Abstract
Background:
Dural metastases have been found in about 8–9% of patients who died of cancer, in most autopsy series. Dural metastases presenting with chronic subdural hematoma are rare, with only about 55 cases reported in the literature.
Case Description:
We discuss the case of a 72 year old gentleman with prostate cancer who presented with a chronic subdural hematoma which was drained surgically. He was found to have disseminated intravascular coagulation (DIC) and recurrence of the subdural hematoma for which further drainage was required. After the second drainage of the chronic subdural hematoma, dural metastases were diagnosed from the pathology specimens.
Conclusion:
On reviewing the literature, 25 cases of dural metastases with chronic subdural hematoma and coagulopathy were found. These cases were characterized by the fact that they had a very poor clinical outcome in spite of surgical drainage. This combination could be a distinct entity and its recognition is important to guide management of this rare condition.
Keywords: Coagulopathy, disseminated intravascular coagulation, dural metastases, subdural hematoma
INTRODUCTION
Dural metastases have been found in about 8–9% of patients who died of cancer, in most autopsy series.[16] Dural metastases presenting with chronic subdural hematoma are rare, with only about 55 cases reported in the literature.[1–22]
We discuss the case of a 72-year-old gentleman with prostate cancer who presented with a chronic subdural hematoma which was drained surgically. He was found to have disseminated intravascular coagulation (DIC) and recurrence of the subdural hematoma for which further drainage was required. After the second drainage of the chronic subdural hematoma, the diagnosis of dural metastasis was made from the pathology specimen.
CASE REPORT
A 72 year old gentleman presented to his family doctor with mild confusion of 3 weeks duration. He had a history of locally aggressive prostate cancer diagnosed a few months previously and had finished a course of chemotherapy. He was also on warfarin for atrial fibrillation. Neurological examination was unremarkable. A magnetic resonance imaging (MRI) scan was arranged by the family doctor to look for intracranial metastases. This was done at his local hospital and reportedly did not reveal any evidence of malignancy, but revealed a small left-sided chronic subdural hematoma. This was confirmed on a computed tomography (CT) scan [Figure 1a]. He was initially managed conservatively, though warfarin was stopped. Four weeks later, he had worsening confusion and a repeat CT scan [Figure 1b] showed that the left-sided chronic subdural hematoma was now larger and causing significant mass effect and midline shift. There was also some new acute hemorrhage in the parietal region. His International Normalized Ratio (INR) was 1.7 at the time and activated partial thromboplastin time (APTT) was also slightly deranged, even though warfarin had been stopped. He was transferred to the neurosurgical center where he subsequently underwent emergent surgery after receiving fresh-frozen plasma and platelets. A frontal burr hole and parietal minicraniotomy were performed. At surgery, oil-black liquid typical of chronic subdural hematoma mixed with some fresh clot was evacuated. Hemorrhagic subdural membranes were found at the parietal site and gently removed, but not sent for pathology as this is not the common practice in our institution.
Figure 1.
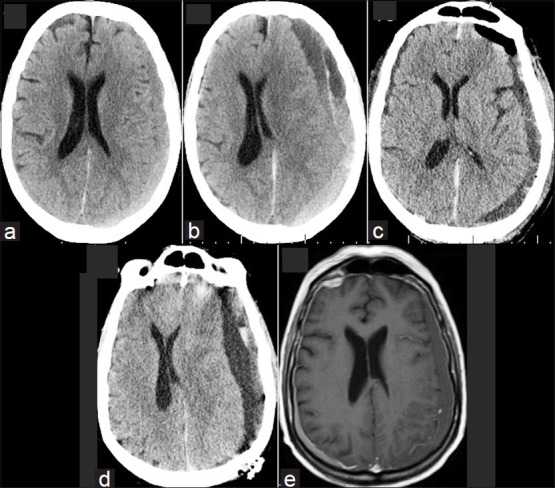
CT and MRI images. (a) Initial CT with a small left-sided chronic subdural hematoma. (b) CT prior to the first surgery which shows the subdural hematoma has expanded. (c) CT after the first surgery with good evacuation of the subdural hematoma. (d) CT prior to the second surgery where the subdural hematoma has recollected. (e) MRI. This was initially reported as showing only a small left-sided subdural hematoma and ruled out any intracranial metastases. However, on closer inspection, the dural enhancement is evident, especially on the right side. On the left side, the subdural hematoma is masking this
Postoperatively, he initially did well, with improvement in his confusion. Postoperative CT [Figure 1c] showed satisfactory evacuation of the subdural hematoma and resolution of the midline shift. However, after 1 week, his coagulation profile showed that he was in DIC, which was treated with fibrinogen. He became drowsier and also developed mild right hemiparesis. Repeat CT [Figure 1d] showed that there was a significant recurrent left-sided chronic subdural hematoma with mass effect and midline shift. He then had re-evacuation of the chronic subdural hematoma. This time, the surgery was performed by a different surgeon, and the clot and some of the subdural membranes from the parietal region were sent for histopathology as the membranes appeared very hemorrhagic. Postoperatively, the patient failed to improve neurologically and remained in DIC in spite of aggressive intensive care therapy. He also developed pneumonia and remained on the ventilator. The pathology [Figure 2] report showed that he had dural metastases and malignant cells in the subdural fluid. The patient died a week after the second surgery. No autopsy was performed at the request of the family. A review of the original MRI [Figure 1e] performed at his local hospital showed that despite the initial report, there was infact, evidence of dural enhancement suggestive of dural metastases.
