Abstract
Isolates of Ophiocordyceps heteropoda (Kobayasi) collected from Mt. Halla on Jeju-do, Korea were tested for mycelial growth on different agar media and in the presence of different carbon and nitrogen sources. Similarly, isolates were also incubated at different temperatures as well as under continuous light and dark conditions. Growth was better on Hamada agar, basal medium, and malt-yeast agar, but poor on Czapek-Dox agar. Different carbon sources such as dextrin, saccharose, starch, lactose, maltose, fructose, and dextrose resulted in better growth. Complex organic nitrogen sources such as yeast extract and peptone revealed the most effective growth. Mycelial growth was best at 25℃. The growth rate was faster in the dark than the light, but mycelial density was less compact in the dark.
Keywords: Carbon source, Cordyceps heteropoda, Medium test, Nitrogen source, Ophiocordyceps heteropoda
Ophiocordyceps heteropoda is a relatively rare species that was first described by Kobayasi as Cordyceps heteropoda from north Japan, growing on hypogaeous cicada nymphs (Figs. 1 and 2) [1]. It was then reported from the Congo, a central African country [2]. Besides Japan, it has been recently reported from other east Asian countries such as Korea and China [3-5]. C. heteropoda var. haiirooosemitake, and two form species, C. heteropoda f. sp. tsutsunagaoosemitake and C. heteropoda f. sp. Usuirooosemitake, were also reported by Shimizu [6]. A different variety, C. heteropoda var. langyashanensis, and its anamorph, Hirsutella heteropoda, have been reported recently from China [4]. Korean C. heteropoda is more similar to the Japanese species [1, 3]. This species has a very patchy distribution, as it has been reported only from east Asia and central Africa, probably due to a lack of exploration. C. heteropoda was previously confused with another Cordyceps species, C. scottianus [1, 7, 8]. Cordyceps species growing on cicadas, including C. heteropoda, have been explicitly described, beautifully illustrated, and well reviewed in different pictorial books [1, 3, 5, 6, 8-11]. Recently, C. heteropoda was transferred to Ophiocordyceps by Sung et al. [12], hence, it was renamed O. heteropoda (Kobayasi) Sung et al. [12]. This species is particularly characterized by the epigaeous part of its stem, which is distinct from the hypogaeous part. The head is oval to spherical and is quite distinct from the stem.
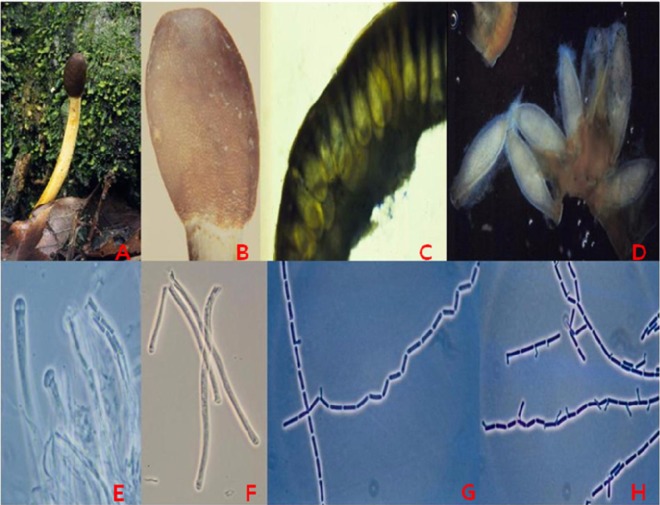
Fig. 1.
Morphological characteristics of Ophiocordyceps heteropoda. Various natural specimens.
Fig. 2.
Morphological characteristics of Ophiocordyceps heteropoda. A, Stroma; B, Magnified head; C, Immersed perithecia; D, Perithecia; E, Ascus head; F, Asci; G, Threadlike ascospores; H, Germination of ascospores.
This fungus produces anti-bacterial and anti-fungal compounds [13]. In the context of growing studies on Cordyceps and allied species [14-23], isolates of this species were tested for growth on different agar media, at different temperatures, and under light and dark conditions. This species showed moderate growth on agar media with the possibility of growing to a larger scale under optimum cultural conditions and nutrition sources.
