Abstract
Nuclear distribution within the extra-radical fungal structures and during spore production in the arbuscular mycorrhizae fungus Glomus intraradices was examined using an in vitro monoxenic culture system. A di-compartmental monoxenic culture system was modified using a nitrocellulose membrane and a coverglass slip for detailed observations. Nuclear distribution was observed using the fluorescent DNA binding probes SYBR Green I and DAPI. Both septate and non-septate mycelial regions were observed, but cytoplasmic contents were only found within non-septate mycelia. Nuclear fluorescent staining revealed that the non-septate hyphal region contained nuclei only with cytoplasm, and that nuclear distribution was limited by septa. Swollen hyphal bodies were often associated with septate and empty-looking hyphae. Cytoplasmic contents filled the swollen hyphal body from the non-septate hyphal region following removal of the septa. As a consequence, the swollen body developed into a new spore. These observations provide understanding about the distribution of AM fungal nuclei within extra-radical mycelia and during spore formation. The results suggest a mechanism by which the development of a cytoplasm-containing mycelium is controlled by the formation or removal of septa to efficiently maintain and proliferate essential contents. This mechanism may provide a survival strategy to the fungus.
Keywords: Arbuscular mycorrhizae fungus spore, Cytoplasm, Glomus, Nuclei, Septum
The arbuscular mycorrhizae (AM) association is the most widespread plant-fungal symbiosis. The ecological and economic importance of this symbiosis has led to a great interest in various aspects of AM fungal biology. AM fungi have existed morphologically unaltered for more than 400 million yr [1]. Their mycelia are usually aseptate and coenocytic; many nuclei coexist within the same cytoplasm [2, 3]. After root colonisation, AM fungus (AMF) develop extra-radical mycelial networks in the soil matrix. The widespread nature of the extra-radical mycelia of AM fungi within the soil substrate plays a role in nutrient uptake and translocation [4]. In addition, their structural development is related to spore production [4, 5]. Intra-cellular organelles such as nuclei and mitochondria can move and translocate within the coenocytic mycelium and, eventually, they may be allocated into new spores, resulting in hundreds of nuclei in an individual spore [6]. Whether all nuclei in a new spore are congregated from the coenocytic mycelium or only a few nuclei (or single nucleus) migrate and undergo nuclear division within the new spore is still unknown. Moreover, whether the nuclei within a single spore are genetically different or identical has not been clearly described. Although morphological observations alone are limited for answering these questions, a detailed description of the process of spore formation and hyphal development regarding nuclear distribution can provide important insight into these questions. Despite this importance, surprisingly little has been studied in this field to date, primarily because of a lack of an appropriate experimental system. Small sized fungal nuclei, thick hyphal (or spore) wall structures and auto-fluorescence from the fungal tissues [7-9] also hamper long-term observations of nuclear behaviour.
Direct observations of the distribution of nuclei using in vitro culture is inefficient, because most hyphal regions are opaque in previous studies [5, 10]. Only a few regions in which the plasma membrane might be particularly permeable reveal nuclei and fluorescing debris that could be deforming nuclei. Bago et al. [10] observed the formation of septa limiting the damaged site and a deformed DNA mass, shortly after damaging a region of hyphae using high laser power. Therefore, septum formation was considered a healing mechanism against ageing or lytic events in this fungus [10]. During development of the branched absorbing structures (BAS), Bago et al. [5] also observed that septa formed progressively from the hyphal apices to the BAS trunk after full development of the BAS [5]. Based on these observations, some authors concluded that a progressive process of cytoplasm retraction occurred after the full development of the BAS, eventually forming the empty-looking, septate BASs. Because this process looked similar to the degeneration process of arbuscules within root cortical cells, it was assumed to be BAS degeneration [5]. In contrast to these BASs, some BASs that were associated with spores or a group of spores were retained longer without forming septa and developed into subtending hyphae [5]. However, the presence of cytoplasm and nuclear distribution in these BASs was not clearly shown in their data.
