Abstract
Cellular damage caused by reactive oxygen species has been implicated in several diseases, thus establishing a significant role for antioxidants in maintaining human health. Acetone, methanol, and hot water extracts of Pleurotus citrinopileatus were evaluated for their antioxidant activities against β-carotene-linoleic acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, reducing power, ferrous ion-chelating abilities, and xanthine oxidase inhibitory activities. In addition, the tyrosinase inhibitory effects and phenolic compound contents of the extracts were also analyzed. Methanol and acetone extracts of P. citrinopileatus showed stronger inhibition of β-carotene-linoleic acid compared to the hot water extract. Methanol extract (8 mg/mL) showed a significantly high reducing power of 2.92 compared to the other extracts. The hot water extract was more effective than the acetone and methanole extracts for scavenging DPPH radicals. The strongest chelating effect (92.72%) was obtained with 1.0 mg/mL of acetone extract. High performance liquid chromatography analysis detected eight phenolic compounds, including gallic acid, protocatechuic acid, chlorogenic acid, ferulic acid, naringenin, hesperetin, formononetin, and biochanin-A, in an acetonitrile and hydrochloric acid (5 : 1) solvent extract. Xanthine oxidase and tyrosinase inhibitory activities of the acetone, methanol, and hot water extracts increased with increasing concentration. This study suggests that fruiting bodies of P. citrinopileatus can potentially be used as a readily accessible source of natural antioxidants.
Keywords: Antioxidant, Phenolic compounds, Pleurotus citrinopileatus, Tyrosinase inhibition, Xanthine oxidase
Pleurotus citrinopileatus, which belongs to the family pleurotaceae and order agaricales, is a popular edible mushroom in China and Japan due to its bright color, unique flavor, and texture [1]. Recently, this mushroom was successfully cultivated and made commercially available in Korea. This mushroom grows on fallen trees and stumps of broad-leaf trees and is commercially cultivated on sawdust, rice straw, sugarcane bagasse, cotton seed, and peanut hulls [2].
Medicinally-edible mushrooms are an important source of natural antioxidants [3]. Natural antioxidants increase the antioxidative capacity of plasma and reduce the risk of certain diseases such as cancer, heart diseases, and stroke [4]. Secondary metabolites such as phenolics and flavonoids from mushrooms have been reported to be potent free radical scavengers [5]. The catalysis of xanthine by the enzyme xanthine oxidase (XO) can lead to the accumulation of uric acid and ultimately cause gout. Allopurinol, a XO inhibitor prescribed for the treatment of chronic gout, acts as a substrate for the competitive inhibitor of the enzyme [6]. A potential source of such compounds can be obtained from edible mushrooms. Flavonoids and polyphenolic crude extracts of mushrooms have been reported to possess XO inhibitory activity [7].
Tyrosinase is a copper-containing enzyme widely distributed in mushrooms, plants, and animals and is responsible for melanization. The formation of melanin in the human body can be influenced or reduced by several mechanisms, including anti-oxidation, direct tyrosinase inhibition, and hormonal activities, etc. [8]. Tyrosinase inhibitors are used frequently in cosmetics and depigmenting agents for the hyperpigmentation [9]. Therefore, a concerted effort has been made to search for naturally occurring tyrosinase inhibitors in P. citrinopileatus. Although the nutritional value and taste components of P. citrinopileatus have been thoroughly studied, little information is available on its antioxidant and antityrosinase properties. Therefore, the aim of the present study was to investigate the antioxidant and antityrosinase activities of the acetone, methanol, and hot water extracts of P. citrinopileatus fruiting bodies.
Materials and Methods
Chemicals and reagents
β-carotene, linoleic acid, chloroform, polyoxyethylene sorbitan monopalmitate (Tween 40), butylated hydroxytoluene (BHT), α-tocopherol (TOC), 1,1-diphenyl-2-picrylhydrazyl (DPPH), L-ascorbic acid, potassium ferricyanide, trichloroacetic acid, ferrous chloride, ferric chloride, ferrozine, Folin-Ciocalteu reagent, gallic acid, methanol, 3,4-dihydroxy-L-phenylalanine (L-DOPA), xanthine, allopurinol, mushroom tyrosinase, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All chemicals and solvents were used as high-performance liquid chromatography (HPLC) or analytical grade.