Materials and Methods
Fungal isolates
Multi-ascospore isolates of O. heteropoda specimens CRI C-11247, CRI C-12565, and CRI C-12567, which were preserved at the Cordyceps Research Institute (CRI), Mushtech, Korea, were used. The isolates were grown on Sabouraud dextrose agar plus yeast extract (SDAY; dextrose 40 g, yeast extract 10 g, peptone 10 g, and agar 20 g per 1,000 mL; pH 5.6) plates at 25℃ for 30 days and were used for further experiments. Specimen CRI C-11247 was collected on May 21, 2004. Similarly, two other specimens, CRI C-12565 and CRI C-12567, were collected on May 20, 2005. All specimens were collected from Mt. Halla on Jeju-do.
Effect of medium on O. heteropoda mycelial growth
Ten different types of agar media, including malt-extract agar, oatmeal agar (OA), malt-yeast agar (MYA), Martin's peptone dextrose agar (MPDA), basal medium (BM), potato dextrose agar (PDA), Schizophyllum (mushroom) genetics complete medium plus yeast extract (MCM), Hamada agar (HA), Czapek-Dox agar (CDA), and SDAY were used to observe the effect of medium on O. heteropoda mycelial growth (Table 1). Agar was added to all media at a 2% concentration (w/v). Mycelial discs (5 mm) were cut from the isolates and were inoculated on all experimental agar plates. The agar plates were then incubated at 25℃ for 30 days under white fluorescent light and were observed for colony diameter (CD) and mycelial density (MD).
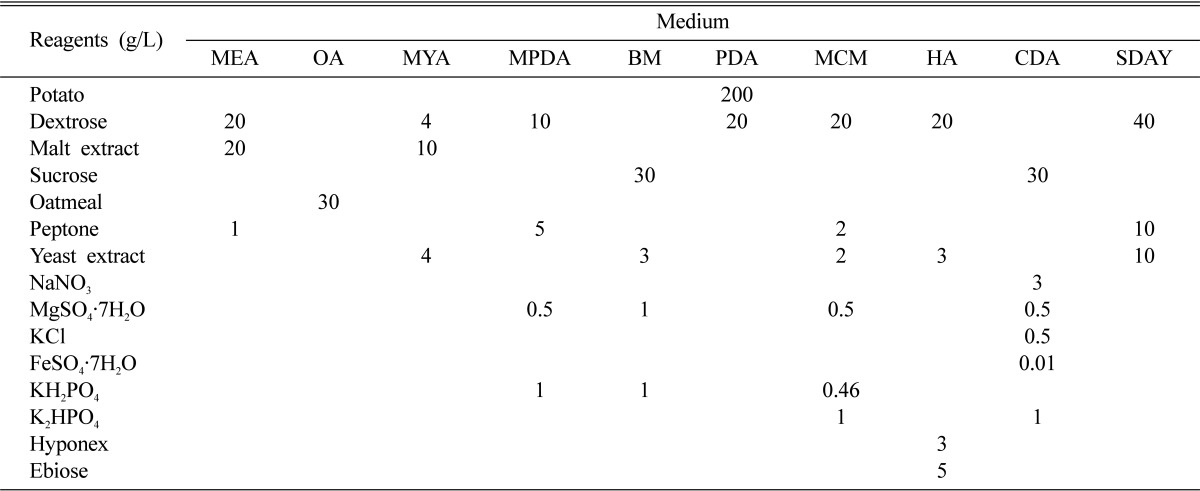
Table 1.
Culture media composition
MEA, malt-extract agar; OA, oatmeal agar; MYA, malt-yeast agar; MPDA, Martin's peptone dextrose agar; BM, basal medium; PDA, potato dextrose agar; MCM, Schizophyllum (mushroom) genetics complete medium plus yeast extract; HA, Hamada agar; CDA, Czapek-Dox agar; SDAY, Sabouraud's dextrose agar plus yeast extract.
Different carbon and nitrogen sources were tested for their effect on O. heteropoda mycelial growth. Ten different carbon sources, including arabinose, dextrin, dextrose, fructose, galactose, lactose, maltose, saccharose, starch, and xylose were individually added to 2% water agar (WA) at a 2% concentration (w/v). Similarly, ten different nitrogen sources, including NH4NO3, (NH4)3PO4, (NH4)2SO4, ammonium tartrate, KNO3, arginine, asparagine, glycine, peptone, and yeast extract were individually added to WA at a 1% concentration (w/v). The isolates were inoculated on WA plates supplemented with carbon and nitrogen sources and incubated at 25℃ for 30 days under white fluorescent light. CD was measured in mm and MD was qualitatively categorized as thin (+), moderate (++), or compact (+++).