In this study, nuclear distribution and morphological details of a spore cluster in Glomus intraradices were investigated. Together with fluorescent DNA binding probes and an in vitro monoxenic culture, this study aims to observe: 1) the distribution of AM fungal nuclei within the extra-radical mycelium and 2) the distribution of cytoplasm and nuclei during spore formation. To achieve these aims, an in vitro monoxenic culture system [11] was modified to facilitate observation of the fungal architecture and nuclear distribution within mycelia using a coverslip and a nitrocellulose membrane.
Materials and Methods
In vitro monoxenic culture
A minimal medium (M) described by Bécard and Fortin [11] was used to maintain the in vitro cultures. The composition of the medium in mg/L of distilled water was: MgSO4·7H2O, 731; KNO3, 80; KCl, 65; KH2PO4, 4.8; Ca(NO3)2·4H2O, 288; Na-FeEDTA, 8; KI, 0.75; MnCl2·4H2O, 6; ZnSO4·7H2O, 2.65; H3BO3, 1.5; CuSO4·5H2O, 0.13; Na2MoO4·2H2O, 0.0024; glycine, 3; thiamine, 0.1; pyridoxine, 0.1; nicotinic acid, 0.5; myoinositol, 50 and sucrose, 10,000. The pH of the medium was adjusted to 5.5 before sterilisation at 121℃ for 15 min. Phytogel (Sigma-Aldrich Inc., St. Louis, MO, USA) was used for the gelling material at a concentration of 0.4%. In vitro Ri-T DNA plates transformed with carrot roots and colonised with Glomus intraradices FACE#494 were gifted by Hannes Gamper. The transformed roots have been routinely propagated on M medium by transferring a fragment of young healthy roots into new medium each time. New non-AMF roots were inoculated with a block of gelling material containing colonised roots, hyphae and spores every 5 mon.
Modification of the in vitro root-organ culture system
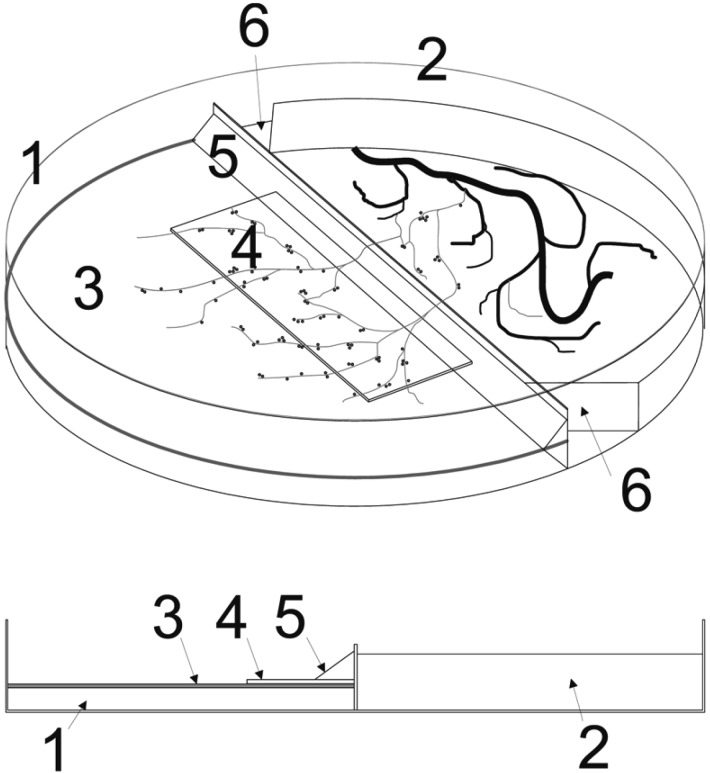
To observe the extra-radical mycelia of the fungus efficiently, modifications were made from the standard di-compartment in vitro system [12]. At first, an autoclaved 22 × 50 mm coverslip (0.13~0.17 mm thick) was added to the top of the solid media of a standard di-compartment in vitro system that had been prepared in advance. To induce the fungal hyphae to grow on top of the coverslip, they were closely set to the middle partition in the fungal compartment and then a slant was made to facilitate the extraradical mycelia of the fungus to grow over the middle partition easily (5 in Fig. 1). Gel medium at both edges of the roots and the fungal side was cut out to prevent host roots from growing over the middle partition of the fungal compartment (6 in Fig. 1). Second, although the first modification made it possible to observe the details of the extra-radical mycelial architecture and spores, fungal growth was not favourable on the glass surface. To obtain more fungal material, a nitrocellulose membrane (hydrophilic, pore size 0.45 µm; Millipore Inc., Billerica, MA, USA) was cut to fit the fungal side of the di-compartment Petri dish. This membrane was sterilised by autoclaving and substituted for the coverslip described above.