Mushroom and extraction
Fresh, mature fruiting bodies of P. citrinopileatus were obtained from Mushroom Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, Suwon, Korea. A pure culture was deposited in Culture Collection DNA Bank of Mushroom (CCDBM), Division of Life Sciences, University of Incheon, Korea with the acquired accession No. IUM-3969. Fruiting bodies were dried with hot air at 40℃ for 48 hr and finely pulverized. Five grams of powdered samples were extracted with 100 mL of 60% acetone and 80% methanol with stirring at 150 rpm for 24 hr at 25℃ to obtain the acetone and methanol extracts, respectively. The mixture was then filtered through two layers of Whatman No. 1 filter paper (Whatman, Maidstone, UK). Following this, the same quantity of sample was boiled at 100℃ for 3 hr with 100 mL of deionized distilled water to obtain the hot water extract. The mixture was cooled to room temperature and filtered through Whatman No. 1 filter paper. The residues were then extracted with two additional 100 mL aliquots of acetone, methanol, and deionized water, as described above. Then, the combined extracts were evaporated using a rotary evaporator (Eyela, Saitama, Japan) at 40℃, after which the remaining solvent was removed with a freeze-drier (Optizen, Daejeon, Korea). The yields of the acetone, methanol, and hot water extracts of P. citrinopileatus were 24.42, 23.81, and 19.58% (w/w), respectively.
Antioxidant activity by β-carotene-linoleic acid
Antioxidant activity was determined by measuring the inhibition of volatile organic compounds and conjugated diene hydroperoxides arising from linoleic acid oxidation [10]. A stock β-carotene-linoleic acid mixture was prepared as follows: 0.5 mg of β-carotene was dissolved in 1 mL of chloroform, after which 25 µL of linoleic acid and 200 mg Tween 40 were added. The chloroform was removed completely using a vacuum evaporator. Then, 100 mL of oxygenated distilled water was added with vigorous shaking, and 2.5 mL of this reaction mixture was dispensed into test tubes. Following this, 0.5 mL each of various concentrations (0.5~20.0 mg/mL) of the extracts in methanol was added, and the reaction mixture was incubated for up to 2 hr at 50℃. The same procedure was repeated with the positive controls BHT and TOC and a blank. After incubation, the absorbance levels of the mixtures were measured at 490 nm using a spectrophotometer (Optizen POP; Mecasys Co. Ltd., Daejeon, Korea). Absorbance was measured until the β-carotene color disappeared. The β-carotene bleaching rate (R) was calculated according to Eq. (1).
R = ln (a/b)/t (1)
where ln = natural log, a = absorbance at time t (0), and b = absorbance at time t (120 min). The antioxidant activity (AA) was calculated as the percent inhibition relative to the control using Eq. (2).
AA = [(Rcontrol - Rsample)/Rcontrol] × 100 (2)
AAs of the extracts were compared to those of BHT and TOC at 0.5 mg/mL and a blank consisting of 0.5 mL of methanol.
Reducing power
Reducing power was determined according to the method of Gülçin et al. [11]. Each extract (1~8 mg/mL) in methanol (2.5 mL) was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricynide, followed by incubation at 50℃ for 20 min. Then, 2.5 mL of 10% trichloroacetic acid was added, and the mixture was centrifuged at 200 ×g (6K 15; Sigma, Mannchein, Germany) for 10 min. The upper layer (2.5 mL) was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% ferric chloride. Finally, the absorbance was measured at 700 nm against a blank. BHT and TOC were used as positive controls.
Scavenging of DPPH radical
The hydrogen atom content or electron donation ability of the corresponding extracts as well as some pure compounds were measured based on bleaching of the purple-colored DPPH methanol solution [12]. Four mL each of various concentrations (0.125~2.0 mg/mL) of the extracts in methanol was added to 1 mL of DPPH radical solution in methanol (final concentration of DPPH was 0.2 mM). The mixture was shaken vigorously and allowed to stand for 30 min, after which the absorbance of the resulting solution was measured at 517 nm using a spectrophotometer. Inhibition of DPPH free radical as a percent (I%) was calculated as:
I% = [(Acontrol - Asample)/Acontrol] × 100
where Acontrol is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compound. BHT, TOC, and L-ascorbic acid were used as positive controls.
Chelation of ferrous ions
Chelating activity was determined according to the method of Dinis et al. [13]. Briefly, 2 mL each of various concentrations (0.063~1.0 mg/mL) of the extracts in methanol was added to a solution of 2 mM FeCl2 (0.05mL). The reaction was initiated by adding 5mM ferrozine (0.2mL). The total volume was adjusted to 5 mL with methanol, after which the mixture was shaken vigorously and left at room temperature for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm. The percent inhibition of ferrozine-Fe2+ complex formation was calculated using the following formula:
Metal chelating effect (%) = [(Acontrol - Asample)/Acontrol] × 100
where Acontrol is the absorbance of the control (control contained FeCl2 and ferrozine; complex formation molecules) and Asample is the absorbance of the test compound. BHT and TOC were used as positive controls.
Analysis of phenolic compounds
Fifteen standard phenolic compounds, including gallic acid, pyrogallol, homogentisic acid, protocatechuic acid, (+) catechin, chlorogenic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin, and biochanin-A, were purchased from Sigma Aldrich and used for calibration curves. The standard stock solutions (50, 100, 250, and 500 ppm) were prepared in DMSO. Sample compounds were identified based on retention times of authentic standards and were quantified by comparing their peak areas with those of the standard curves.