Effect of temperature and light on O. heteropoda mycelial growth
The isolates were inoculated on PDA, MCM, and BM agar plates and incubated at 15~30℃ at intervals of 5℃ for 30 days under white fluorescent light. Similarly, the isolates were inoculated on PDA, MCM, and BM agar plates and incubated at 25℃ for 30 days under white fluorescent light as well as under dark conditions. CD and MD were observed.
Results and Discussion
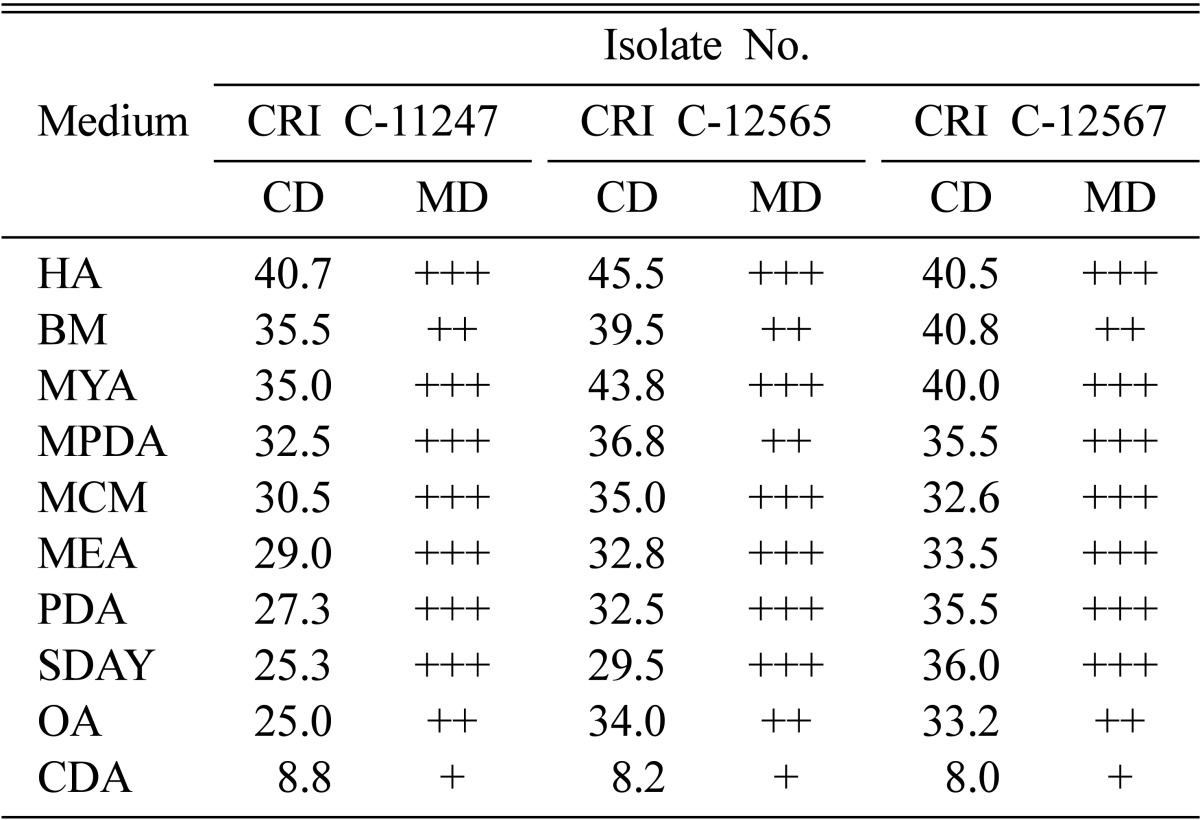
CD was longer on HA, BM, and MYA followed by MPDA, MCM, SDAY, and PDA (Table 2). The isolates produced compact to moderate MD on all media, except CDA in which a thin MD was produced (Table 2). CDA also resulted in the shortest CD. The major difference between CDA and other media is that the former does not contain any organic nitrogen source. OA and PDA also do not contain an extra organic nitrogen source, but oatmeal and potato are complex organic substances that contain organic nitrogen sources. However, all remaining media were supplemented with either peptone, yeast extract, or both. From this observation, it can be concluded that organic nitrogen sources are the most important factor for rich mycelial growth in O. heteropoda. Besides CDA, OA produced shorter CD as well as a moderate MD.
Table 2.