Fig. 1.
Modified di-compartmental in vitro organ culture system. 1, side without sucrose for fungi only; 2, side with sucrose for plants and fungi; 3, nitrocellulose membrane (0.45 µm); 4, coverslip; 5, slant; 6, cutting out the edges of solid media on the plant and fungal side. Either a coverslip or nitrocellulose membrane, or both were used for the modifications (see text).
Observations of extra-radical mycelia and spores
Observations were conducted twice per month using 1~8 mon old cultures. The standard in vitro monoxenic culture, in which G. intraradices had been growing, was directly set on a Nikon EFD-3 microscope (Nikon Inc., Tokyo, Japan). To adjust the specimen to the gap between the objective lens (×4 or ×10) and the microscope stage, the fungal side of the di-compartment culture was made with a minimum volume of M medium, and the fungal structures were observed with the lid opened. For the modified in vitro culture with a coverslip, when fungal hyphae and spores were found on the coverslip of the first modified in vitro culture, the extra-radical hyphae and spores were fixed with Helly's fluid solution (K2Cr2O7 2.5 g, HgCl2 5.0 g, Na2SO4 1.0 g dissolved in 100 mL distilled water). The fungus was fixed by adding a few drops of Helly's fluid solution directly onto the coverslip where the fungal hyphae and spores were growing. Samples were washed for 24 hr by filling the fungal side of the di-compartment Petri dish with distilled water. Water was replaced every 8 hr. The fixed samples were stained with hematoxylin following the procedure of Robinow [13]. The stained fungal extra-radical mycelia and spores were collected with the coverslip from the Petri dish. Detailed hyphal networks and spores were observed under a Nikon EFD-3.
Observation of nuclear distribution within extra-radical mycelia. The modified in vitro AMF culture with the nitrocellulose membrane was used to observe the nuclei. The nuclei were fluorescently stained using either SYBR Green I (Molecular Probes, Inc., Eugene, OR, USA) or 4',6-diamidino-2-phenylindole (DAPI, Molecular Probes, Inc.), independently. The membrane on which the fungus had been growing was removed from the culture. For the SYBR Green I stain, the membrane was soaked in 0.25 Tris-Borate-EDTA (TBE) buffer (pH 8.3) for 30 min to stabilize the pH and was transferred to a new Petri dish. Fungal materials were stained by adding 5 µg/mL SYBR Green I. Five µg/mL DAPI solution was added to the fungal mycelia growing on the membrane. After a room temperature incubation in the dark for 30 min, the stained fungal materials were observed under an epi-fluorescence microscope, Nikon EFD-3. A UV-2A filter (excitation wavelength: 330~380 nm) and a B-2A filter (excitation wavelength: 450~490 nm) were used for the DAPI and SYBR Green I staining, respectively.