Sample preparation for the phenolic compound analysis followed Kim et al. [14]. Two grams of dried mushroom powder was mixed with 10 mL of acetonitrile and 2 mL of 0.1 N hydrochloric acid, followed by stirring at 150 rpm for 2 hr at room temperature and filtration through Whatman No. 42 filter paper. The extract was freeze-dried, and the residues were redissolved in 10 mL of 80% aqueous methanol (HPLC grade) and filtered through a 0.45 µm nylon membrane filter (Titan, Rockwood, TN, USA). The filtrate (20 µL) was then loaded onto an Agilent-1100 series liquid chromatography HPLC system (Agilent Technologies, Waldbronn, Germany). Separation was achieved on a 250 nm × 4.6mm i.d., 5 µm, YMC-Pack ODS AM (YMC Co. Ltd., Kyoto, Japan) column. The mobile phase was distilled water with 0.1% glacial acetic acid (solvent A) and acetonitrile with 0.1% glacial acetic acid (solvent B). The gradient was 0min, 92% A; 0~2min, 90% A; 2~27 min, 70% A; 27~50 min, 10% A; 50~51 min, 0% A; 51~60min, 0% A; 60~63min, 92% A. The run time was 60min at a flow rate of 1mL/min. Detection was performed using a diode array detector at a wavelength of 280 nm.
XO inhibition
In vitro XO inhibitory activities of various extracts of P. citrinopileatus fruiting bodies were assayed spectrophotometrically under aerobic conditions using xanthine as the substrate [6]. The assay mixture consisted of 1 mL of extract at different concentrations (0.5~8.0 mg/mL), 2.9 mL of phosphate buffer (pH 7.5), and 0.1 mL of XO enzyme solution (0.1 units/mL in phosphate buffer, pH 7.5), which was prepared immediately before use. After pre-incubation at 25℃ for 15 min, the reaction was initiated by the addition of 2 mL of the substrate solution (150 µM xanthine in the same buffer). The assay mixture was then incubated at 25℃ for 30 min. The reaction was stopped by the addition of 1 mL of 1 N hydrochloric acid, and the absorbance was measured at 290 nm using a spectrophotometer. Different concentrations of the extracts were dissolved in DMSO, and the final concentration of DMSO was 5%, which did not affect the enzyme assay. Proper controls with DMSO were carried out. Allopurinol (0.5~8.0 mg/mL), a known inhibitor of XO, was used as a positive control. One unit of XO is the amount of enzyme required to produce 1 mmol of uric acid/min at 25℃. XO inhibitory activity is expressed as the percent inhibition of XO in the above assay system calculated as
Inhibition (%) = [(A - B) - (C - D)/(A - B)] × 100
where A is the activity of the enzyme without the extraction, B is the control of A without the extraction and enzyme, and C and D are the activities of the extraction with and without XO, respectively.
Tyrosinase inhibition
Tyrosinase inhibition activity was determined using the modified dopachrome method with L-DOPA as the substrate [15]. A 96-well microtiter plate was used to measure absorbance at 475 nm with 700 nm as a reference. Extract fractions were dissolved in 50% DMSO. Each well contained 40 µL of sample with 80 µL of phosphate buffer (0.1M, pH 6.8), 40 µL of tyrosinase (31 units/mL), and 40 µL of L-DOPA (2.5 mM). The mixture was incubated for 10 min at 37℃, and the absorbance was measured at 475 nm using a UVM 340 microplate reader (Asys, Eugendrof, Austria). Each sample was accompanied by a blank containing all of the components except L-DOPA. L-ascorbic acid and kojic acid were used as positive controls. The results were compared with a control consisting of 50% DMSO in place of the sample. The percentage of tyrosinase inhibition was calculated as follows:
[(Acontrol - Asample)/Acontrol] × 100
Statistical analysis
Data were expressed as means ± SDs of three replicate determinations and were analyzed by SPSS ver. 13 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance and Duncan's new multiple-range test were used to determine the differences among the means.
