylation of mosquito DNA. Biochim Biophys Acta. 1979 Jun 20;563(1):72–81. doi: 10.1016/0005-2787(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Andrews P. W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984 Jun;103(2):285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Purification and characterization of chicken ovalbumin gene upstream promoter transcription factor from homologous oviduct cells. Mol Cell Biol. 1987 Dec;7(12):4151–4158. doi: 10.1128/mcb.7.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Benvenisty N., Mencher D., Meyuhas O., Razin A., Reshef L. Sequential changes in DNA methylation patterns of the rat phosphoenolpyruvate carboxykinase gene during development. Proc Natl Acad Sci U S A. 1985 Jan;82(2):267–271. doi: 10.1073/pnas.82.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi N. O., Vidal-Rioja L., Cleaver J. E. Direct visualization of the sites of DNA methylation in human, and mosquito chromosomes. Chromosoma. 1986;94(5):362–366. doi: 10.1007/BF00328636. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Keller W., Dale T., Schöler H. R., Tebb G., Mattaj I. W. A transcription factor which binds to the enhancers of SV40, immunoglobulin heavy chain and U2 snRNA genes. Nature. 1987 Jan 15;325(6101):268–272. doi: 10.1038/325268a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper D. N. Eukaryotic DNA methylation. Hum Genet. 1983;64(4):315–333. doi: 10.1007/BF00292363. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Elias P., O'Donnell M. E., Mocarski E. S., Lehman I. R. A DNA binding protein specific for an origin of replication of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6322–6326. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. P., Coca-Prados M., Hsu M. T. Enzymatic analysis of 5-methylcytosine content in eukaryotic DNA. Study of intracellular Simian Virus 40 DNA. J Biol Chem. 1980 Aug 25;255(16):7544–7547. [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Gowda S., Wagner H., Simon D. Methylation of the enhancer region of avian sarcoma virus long terminal repeat suppresses transcription. FEBS Lett. 1987 Sep 14;221(2):332–336. doi: 10.1016/0014-5793(87)80951-1. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Desiderio S. V. Identification of a DNA binding protein that recognizes the nonamer recombinational signal sequence of immunoglobulin genes. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7019–7023. doi: 10.1073/pnas.84.20.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., Bollekens J., Dorn A., Benoist C., Mathis D. Properties of a CCAAT box-binding protein. Nucleic Acids Res. 1987 Sep 25;15(18):7265–7282. doi: 10.1093/nar/15.18.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Wang R., Gama-Sosa M. A., Shenoy S., Ehrlich M. A protein from human placental nuclei binds preferentially to 5-methylcytosine-rich DNA. Nature. 1984 Mar 15;308(5956):293–295. doi: 10.1038/308293a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Jackson P. D., Felsenfeld G. Protein-binding sites within the 5' DNase I-hypersensitive region of the chicken alpha D-globin gene. Mol Cell Biol. 1987 Jun;7(6):2059–2069. doi: 10.1128/mcb.7.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo K. C., McCune R. A., Gehrke C. W., Midgett R., Ehrlich M. Quantitative reversed-phase high performance liquid chromatographic determination of major and modified deoxyribonucleosides in DNA. Nucleic Acids Res. 1980 Oct 24;8(20):4763–4776. doi: 10.1093/nar/8.20.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Huang T. C., Teng M. H. 5-Methylcytosine replacing cytosine in the deoxyribonucleic acid of a bacteriophage for Xanthomonas oryzae. J Mol Biol. 1968 Jul 14;34(2):373–375. doi: 10.1016/0022-2836(68)90263-5. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Paterson B. M., Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986 Dec 5;47(5):649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Murray E. J., Grosveld F. Site specific demethylation in the promoter of human gamma-globin gene does not alleviate methylation mediated suppression. EMBO J. 1987 Aug;6(8):2329–2335. doi: 10.1002/j.1460-2075.1987.tb02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Strobl J. S., Dannies P. S., Thompson E. B. Rat growth hormone gene expression is correlated with an unmethylated CGCG sequence near the transcription initiation site. Biochemistry. 1986 Jun 17;25(12):3640–3648. doi: 10.1021/bi00360a025. [DOI] [PubMed] [Google Scholar]
- Tribioli C., Biamonti G., Giacca M., Colonna M., Riva S., Falaschi A. Characterization of human DNA sequences synthesized at the onset of S-phase. Nucleic Acids Res. 1987 Dec 23;15(24):10211–10232. doi: 10.1093/nar/15.24.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G. Two forms of transcription factor TFIIIC in extracts from HeLa cells. Nucleic Acids Res. 1987 Jul 10;15(13):5031–5039. doi: 10.1093/nar/15.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Huang L. H., Ehrlich M. Human placental DNA methyltransferase: DNA substrate and DNA binding specificity. Nucleic Acids Res. 1984 Apr 25;12(8):3473–3490. doi: 10.1093/nar/12.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Zhang X. Y., Ehrlich M. A human DNA-binding protein is methylation-specific and sequence-specific. Nucleic Acids Res. 1986 Feb 25;14(4):1599–1614. doi: 10.1093/nar/14.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Zhang X. Y., Khan R., Zhou Y. W., Huang L. H., Ehrlich M. Methylated DNA-binding protein from human placenta recognizes specific methylated sites on several prokaryotic DNAs. Nucleic Acids Res. 1986 Dec 22;14(24):9843–9860. doi: 10.1093/nar/14.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. G. Methylation of specific cytosine residues enhances simian virus 40 T-antigen binding to origin region DNA. J Virol. 1987 Jul;61(7):2344–2348. doi: 10.1128/jvi.61.7.2344-2348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli J., Adelstein R. S., Melloul D., Nudel U., Yaffe D., Cedar H. Muscle-specific activation of a methylated chimeric actin gene. Cell. 1986 Aug 1;46(3):409–416. doi: 10.1016/0092-8674(86)90661-6. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Ehrlich K. C., Wang R. Y., Ehrlich M. Effect of site-specific DNA methylation and mutagenesis on recognition by methylated DNA-binding protein from human placenta. Nucleic Acids Res. 1986 Nov 11;14(21):8387–8397. doi: 10.1093/nar/14.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Loflin P. T., Gehrke C. W., Andrews P. A., Ehrlich M. Hypermethylation of human DNA sequences in embryonal carcinoma cells and somatic tissues but not in sperm. Nucleic Acids Res. 1987 Nov 25;15(22):9429–9449. doi: 10.1093/nar/15.22.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]
- van der Hoorn F. A. c-mos upstream sequence exhibits species-specific enhancer activity and binds murine-specific nuclear proteins. J Mol Biol. 1987 Jan 20;193(2):255–266. doi: 10.1016/0022-2836(87)90217-8. [DOI] [PubMed] [Google Scholar]