Results
Development of the extra-radical mycelia and spores. The developmental stages of the extra-radical mycelia of G. intraradices followed the same process as the observations from Bago et al. [4, 10]. Runner hyphae rapidly colonised new regions of the medium in straight lines. While the runner hyphae were developing, they dichotomously branched into sub-ordered runner hyphae, and some subsequently divided shortly. Hyphal thickness decreased as branch order increased. After colonising a new area of the medium, the runner hyphae stopped expanding and differentiated into highly branching structures. The hyphal branches were far more dichotomously divided into much finer mycelium, forming a BAS. Some G. intraradices spores developed from the runner hyphae, but most of the spores formed after extensive development of the runner hyphae and BAS.
A conventional nuclear stain with hematoxylin did not reveal the fungal nuclei but hyphae and spores that contained cytoplasm and those that did not were clearly distinguished by their transparency (Fig. 3). Cytoplasm was only observed in non-septate hyphal areas, and the areas of septate hyphae always looked empty. Spores with cytoplasm were dark and cloudy, whereas the non-cytoplasmic spores appeared empty (Fig. 2). The empty-looking spores were connected to the septate hyphae, whereas cytoplasmic spores were always connected to one of the non-septate, cytoplasmic mycelium (Figs. 2 and 3). Some of the old-looking septate hyphae appeared to shrink (Fig. 3). Septa seemed to block the cytoplasm, which may have been expanding into the new mycelium (Fig. 3). Finely branched structures were observed in a spore cluster, and most had septa at intervals of 20~30 µm (Fig. 3). A swollen hyphal body, which might become a new spore, was found at the apical position of the newly branched hyphae. These hyphae had septa only on the side of a swollen body (Fig. 3).
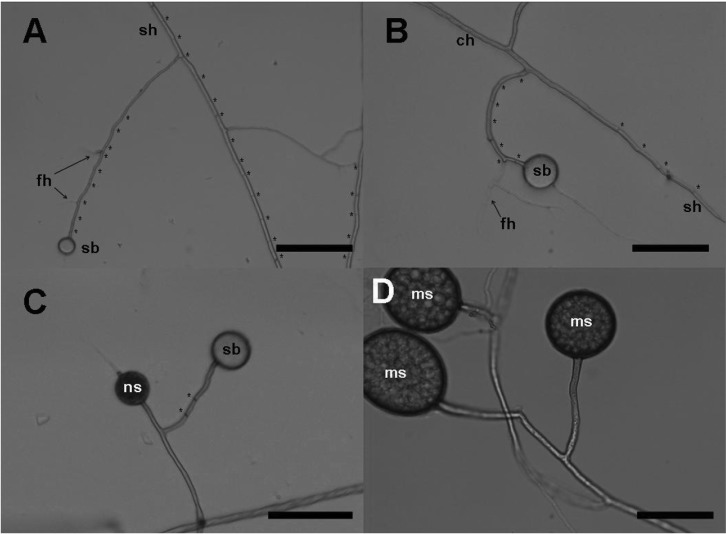
Fig. 3.
Observations of the extra-radical hyphae and spore formation with hematoxylin stain. A, D, Developing spores; B, C, E, F, Different stages of the extra-radical hyphae. Spores (ms) were associated with finely branching hyphae (fh). Cytoplasmic area was distinguished from the non-cytoplasmic area by transparency. A new swollen hyphal apex (ns) formed at a new branch. B, Cytoplasmic contents seemed to migrate from the runner hyphae (rh) into the septate hyphae by removing the septa; C, E, Some of lateral hyphal branches remained septate; C, F, Shrunk-looking structures (ss) were found with the septate hyphae. White arrows indicate the positions where cytoplasm was limited by the septum. Background blots appeared due to the Helly's fluid fixation process (scale bars = 50 µm).