Figure 2.
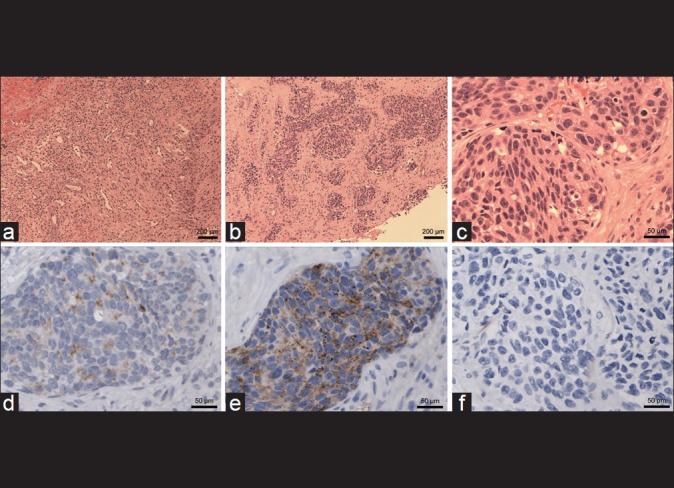
Histology slides. (a) Low-power (×10 magnification) view shows typical subdural hematoma with ectatic blood vessels, mixed inflammation, and fibroblasts. (b) In addition, there is a widely infiltrating carcinoma that grows in nodules (×10). (c) Higher magnification (×40) shows pleomorphic, often round nuclei with prominent single nucleoli and brisk mitotic activity. (d) Immunohistochemistry for prostate-specific antigen (PSA) shows mild positivity in the carcinoma but not anywhere else (×40). (e) There is intense immunoreactivity for prostate-specific acid phosphatase (PSAP, ×40). (f) In contrast, no staining is seen for cytokeratin 7 (×40)
DISCUSSION
The overall incidence of chronic subdural hematoma has been estimated to be 13.1 per 100,000 per year, with an incidence of 3.1 per 100,000 per year in patients <65 years of age and an incidence of 58.1 per 100,000 per year in older patients.[13]
Chronic subdural hematoma occurring in the setting of dural metastases is very uncommon. Since 1904, when Westenhoeffer et al.[24] first described a subdural hematoma associated with a dural metastasis of gastric carcinoma, only a few cases have been reported. Most of these reports are from the pre-1990s when larger numbers of autopsies were performed routinely. The true incidence is unknown as it is not common practice to send the chronic subdural hematoma or the subdural membrane for pathology after chronic subdural hematoma evacuation.
A review of the published medical literature reveals about 55 cases of chronic subdural hematoma associated with dural metastases. Of these, 25 had reported coagulation disorders.
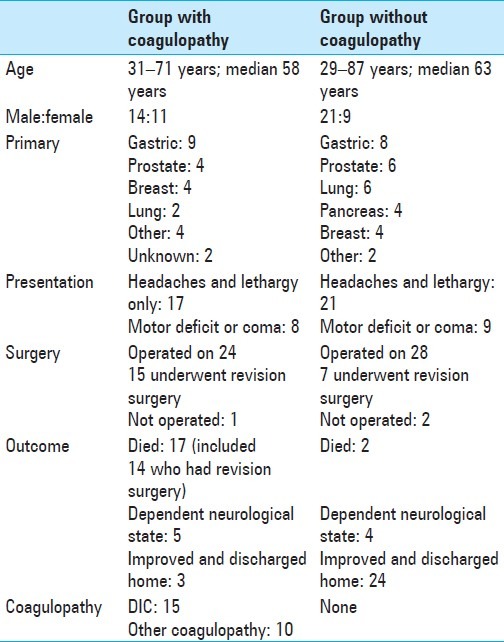
The clinical features of these patients with metastatic subdural hematoma with and without coagulopathy are summarized in Table 1.