Results and Discussion
Antioxidant activity against β-carotene-linoleic acid
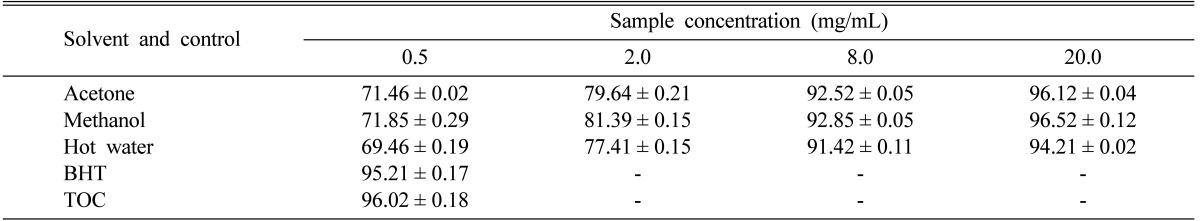
The antioxidant activity of carotenoids is based on the radical adducts of carotenoids with free radicals from linoleic acid. Linoleic acid free radical attacks highly unsaturated β-carotene. The antioxidant activities of the acetone, methanol, and hot water extracts of P. citrinopileatus fruiting bodies against β-carotene-linoleic acid gradually increased with increasing concentration. At 0.5~20.0 mg/mL, the antioxidant activities of the acetone, methanol, and hot water extracts of P. citrinopileatus ranged from 71.46~96.12, 71.85~96.52, and 69.46~91.42%, respectively (Table 1). The results indicate that the antioxidant activities of P. citrinopileatus were lower than those of synthetic antioxidants, BHT and TOC, at 0.5 mg/mL. However, the methanol and acetone extracts showed high antioxidant activities at the concentrations tested, whereas hot water extract showed moderate activity. The presence of carotenoids not only decreased the concentration of free radicals but also reduced Fe3+ to Fe2+. The antioxidative components in mushroom extracts can reduce the extent of β-carotene destruction by neutralizing linoleate free radical and other free radicals formed in the system [16]. Barros et al. [17] reported that the antioxidant activities of various extracts of Leucopaxillus giganteus, Sarcodon imbricatus, and Agaricus arvensis increased with increasing concentration. Their antioxidant activities were 61.4, 54.3, and 46.7% at 5 mg/mL, respectively, whereas the antioxidant activity of tertiary butylhydroquinone standard reached 82.2% at 2 mg/mL. It seems that the antioxidant activity of P. citrinopileatus fruiting bodies was more effective than those mentioned above.
Table 1.
Antioxidant activities of various extracts of Pleurotus citrinopileatus fruiting bodies at various concentrations against β-carotene-linoleic acid
Values expressed as means ± SDs (n = 3).
-, not analyzed; BHT, butylated hydroxytoluene; TOC, α-tocopherol.
Reducing power
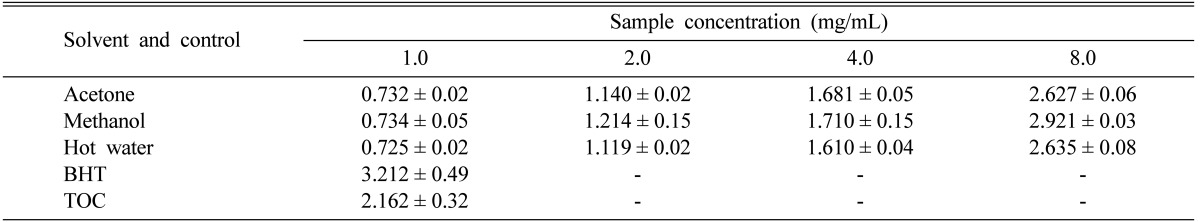
The reducing power of the acetone, methanol, and hot water extracts of P. citrinopileatus fruiting bodies increased readily with increasing concentration. At 8 mg/mL, the strongest reducing power was observed in the methanol extract, with a value of 2.92, whereas the lowest reducing power (2.63) was exhibited by acetone extract. However, the reducing power values of BHT and TOC at 1.0 mg/mL were 3.21 and 2.16, respectively (Table 2).
Table 2.
Reducing power levels of various extracts of Pleurotus citrinopileatus fruiting bodies at various concentrations
Values expressed as means ± SDs (n = 3).
-, not analyzed; BHT, butylated hydroxytoluene; TOC, α-tocopherol.
Regarding the hot water extracts, the reducing power of Hypsizygus marmoreus was 0.99 at 5 mg/mL, whereas Agricus bisporus, Pleurotus eryngii, Pleurotus ferulae, and Pleurotus ostreatus showed reducing power levels of 0.76, 0.75, 0.70, and 0.61, respectively, at 20 mg/mL [18]. Our results indicate that the reducing power of P. citrinopileatus was higher and significantly more effective compared to those of other mushrooms. It was reported that reducing power properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by donating a hydrogen atom and breaking the free radical chain [5, 19].