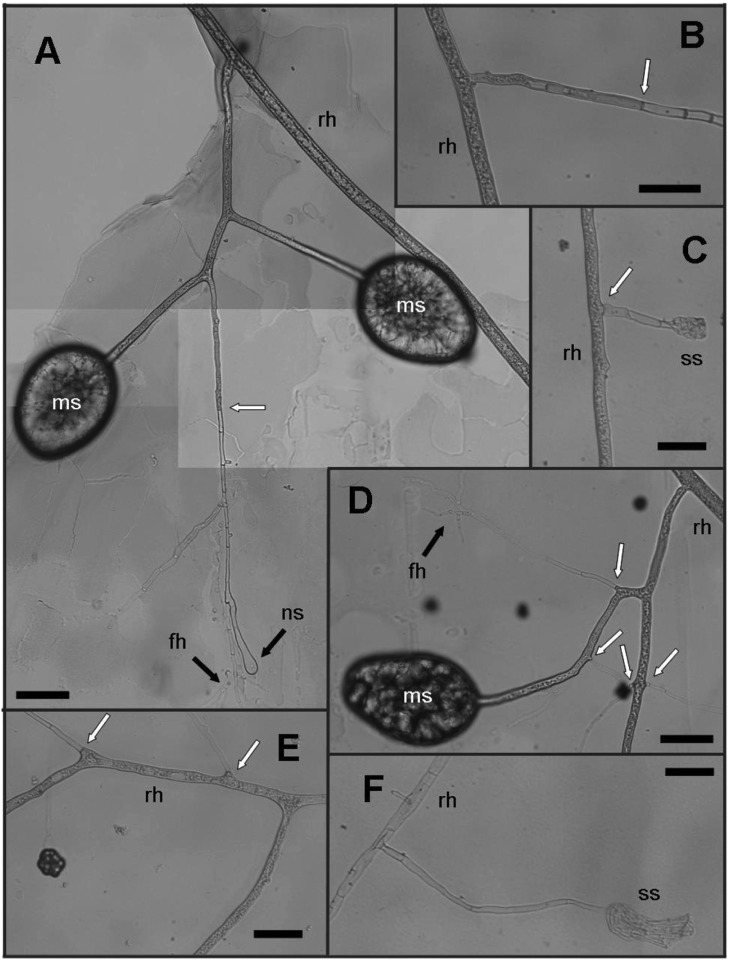
Fig. 2.
Observation of spore differentiation using the monoxenic culture. Newly developing spores (ns) and swollen hyphal bodies (sb) were distinguished by their transparency, as the cytoplasm was dark and cloudy in appearance. Spores containing cytoplasm were always connected to non-septate hyphae, whereas empty-looking spores were connected to septate hyphae. A~C, Images of runner hyphae and the swollen hyphal body developed from the hyphae. The hyphae connected to the empty-looking swollen hyphal bodies were septate. One of the two swollen mycelia was filled with cytoplasm and became a new spore, whereas the other remained as a swollen body in the image of (C). At the end of spore formation, mature spores (ms) were produced in the image of (D). Septa are represented with '*.' 'sh', 'ch,' and 'fh' which represent septate hyphae, cytoplasmic hyphae (non-septate), and finely branching hyphae, respectively (scale bars = 100 µm).
Nuclear fluorescence staining using SYBR Green I and DAPI. SYBR Green I stained G. intraradices nuclei with a bright green fluorescence from the very young spore stage, in relatively thin hyphae, or when matured spores were crushed (Figs. 4 and 5). However, the nuclei from mature intact spores and thick hyphae did not stain well. Cytoplasm was also coloured with irregular dim green, which allowed us to recognise the cytoplasm. An examination of staining continuity showed that SYBR Green I stained brightly for about 5 hr but gradually faded after 7 hr 30 min under the experimental conditions (less than 3 min exposure every 1 hr). Positions of nuclei did not change much during the 7 hr, indicating that SYBR Green I may not be suitable for long-term observations of fungi. Spore outer layers (L1 and L2 in the description in INVAM) and thick hyphae had stronger auto-fluorescence than that of the inner layer (L3) at 488 nm excitation (Fig. 4B).