Effect of medium type on Ophiocordyceps heteropoda mycelial growth
CRI, Cordyceps Research Institute; CD, colony diameter; MD, mycelial density; HA, Hamada agar; BM, basal medium; MYA, malt-yeast agar; MPDA, Martin's peptone dextrose agar; MCM, Schizophyllum (mushroom) genetics complete medium plus yeast extract; MEA, malt-extract agar; PDA, potato dextrose agar; SDAY, Sabouraud's dextrose agar plus yeast extract; OA, oatmeal agar; CDA, Czapek-Dox agar.
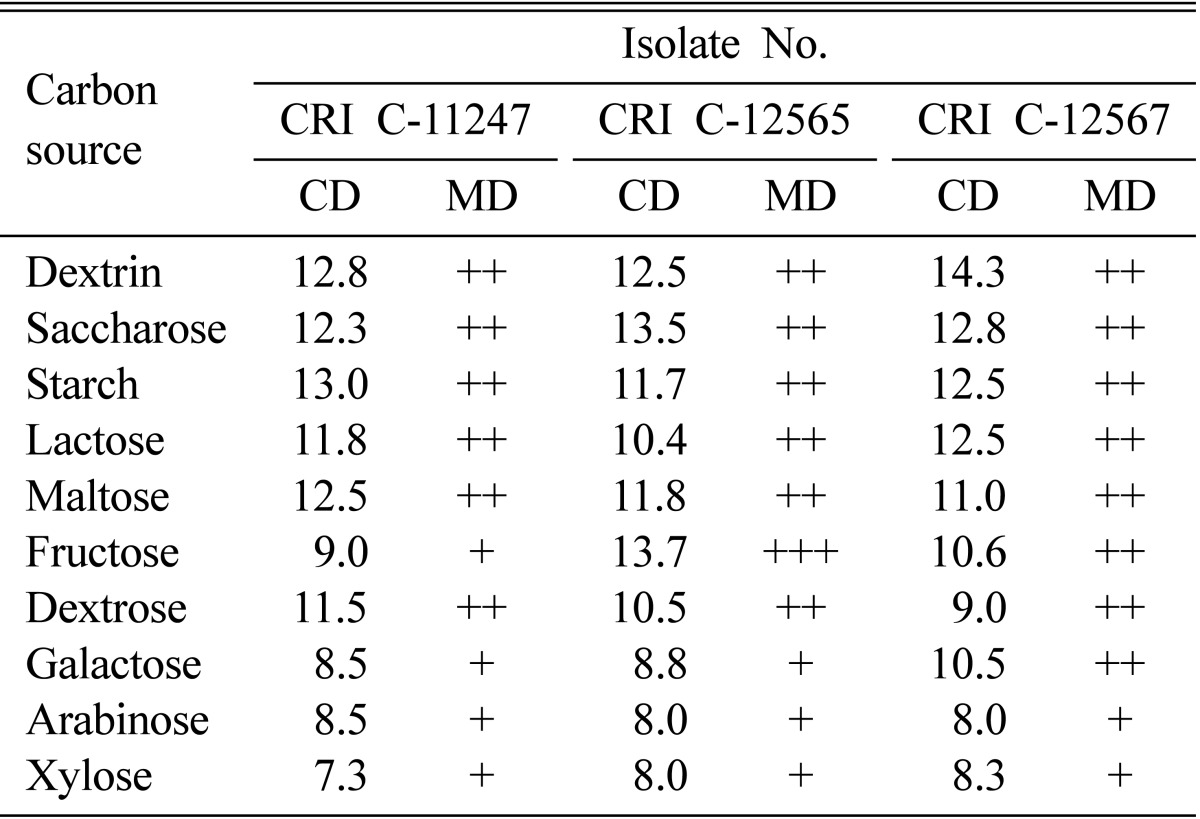
Dextrin, saccharose, starch, lactose, maltose, fructose, and dextrose resulted in better growth than the remaining carbon sources both in terms of CD and MD (Table 3). In general, dextrin, saccharose, and starch were more favorable carbon sources. The results showed that carbon sources alone could not sustain growth when compared to growth on complete media (Tables 2 and 3). However, it was unclear why CDA performed worse than the simple carbon sources despite being supplemented with inorganic nitrogen sources and mineral salts.
Table 3.
Effect of carbon source on Ophiocordyceps heteropoda mycelial growth
CRI, Cordyceps Research Institute; CD, colony diameter; MD, mycelial density.