Table 1.
Clinical features of patients with metastatic subdural hematoma with and without coagulopathy
In a review of the literature, Laigle-Donadey et al. identified 198 cases of dural metastases.[16]
Dural metastasis is differentiated from leptomeningeal metastatic disease in that the latter affects the the pia and arachnoid only and can be seen to follow the gyral convolutions on MRI, compared to the former. The mean age of the patients was 59 years (from 4 months to 84 years). The main primary tumors in order of decreasing frequency were: prostate (19.5%), breast (16.5%), lung (11%), and stomach carcinomas (7.5%). Dural metastases may arise from direct extension of skull metastases (57%) or from hematogenous metastases (43%). Exceptionally, dural involvement results from outward progression of a cortical brain metastasis. Direct extension from calvarial metastases predominates in lung, prostate, breast carcinomas, and in Ewing's sarcoma. In the absence of skull invasion, dissemination occurs through the systemic, mainly arterial, circulation.
Retrograde seeding by the valveless vertebral venous system (Batson's plexus) has been advocated in prostate cancer to explain the high frequency of skull and dural metastases in this tumor.[4] Extension via the lymphatic circulation is also possible.
Several hypotheses have been proposed to explain the propensity of dural metastases to cause subdural hematoma. Firstly, the bleeding could be due to the rupture of fragile tumor neo-vessels. Secondly, expanding skull metastases could cause mechanical obstruction of external dural vessels, leading to the dilatation and eventually the rupture of the capillaries of the inner dural layer. Thirdly, chronic subdural hematoma could be the mediator rather than a consequence of subdural invasion.[23]
Although laboratory evidence of abnormal coagulation is common in cancer patients, clinically evident bleeding is rare in patients with solid tumors. One study group recently reported on the occurrence of symptomatic DIC in 1117 patients with solid tumors.[10] Seventy-six patients (6.8%) had laboratory evidence of DIC and clinical evidence of altered hemostasis. Unknown primary cancers and cancers originating in the lung, breast, and prostate were responsible for almost half of the cases. Gastrointestinal carcinomas (colorectal, gastric, and pancreatic) were responsible for another 22%. The majority of cancers were adenocarcinomas.
A variety of coagulation disorders in cancer patients arise from tumor-specific growth characteristics, neo-angiogenesis with impaired endothelial lining, defective myelopoiesis, hypoproteinemia or metastatic lesion growth with organ dysfunction. A coagulation homeostasis may become further impaired after non-surgical cancer therapy, especially after preoperative irradiation, which produces lesions precipitating both bleeding and thrombosis. Anti-cancer chemotherapy may affect liver function and decrease the synthesis of both procoagulative and anticoagulative factors. Most chemotherapeutic protocols affect platelet synthesis, which presents a principal dose-limiting side effect.[15]
Tasaki et al. examined the presence or absence of tumor embolism in the dura as well as blood coagulation impairment in patients with dural metastasis, indicating that 70% of these patients had tumor embolism and half of them also had blood coagulation impairment.[21]
This is in contrast to most chronic subdural hematomas seen in routine clinical practice.
In a series of 500 consecutive patients treated surgically for chronic subdural hematoma, non–drug-induced coagulopathy was seen only in 14 patients (2.8%).[18] Seven of the 14 (50%) had poor outcome. Half of the mortality rate of 1.2% in this series was of patients with DIC. Patients with drug-induced coagulopathy had poor outcome in only 18.5% cases. Their overall recurrence rate was 9.8% and overall good recovery for the whole series was achieved by 89%.
In our review of patients with metastatic coagulopathic subdural hematoma, 88% had poor outcome and the mortality rate was 68%. At least 60% had revision surgery for recurrence, though some more patients were sent for palliation rather than revision surgery. In our review of those with metastatic subdural hematoma without coagulopathy, 80% had good outcome from the surgery.