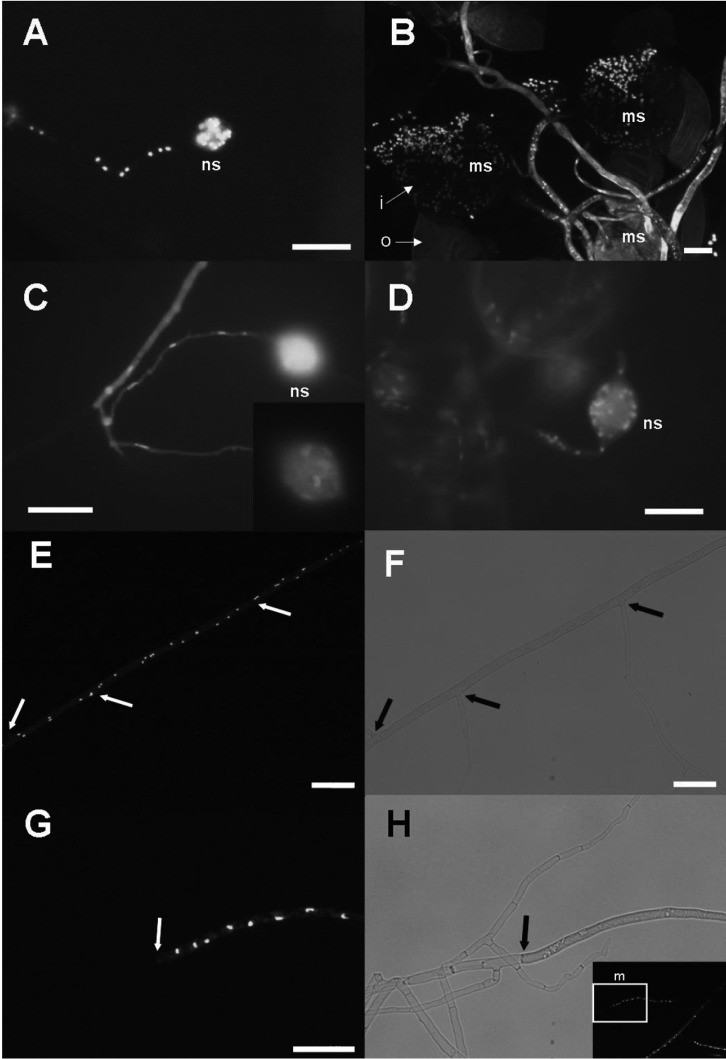
Fig. 4.
Observations of the extra-radical hyphae and nuclear distribution using DAPI (B, C) and SYBR Green I (A, B, E, G). Nuclei fluoresced with blue spots in DAPI staining and bright green spots in SYBR Green I staining. A, Nuclei were observed within a new spore (ns) and within the hyphae connecting to the spore; B, As mature spores (ms) were crushed, many nuclei were detected within the spores as well as the hyphae but auto-fluorescence was strong in the spore coats and thick hyphal walls. 'i' and 'o' indicate inner layer and outer layer of spore wall, respectively; C, Very early stage of spore development; only a few nuclei were observed within the spore (in a small window at top right of the image), and squashed nuclei were found in a row along the fine hyphae; D, Juvenile stage of a developing spore that contains tens of nuclei. A nucleus was found at the neck of the spore, indicating nuclei movement into (or out) the spore; E~H, Runner hyphae; E, G, Dark field images for the fluorescence and (F) and (H) are bright field images corresponding to (E) and (G), respectively. G, H, Magnified images of the white boxed area (m) in (H), showing the border between cytoplasmic and non-cytoplasmic hyphal regions. Cytoplasm and nuclei limited by septa. Border between septate and non-septate mycelia are indicated by a white arrow in bright-field images and by a black arrow in dark-field images. Branching hyphae with a septa did not contain cytoplasmic contents or nuclei (scale bars = 20 µm).
Fig. 5.