Scavenging of DPPH radicals
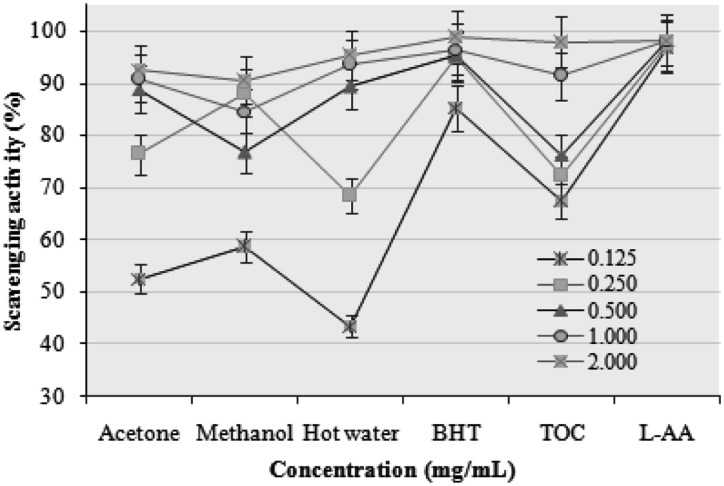
The DPPH radical scavenging activities of the acetone, methanol, and hot water extracts of P. citrinopileatus fruiting bodies increased with increasing concentration. At 0.125~2.0 mg/mL, the DPPH radical scavenging activities of the acetone, methanol, and hot water extracts ranged from 52.56~92.65, 58.65~90.46, and 43.47~95.34%, respectively (Fig. 1). However, at 0.125~2.0 mg/mL, BHT, TOC, and L-ascorbic acid showed excellent scavenging activities of 85.25~98.74, 67.37~97.78, and 96.74~98.23%, respectively.
Fig. 1.
Scavenging activities of various extracts of Pleurotus citrinopileatus fruiting bodies against 1,1-diphenyl-2-picrylhydrazyl. Values expressed as mean ± SDs (n = 3). BHT, butylated hydroxytoluene; TOC, α-tocopherol; L-AA, L-ascorbic acid.
Lee et al. [18] reported that the ethanol extracts of H. marmoreus and A. bisporus fruiting bodies scavenge DPPH radicals at rates between 46.6~68.4% at 5 mg/mL. The scavenging activities of the fruiting bodies, mycelia, and filtrate of the cold and hot water extracts were 20.7~52.3, 37.6~48.3, and 19.6~23.3%, respectively, at 20 mg/mL. It seems that the scavenging activity of P. citrinopileatus fruiting bodies was more effective than those mentioned above. Various extracts might react with free radicals, particularly peroxy radicals, which are the major propagators of the fat autoxidation chain, thereby terminating the chain reaction [20]. The antioxidant capacity of natural antioxidants is due to the termination of the free radical reaction [19]. Furthermore, Herraiz et al. [21] found that the essential amino acid L-tryptophan could react with phenolic aldehydes in food to form phenolic tetrahydro-β-carboline alkaloids, which scavenges 2,2-azinobis (3-ethylbenzothiazoline)-6-sulfonic acid effectively. Therefore, the presence of L-tryptophan in various extracts might account for the scavenging activity of DPPH radicals. However, the higher scavenging activity of the acetone extract might have been due to more hydrogen-donating components contained within the extracts.
Chelation of ferrous ions
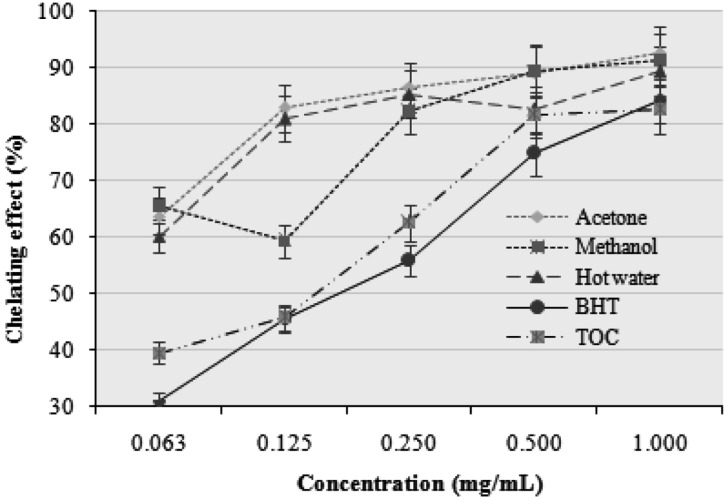
The chelating activities of the acetone, methanol, and hot water extracts at five different concentrations (0.063, 0.125, 0.250, 0.500, and 1.000 mg/mL) of the P. citrinopileatus fruiting bodies toward ferrous ion were investigated. BHT and TOC were used as a ferrous ion standard. As shown in Fig. 2, the chelating capacity of the extracts increased with increasing concentration. The strongest chelating effect (92.72%) was obtained with the acetone extract at 1.0 mg/mL. At this concentration, the lowest chelating effect was exhibited by the hot water extract (89.45%).
Fig. 2.
Chelating effects of various extracts of Pleurotus citrinopileatus fruiting bodies. Values expressed as mean ± SDs (n = 3). BHT, butylated hydroxytoluene; TOC, α-tocopherol.