Dural metastases with subdural hematoma had poor outcome in 20%. Coagulopathy with subdural hematoma had poor outcome in 50%. Combination of all the three led to poor outcome in 88%. It is likely that coagulopathy is the predominant factor in mediating the poor outcome, at least in the short term. Coagulopathy, especially in the setting of DIC, is difficult to manage without control of the primary cause, i.e. the advanced metastatic disease process.
Our patient had been on warfarin, which may have predisposed him to develop the chronic subdural hematoma in the first place. However, in spite of stopping warfarin, the subdural hematoma progressed. The dural metastases could have played a role as well, as the membranes appeared hemorrhagic at the time of surgery. Dural metastases have been shown to have produced subdural effusions without obvious hemorrhage.[21] This would suggest that dural metastases are not a benign and incidental finding in patients with chronic subdural hematomas. However, as autopsy reports suggest a high rate of dural metastasis of 8–9% and we see a much lower in cidence of chronic subdural hematomas (13/10,000), it is likely that the vast majority of dural metastases remain asymptomatic.[13,16] The DIC, however, could have set the dural metastases from a mild propensity to bleed to a more aggressive course. While the exact etiology of his hematoma is likely to have been mutifactorial, the uncontrollable DIC triggered by his malignancy in the setting of dural metastases would have been the main contributor to the poor outcome from this patient's chronic subdural hematoma.
This report illustrates an uncommon finding in a very common neurosurgical condition. When the patient initially presented to us, the original MRI scan was not available to us. Even though we were aware that he had prostate cancer, we were not aware at the time that this had been locally invasive prostate cancer. It is estimated that 80% of men by the age of 80 have prostate cancer and drainage of a chronic subdural hematoma in a patient with prostate cancer or some other malignancy would have been performed by almost all neurosurgeons at some point.[20] Yet, sending the subdural hematoma and dura for histology even in these circumstances is not standard neurosurgical practice in many institutions.
Awareness of this case has changed the practice in our institution in that all chronic subdural hematomas in a patient with malignancy are screened for coagulopathy before and after surgery and the subdural hematoma and dura are sent for pathology at surgery.
CONCLUSION
Patients with chronic subdural hematoma, who have a history of malignancy, should be investigated for metastatic disease and coagulation disorders. If both are found, the prognosis is likely to be dismal. If surgery is performed, the subdural membrane and subdural hematoma should be sent for histopathologic analysis. This is essential for diagnostic and prognostic purposes. If there is recurrence of the subdural hematoma, revision surgery should be undertaken only with the knowledge that the prognosis is very poor despite surgery.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/60/97004
Contributor Information
Kuriakose J. George, Email: joshigeorge@doctors.org.uk.
Anthony Lau, Email: anthony.lau@utoronto.ca.
Michael Ellis, Email: ellis804@yahoo.com.
Tim-Rasmus Kiehl, Email: rasmus.kiehl@uhn.ca.
Michael G. Fehlings, Email: michael.fehlings@uhn.ca.
REFERENCES
- 1.Ambiavagar PC, Sher J. Subdural hematoma secondary to metastatic neoplasm: Report of two cases and a review of the literature. Cancer. 1978;42:2015–8. doi: 10.1002/1097-0142(197810)42:4<2015::aid-cncr2820420450>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann M, Puskas Z, Kuchelmeister K. Subdural hematoma due to dural metastasis: Case report and review of the literature. Clin Neurol Neurosurg. 1992;94:235–40. doi: 10.1016/0303-8467(92)90095-k. [DOI] [PubMed] [Google Scholar]
- 3.Braun EM, Burger LJ, Schlang HA. Subdural hematoma from metastatic malignant disease. Cancer. 1973;32:1370–3. doi: 10.1002/1097-0142(197312)32:6<1370::aid-cncr2820320614>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Caputi F, Lamaida E, Gazzeri R. Acute subdural hematoma and pachymeningitis carcinomatosa: Case report. Rev Neurol (Paris) 1999;155:383–5. [PubMed] [Google Scholar]