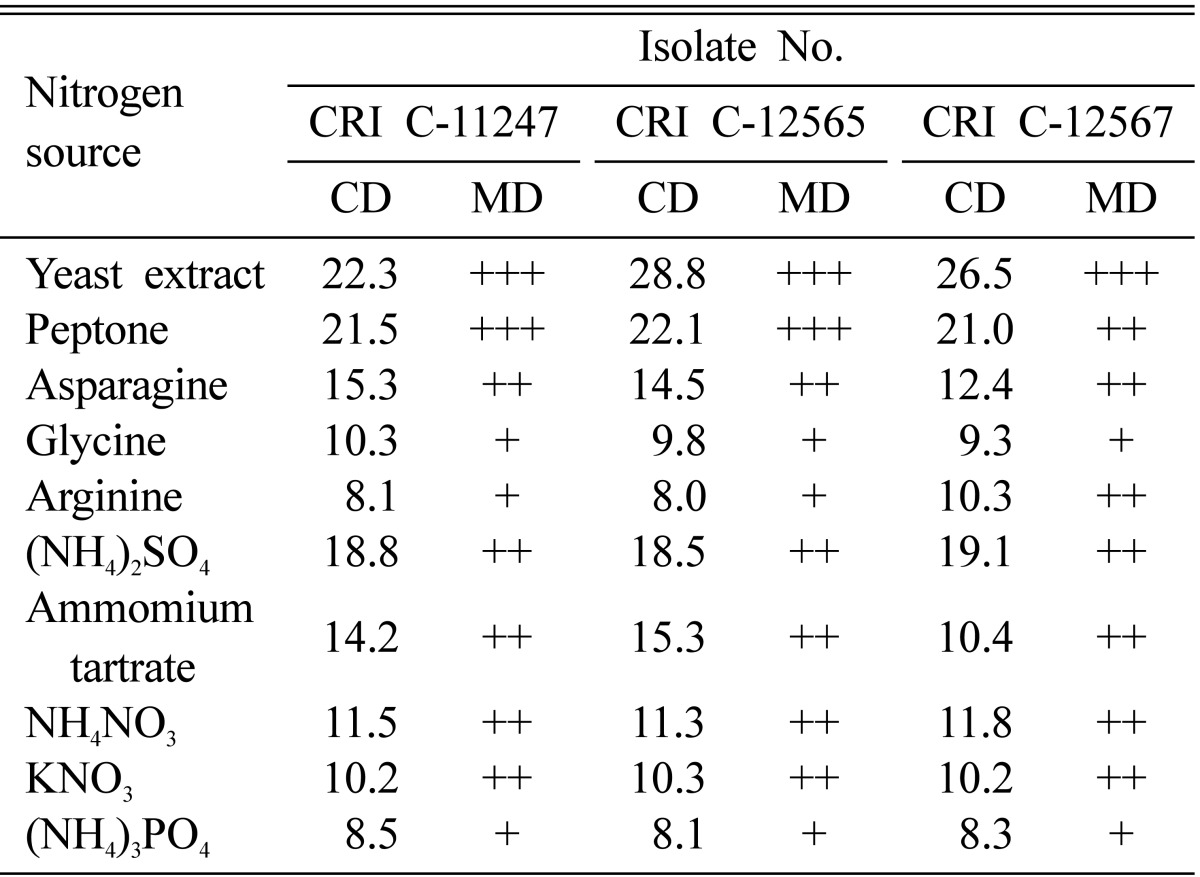
Complex organic nitrogen sources resulted in better growth than inorganic nitrogen sources (Table 4). Furthermore, complex organic nitrogen sources, such as yeast extract and peptone, performed better than amino acids (Table 4), as shown by previous studies [24, 25]. The mycelial growth patterns revealed that only yeast extract and peptone resulted in a compact MD. It was obvious that yeast extract and peptone consisted of many types of amino acids and, hence, resulted in better growth than that provided by individual amino acids. Among the amino acids tested, asparagine was the best and resulted in growth similar to ammonium tartrate and NH4NO3. (NH4)2SO4 showed the best growth among the inorganic nitrogen sources and performed better than any of the individual amino acids (Table 4). NH4NO3 and KNO3 also resulted in better mycelial growth than glycine and arginine both in terms of CD and MD (Table 4). Glycine, arginine, and (NH4)3PO4 all produced a thin MD.
Table 4.
Effect of nitrogen source on Ophiocordyceps heteropoda mycelial growth
CRI, Cordyceps Research Institute; CD, colony diameter; MD, mycelial density.