With regard to hot water extracts at 20 mg/mL, Ganoderma tsugae and Agrocybe cylindracea were observed to chelate ferrous ions at rates of 42.6 and 45.8%, respectively [22, 23]. At 1~5 mg/mL, the chelating ability of H. marmoreus is 75.6~92.6% [18]. It seems that the chelating of ferrous ion by P. citrinopileatus was significantly higher and more effective compared to that of previously mentioned mushroom. Chelating agents may serve as secondary antioxidants, as they reduce redox potential and stabilize the oxidized forms of metal ions. As ferrous ions are the most effective pro-oxidants in the food system [24], the high ferrous ion-chelating ability of the various extracts of P. citrinopileatus fruiting bodies could be beneficial.
Analysis of phenolic compounds
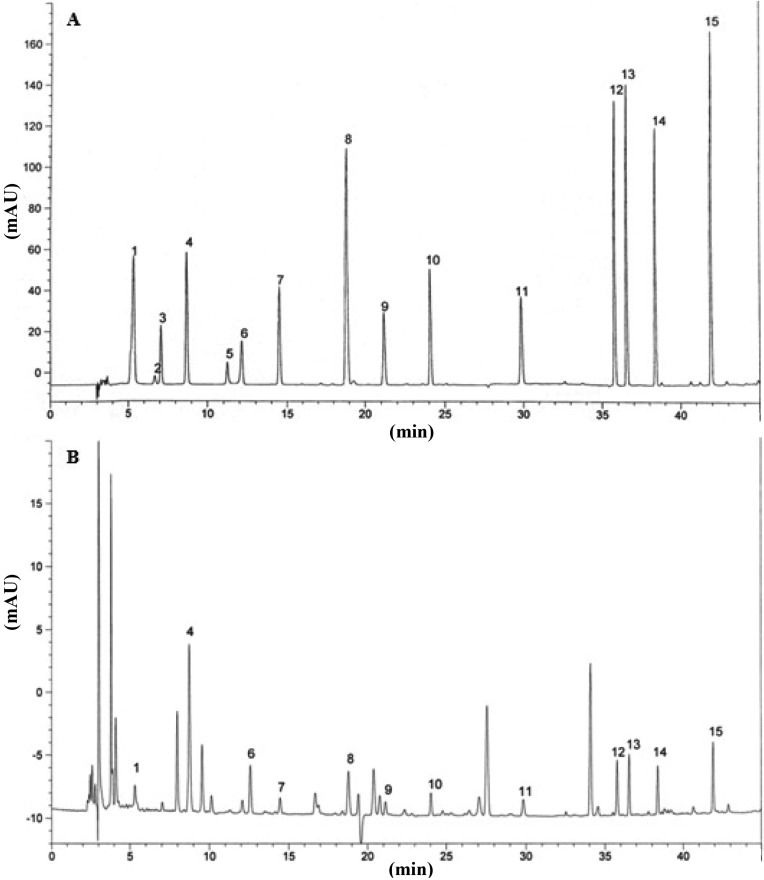
Gallic acid, pyrogallol, homogentisic acid, protocatechuic acid, (+) catechin, chlorogenic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin, and biochanin-A were used as standards to detect phenolic compounds in the P. citrinopileatus extract. Twelve phenolic compounds, gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin, and biochanin-A, were detected in an acetonitrile and hydrochloric acid (5 : 1) solvent extract. The total phenolic compound content was 345 µg/g. The highest and lowest concentrations of phenolic compounds were recorded in protocatechuic acid (85 µg/g) and formononetin and biochanin-A (14 µg/g), respectively (Fig. 3). These findings are comparable to previous studies on edible mushroom [3], in which the total phenolic compound concentration was found to be 174 µg/g. Mushroom species also contain from 3 to 15 phenolic compounds, whereas gallic acid is a commonly reported phenolic compound in mushrooms. Thus, the phenolic compound content could be used as an important indicator of the antioxidant capacity. Several reports have convincingly shown a close relationship between antioxidant activity and phenolic content [25, 26]. Mushroom extracts contain high levels of phenolic compounds, which are composed of one or more aromatic rings bearing one or more hydroxyl groups and can exhibit extensive free radical-scavenging activities as hydrogen donors or electron-donating agents, as well as metal ion-chelating properties. The greater number of hydroxyl groups in phenolics could increase the antioxidant activity [27].
Fig. 3.
High performance liquid chromatography of phenolic compounds. A, Standard mixture of 15 phenolic compounds; B, Pleurotus citrinopileatus extract. 1, gallic acid; 2, pyrogallol; 3, homogentisic acid; 4, protocatechuic acid; 5, (+) catechin; 6, chlorogenic acid; 7, caffeic acid; 8, vanillin; 9, ferulic acid; 10, naringin; 11, resveratrol; 12, naringenin; 13, hesperetin; 14, formononetin; 15, biochanin-A.