- 5.Comãnescu A, Roşca E, Bota M, Ninulescu G. Chronic subdural hematoma in a patient with acute myeloid leukemia and dural metastatic infiltration. Rom J Morphol Embryol. 2008;49:259–62. [PubMed] [Google Scholar]
- 6.Fukino K, Terao T, Kojima T, Adachi K, Teramoto A. Chronic subdural hematoma following dural metastasis of gastric cancer: Measurement of pre- and postoperative cerebral blood flow with N-isopropyl-p-[123I]iodoamphetamine--Case report. Neurol Med Chir (Tokyo) 2004;44:646–9. doi: 10.2176/nmc.44.646. [DOI] [PubMed] [Google Scholar]
- 7.Hirano A, Hojo S. [Metastatic tumors in the central nervous system. The neuropathological point of view (Part 2) (author's transl)] No Shinkei Geka. 1980;8:599–603. [PubMed] [Google Scholar]
- 8.Hirashima Y, Kamiyama K, Endo S, Takaku A. [Subdural hematoma due to metastatic dural carcinomatosis associated with DIC--A case report] No Shinkei Geka. 1983;11:651–6. [PubMed] [Google Scholar]
- 9.Kamiya K, Inagawa T, Nagasako R. [Chronic subdural hematoma due to dural metastasis: Demonstration of adenocarcinoma cell nests in the fluid. Case report] Neurol Med Chir (Tokyo) 1987;27:892–8. doi: 10.2176/nmc.27.892. [DOI] [PubMed] [Google Scholar]
- 10.Kampel LJ. Challenging problems in advanced malignancy: Case 2. Disseminated intravascular coagulation in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2003;21:3170–1. doi: 10.1200/JCO.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 11.Katsube T, Kikuchi T, Konnno S, Murayama M, Kobayashi R, Kuhara K, et al. Subdural hematoma associated with Dural metastasis of gastric carcinoma: Report of two cases. Anticancer Res. 2007;27:4339–44. [PubMed] [Google Scholar]
- 12.Kinjo T, Mukawa J, Takara E, Horikawa K. [Chronic subdural hematoma following advanced cancer: Report of three cases] No Shinkei Geka. 1989;17:763–8. [PubMed] [Google Scholar]
- 13.Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: Present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo) 1992;32:207–9. doi: 10.2176/nmc.32.207. [DOI] [PubMed] [Google Scholar]
- 14.Kunii N, Morita A, Yoshikawa G, Kirino T. Subdural hematoma associated with dural metastasis--Case report. Neurol Med Chir (Tokyo) 2005;45:519–22. doi: 10.2176/nmc.45.519. [DOI] [PubMed] [Google Scholar]
- 15.Kvolik S, Jukic M, Matijevic M, Marjanovic K, Glavas-Obrovac L. An overview of coagulation disorders in cancer patients. Surg Oncol. 2010;19:e33–46. doi: 10.1016/j.suronc.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005;75:57–61. doi: 10.1007/s11060-004-8098-1. [DOI] [PubMed] [Google Scholar]
- 17.Leech RW, Welch FT, Ojemann GA. Subdural hematoma secondary to metastatic Dural carcinomatosis. Case report. J Neurosurg. 1974;41:610–3. doi: 10.3171/jns.1974.41.5.0610. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001;41:371–81. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 19.Otsuka A, Asakura K, Takahashi K, Tasaki T, Okada K, Suzuki Y. [Nontraumatic chronic subdural hematoma due to dural metastases of breast cancer. Case report] No Shinkei Geka. 1985;13:999–1004. [PubMed] [Google Scholar]
- 20.Sakr WA, Grignon DJ, Haas GP, Heilbrun LK, Pontes JE, Crissman JD. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol. 1996;30:138–44. doi: 10.1159/000474163. [DOI] [PubMed] [Google Scholar]
- 21.Tasaki K, Shima T, Matsumura S, Okada Y, Nishida M, Yamada T, et al. [A case of subdural effusion secondary to dural metastasis of prostatic cancer: Case report] No Shinkei Geka. 1990;18:539–42. [PubMed] [Google Scholar]
- 22.Tseng SH, Liao CC, Lin SM, Chen Y, Shun CT. Dural metastasis in patients with malignant neoplasm and chronic subdural hematoma. Acta Neurol Scand. 2003;108:43–6. doi: 10.1034/j.1600-0404.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 23.Turner DM, Graf CJ. Nontraumatic subdural hematoma secondary to dural metastasis: Case report and review of the literature. Neurosurgery. 1982;11:678–80. doi: 10.1227/00006123-198211000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Westenhoeffer M. Pachymeningitis carcinomatosa haemorrhagia interna productive mit Colibacillosis agonalis. Virchow Arch. 1904;175:364–79. [Google Scholar]