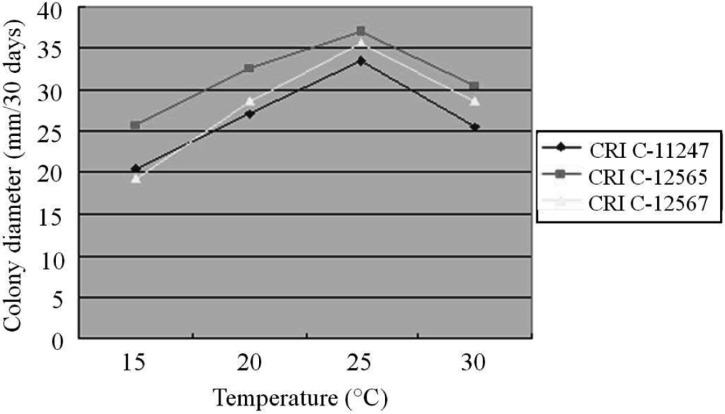
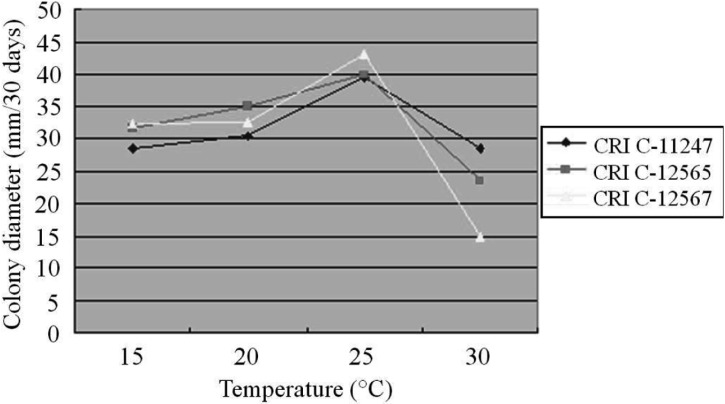
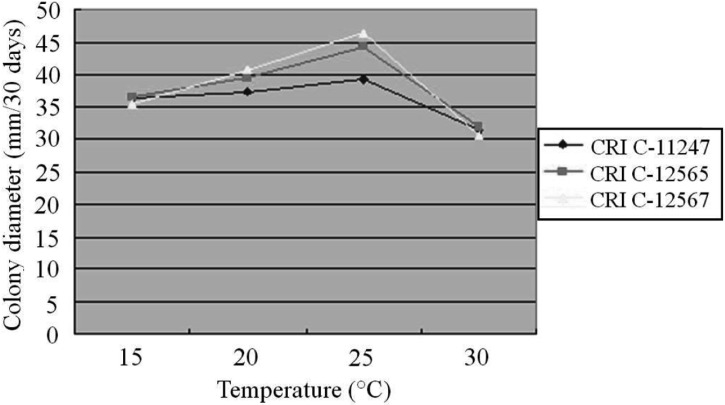
The effect of temperature on O. heteropoda mycelial growth differed from medium to medium. All isolates showed their longest CD at 25℃ on PDA, followed by 20℃, 30℃ and 15℃ (Fig. 3). Similar to PDA, the longest CD was observed at 25℃ on both MCM and BM, whereas the least growth was observed at 30℃ (Figs. 3, 4 and 5). In general, mycelial growth occurred at all the temperatures tested ranging from 15~30℃. A 25℃ temperature has been reported as optimum for Cordyceps species [25-27].
Fig. 3.
Effect of temperature on Ophiocordyceps heteropoda mycelial growth in potato dextrose agar after 30 days of culture. CRI, Cordyceps Research Institute.
Fig. 4.
Effect of temperature on Ophiocordyceps heteropoda mycelial growth in Schizophyllum (mushroom) genetics complete medium plus yeast extract medium after 30 days of culture. CRI, Cordyceps Research Institute.
Fig. 5.
Effect of temperature on Ophiocordyceps heteropoda mycelial growth in basal medium after 30 days of culture. CRI, Cordyceps Research Institute.