XO inhibitory activity
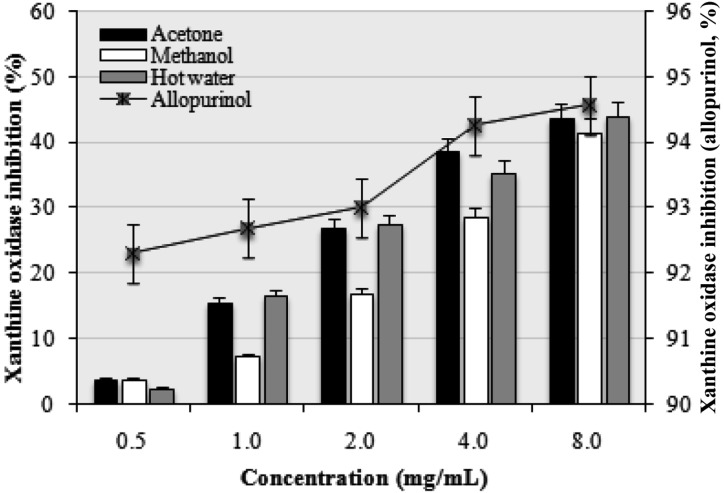
XO inhibitory activities of various extracts of P. citrinopileatus increased with increasing concentration. At 0.5~8.0 mg/mL, the XO inhibitory activities of the acetone, methanol, and hot water extracts were in the range from 3.82~43.86, 3.91~41.63, and 2.52~44.03%, respectively, whereas allopurinol showed excellent XO inhibitory activity from 92.31~94.58% (Fig. 4). However, at higher doses of the extract, XO would be significantly inhibited. Flavonoids are a group of polyphenolic compounds that have been reported to possess XO inhibitory activity [28]. Hence, the presence of phenolic and flavonoid compounds in the extract could have contributed towards XO inhibition.
Fig. 4.
Xanthine oxidase inhibition activity of various extracts of Pleurotus citrinopileatus fruiting bodies. Values expressed as mean ± SDs (n = 3).
Tyrosinase inhibition
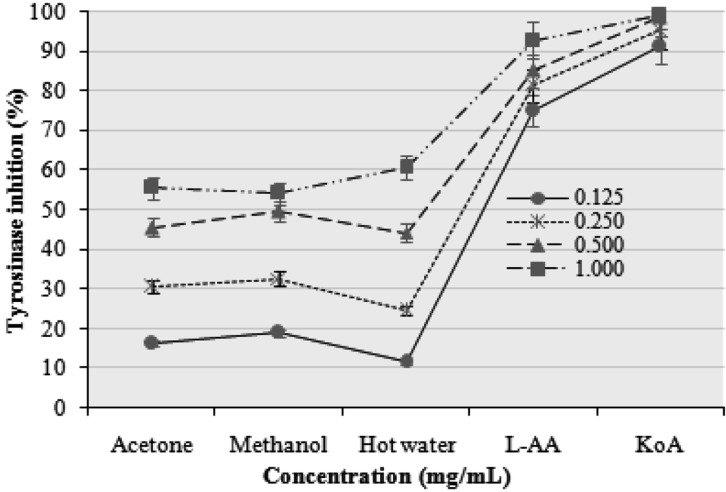
Tyrosinase inhibitory activities of the acetone, methanol, and hot water extracts of the fruiting bodies of P. citrinopileatus increased with increasing concentration. At 0.125~1.0 mg/mL, the tyrosinase inhibitory activities of the acetone, methanol, and hot water extracts ranged from 16.21~55.44, 18.93~54.12, and 11.45~60.68%, respectively (Fig. 5). However, at 0.125~1.0 mg/mL, L-ascorbic acid and kojic acid showed excellent tyrosinase inhibitory activities of 75.12~92.74 and 91.23~99.00%, respectively. The inhibition of tyrosinase might depend on the number of hydroxyl groups in the phenolic compounds from the mushroom extracts that are available to form a hydrogen bond with an enzyme site, leading to lower enzymatic activity. Some tyrosinase inhibitors act through hydroxyl groups that bind to the tyrosinase active site, resulting in steric hindrance or altered conformation [29]. Gallic acid is an effective tyrosinase activity inhibitor [30]. The antioxidant activity mechanism may also be an important reason for the inhibition activity.
Fig. 5.
Tyrosinase inhibition activities of various extracts of Pleurotus citrinopileatus fruiting bodies. Values expressed as mean ± SDs (n = 3). L-AA, L-ascorbic acid; KoA, kojic acid.
Acknowledgements
This work was supported by research grant from the Korea National Research Resource Center Program (2011-0000525) through National Research Foundation of Korea (NRF) for Culture Collection and DNA Bank of Mushroom (CCDBM), University of Incheon and the mutual grant from Rural Development Administration (Agenda 9-27-63; No. 200901OFT092763229).
References
- 1.Zhang J, Wang G, Li H, Zhuang C, Mizuno T, Ito H, Suzuki C, Okamoto H, Li J. Antitumor polysaccharides from a Chinese mushroom, "Yuhuangmo", the fruiting body of Pleurotus citrinopileatus. Biosci Biotechnol Biochem. 1994;58:1195–1201. doi: 10.1271/bbb.58.1195. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh N, Mitra DK, Chakravarty DK. Composition analysis of tropical white oyster mushroom (Pleurotus citrinopileatus) Ann Appl Biol. 1991;118:527–531. [Google Scholar]
- 3.Kim YJ, Kang KS, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 2008;46:2466–2471. doi: 10.1016/j.fct.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Hossain S, Hashimoto M, Choudhury EK, Alam N, Hussain S, Hasan M, Choudhury SK, Mahmud I. Dietary mushroom (Pleurotus ostreatus) ameliorates atherogenic lipid in hypercholesterolaemic rats. Clin Exp Pharmacol Physiol. 2003;30:470–475. doi: 10.1046/j.1440-1681.2003.03857.x. [DOI] [PubMed] [Google Scholar]
- 5.Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Owen PL, Johns T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J Ethnopharmacol. 1999;64:149–160. doi: 10.1016/s0378-8741(98)00119-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhou CX, Kong LD, Ye WC, Cheng CH, Tan RX. Inhibition of xanthine and monoamine oxidases by stillbenoids from Veratrum taliense. Planta Med. 2001;67:158–161. doi: 10.1055/s-2001-11500. [DOI] [PubMed] [Google Scholar]
- 8.Pawelek JM, Körner AM. The biosynthesis of mammalian melanin. Am Sci. 1982;70:136–145. [PubMed] [Google Scholar]
- 9.Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(Suppl 8):170–174. doi: 10.1111/j.0893-5785.2000.130830.x. [DOI] [PubMed] [Google Scholar]
- 10.Dapkevicius A, Venskutonis R, van Beek TA, Linssen JP. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric. 1998;77:140–146. [Google Scholar]
- 11.Gülçin I, Büyükokuroglu ME, Oktay M, Küfrevioglu OI. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J Ethnopharmacol. 2003;86:51–58. doi: 10.1016/s0378-8741(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 12.Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 1997;80:1144–1152. [Google Scholar]
- 13.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 14.Kim EH, Kim SH, Chung JI, Chi HY, Kim JA, Chung IM. Analysis phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur Food Res Technol. 2006;222:201–208. [Google Scholar]
- 15.Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 16.Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001;73:285–290. [Google Scholar]
- 17.Barros L, Ferreira MJ, Queirós B, Ferreira IC, Baptista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103:413–419. [Google Scholar]
- 18.Lee YL, Yen MT, Mau JL. Antioxidant properties of various extracts from Hypsizigus marmoreus. Food Chem. 2007;104:1–9. [Google Scholar]
- 19.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. [Google Scholar]
- 20.Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 21.Herraiz T, Galisteo J, Chamorro C. L-tryptophan reacts with naturally occurring and food-occurring phenolic aldehydes to give phenolic tetrahydro-β-caroline alkaloids: activity as antioxidants and free radical scavengers. J Agric Food Chem. 2003;51:2168–2173. doi: 10.1021/jf0210066. [DOI] [PubMed] [Google Scholar]
- 22.Mau JL, Tsai SY, Tseng YH, Huang SJ. Antioxidant properties of hot water extracts from Ganoderma tsugae Murrill. LWT-Food Sci Technol. 2005;38:589–597. [Google Scholar]
- 23.Tsai SY, Huang SJ, Mau JL. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006;98:670–677. [Google Scholar]
- 24.Yamauchi R, Tatsumi Y, Asano M, Kato K, Ueno Y. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric Biol Chem. 1988;52:849–850. [Google Scholar]
- 25.Duan X, Wu G, Jiang Y. Evaluation of the antioxidant properties of litchi fruit phenolics in relation to pericarp browning prevention. Molecules. 2007;12:759–771. doi: 10.3390/12040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji X, Zhang J, Huang F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus longan Lour.) peel. Food Chem. 2008;106:1264–1270. [Google Scholar]
- 27.Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol. 2007;45:328–336. doi: 10.1016/j.fct.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Costantino L, Albasini A, Rastelli G, Benvenuti S. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Med. 1992;58:342–344. doi: 10.1055/s-2006-961481. [DOI] [PubMed] [Google Scholar]
- 29.Baek HS, Rho HS, Yoo JW, Ahn SM, Lee JY, Lee J, Kim MK, Kim DH, Chang IS. The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. Bull Korean Chem Soc. 2008;29:43–46. [Google Scholar]
- 30.Momtaz S, Mapunya BM, Houghton PJ, Edgerly C, Hussein A, Naidoo S, Lall N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J Ethnopharmacol. 2008;119:507–512. doi: 10.1016/j.jep.2008.06.006. [DOI] [PubMed] [Google Scholar]