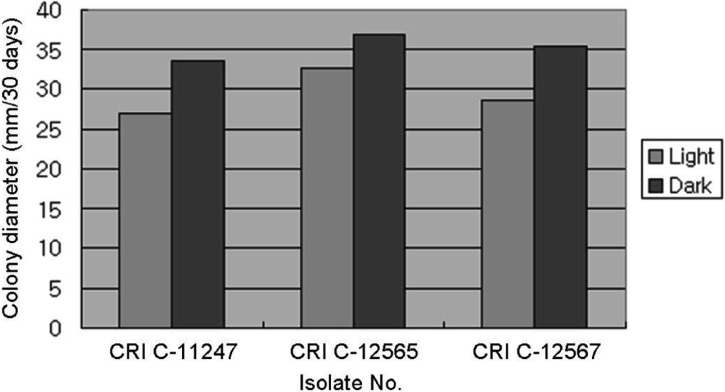
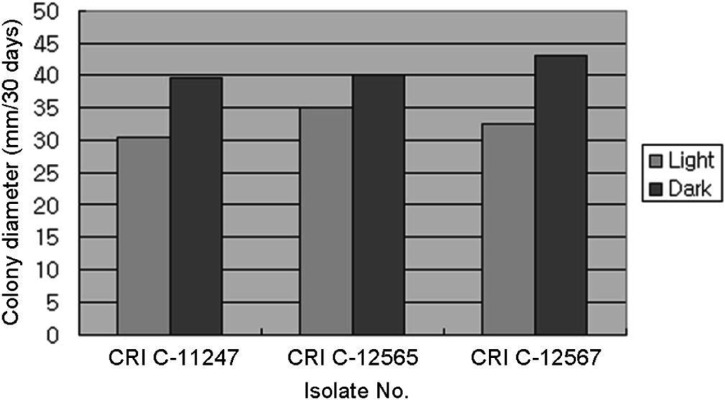
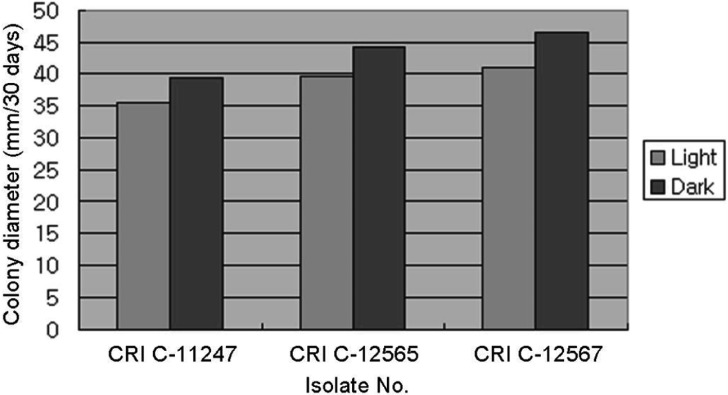
All isolates had a longer CD in the dark than the light (Figs. 6, 7 and 8). However, a difference in MD was observed. PDA and MCM resulted in a compact MD in the light but a moderate density in the dark. Moreover, BM resulted in moderate density in the light, but thin density in the dark. This result was very similar to that of Shrestha et al. [24]. Isolates of O. heteropoda produced yellowish white to yellow colonies with reddish pigmentation on the medium, as shown by Li et al. [4]. However, the growth rate was faster in this study than that of Li et al. [4]. But, the growth rate of O. heteropoda was slower, than that of C. militaris and Metacordyceps yongmunensis [24, 25].
Fig. 6.
Effect of light on Ophiocordyceps heteropoda mycelial growth in potato dextrose agar. CRI, Cordyceps Research Institute.
Fig. 7.
Effect of light on Ophiocordyceps heteropoda mycelial growth in Schizophyllum (mushroom) genetics complete medium plus yeast extract medium. CRI, Cordyceps Research Institute.
Fig. 8.
Effect of light on Ophiocordyceps heteropoda mycelial growth in basal medium. CRI, Cordyceps Research Institute.
Acknowledgements
The authors wish to acknowledge the Cordyceps Research Institute of Mushtech for providing the facilities to conduct this study.
References
- 1.Kobayasi Y. On the genus Cordyceps and its allies on cicadidae from Japan. Bull Biogeogr Soc Jpn. 1939;9:145–176. [Google Scholar]
- 2.Moureau J. Nouveaux Cordyceps du Congo. Lejeunia Mem. 1961;15:1–38. [Google Scholar]
- 3.Sung JM. The insects-born fungus of Korea in color. Seoul: Kyohak Publishing Co., Ltd.; 1996. [Google Scholar]
- 4.Li CR, Chen MJ, Wang M, Lin YR, Fan MZ, Li ZZ. Hirsutella heteropoda sp. nov. and its teleomorph, a new variety of Cordyceps heteropoda. Mycosystema. 2006;25:163–168. [Google Scholar]
- 5.Liang Z. Flora fungorum sinicorum. Vol. 32. Cordyceps. Beijing: Science Press; 2007. [Google Scholar]
- 6.Shimizu D. Color iconography of vegetable wasps and plant worms. Tokyo: Seibundo Shinkosha; 1994. [Google Scholar]
- 7.Willis JH. Australian species of the fungal genus Cordyceps (Fr.) Link with critical notes on collection in Australian herbaria. Muelleria. 1959;1:68–89. [Google Scholar]
- 8.Kobayasi Y, Shimizu D. Monographic studies of Cordyceps 2. Group parasitic on cicadidae. Bull Nat Sci Mus. 1963;6:286–314. [Google Scholar]
- 9.Kobayasi Y, Shimizu D. Iconography of vegetable wasps and plant worms. Osaka: Hoikusha Publishing Co., Ltd.; 1983. [Google Scholar]
- 10.Shimizu D. Illustrated vegetable wasps and plant worms in colour. Tokyo: Ie-No-Hikari Association; 1997. [Google Scholar]
- 11.Aoki J. A key to insect pathogenic fungi. Tokyo: Zenkoku Nouson Kyouiku Kyoukai; 2003. [Google Scholar]
- 12.Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasnoff SB, Reátegui RF, Wagenaar MM, Gloer JB, Gibson DM. Cicadapeptins I and II: new Aib-containing peptides from the entomopathogenic fungus Cordyceps heteropoda. J Nat Prod. 2005;68:50–55. doi: 10.1021/np0497189. [DOI] [PubMed] [Google Scholar]
- 14.Sung JM, Choi YS, Lee HK, Kim SH, Kim YO, Sung GH. Production of fruiting body using cultures of entomopathogenic fungal species. Korean J Mycol. 1999;27:15–19. [Google Scholar]
- 15.Chen R, Ichida M. Infection of the silkworm, Bombyx mori, with Cordyceps militaris. J Insect Biotechnol Sericol. 2002;71:61–63. [Google Scholar]
- 16.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnol Bioprocess Eng. 2004;9:440–446. [Google Scholar]
- 17.Ha NG, Kim SY, Kang JH, Kang PD, Sung GB, Hong IP. Biological activities and cultural characteristics of an entomogenous fungus, Paecilomyces tenuipes (Peck) Samson. Korean J Sericult Sci. 2005;47:12–17. [Google Scholar]
- 18.Li CR, Nam SH, Geng DG, Fan MZ, Li ZZ. Artificial culture of seventeen Cordyceps spp. Mycosystema. 2006;25:639–645. [Google Scholar]
- 19.Hong IP, Kang PD, Kim KY, Nam SH, Lee MY, Choi YS, Kim NS, Kim HK, Lee KG, Humber RA. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology. 2010;38:128–132. doi: 10.4489/MYCO.2010.38.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang PD, Sung GB, Kim KY, Kim MJ, Hong IP, Ha NG. Breeding of a silkworm variety for synnemata production of Isaria tenuipes. Mycobiology. 2010;38:180–183. doi: 10.4489/MYCO.2010.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Shrestha B, Sung GH, Han SK, Sung JM. Optimum conditions for artificial fruiting body formation of Cordyceps cardinalis. Mycobiology. 2010;38:133–136. doi: 10.4489/MYCO.2010.38.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JO, Shrestha B, Sung GH, Han SK, Kim TW, Sung JM. Cultural characteristics and fruiting body production in Cordyceps bassiana. Mycobiology. 2010;38:118–121. doi: 10.4489/MYCO.2010.38.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JO, Shrestha B, Sung GH, Han SK, Sung JM. Successful development of Cordyceps bassiana stromata from Beauveria bassiana. Mycobiology. 2010;38:13–16. doi: 10.4489/MYCO.2010.38.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha B, Lee WH, Han SK, Sung JM. Observations on some of the mycelial growth and pigmentation characteristics of Cordyceps militaris isolates. Mycobiology. 2006;34:83–91. doi: 10.4489/MYCO.2006.34.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung GH, Shrestha B, Sung JM. Characteristics of Metacordyceps yongmunensis, a new species from Korea. Mycobiology. 2010;38:171–175. doi: 10.4489/MYCO.2010.38.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung JM, Choi YS, Shrestha B, Park YJ. Cultural characteristics of mycelial growth by Cordyceps militaris. Korean J Mycol. 2002;30:1–5. [Google Scholar]
- 27.Shin JC, Shrestha B, Lee WH, Park YJ, Kim SY, Jeong GR, Kim HK, Kim TW, Sung JM. Distribution and favorable conditions for mycelial growth of Cordyceps pruinosa in Korea. Korean J Mycol. 2004;32:79–88. [Google Scholar]