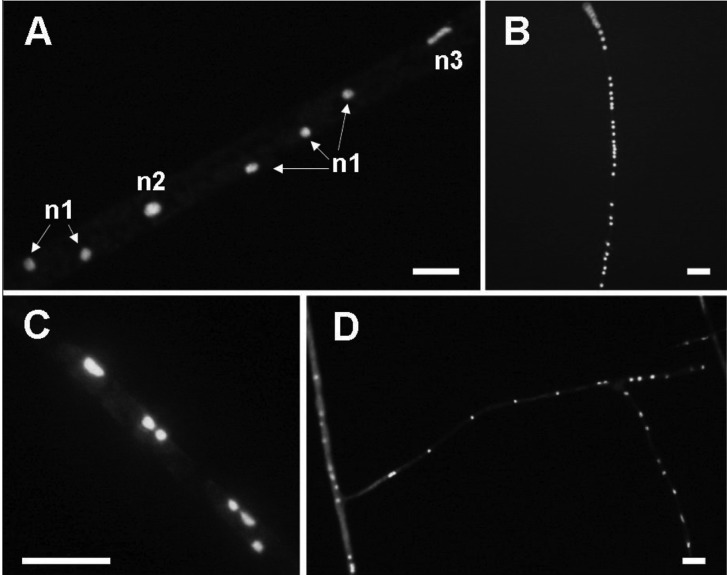
Nuclei within a Glomus intraradices mycelium. A, Magnified image of the centre of (E) in Fig. 4. n1, nuclei of usual size; n2, nuclei about twice the normal size; n3, stretched form of nuclei; B, Nuclei existing densely in a row within a thin mycelium; C, Nuclei existing in pairs within runner hypha; D, Nuclei distributed loosely but in regular intervals within a thin hypha (scale bars = 10 µm).
The G. intraradices nuclei within mature spores and some of the thick runner hyphae rarely fluoresced with DAPI. However, DAPI stained the fungal nuclei within thin hyphae, some of the thick runner hyphae, very early stage spores and juvenile spores, which were smaller in size and had fewer nuclei than that of mature spores. Thus, DAPI performed slightly better than SYBR Green I. Although the auto-fluorescence was stronger, it was difficult to determine whether cytoplasm was present or absent within hyphae compared to the SYBR Green I stain.
A very young spore that had only several nuclei was connected to thin hyphae whose diameter was narrower than that of the nuclei (1.25~1.5 µm, hyphal diameter). The fungal nuclei within the narrow hyphae appeared squashed (Fig. 4C). A nucleus was found in the neck of a juvenile spore in which the nucleus might have been passing through into a new spore (Fig. 4D).
The G. intraradices nuclei within hyphae or from burst spores were round, oval or irregular in shape. Their diameters were 3.23 ± 0.79 µm and 2.88 ± 0.40 µm in hyphae and spores, respectively. Runner hyphae and branching hyphae colonised the membrane surface and most had septa (Fig. 4E~4H). An examination of the border between septate and non-septate mycelia confirmed that non-septate areas only contained cytoplasm, and that fluorescent nuclei always appeared with cytoplasm only in the non-septate regions (Fig. 4E~4H). Consistent with these observations, cytoplasm was blocked by septa. These non-septate hyphae, containing cytoplasm and nuclei, were linked to the cytoplasmic hyphae or thin hyphae that supported spores (Fig. 4A~4D). Nuclei were distributed at regular intervals in some regions, particularly thin hyphae, but were rather irregularly distributed within thick mycelia in most cases (Fig. 5). The density of nuclei also differed among different hyphal regions (Fig. 5B and 5D). Paired nuclei were observed frequently, and some of the nuclei were about twice as large as normal nuclei or stretched forms and seemed to be dividing (Fig. 5A and 5C), suggesting segregation of nuclei within extra-radical hyphae.
Discussion
Improvement in the in vitro AMF monoxenic system to study extra-radical hyphal networks and nuclear behaviour
Although the current in vitro monoxenic system is a useful tool for studies of the extra-radical mycelia of AM fungi [10, 14, 15], modifications to the culture system were necessary to examine more detailed fungal architecture and nuclear behaviour. The fungal nuclei were much more efficiently stained using the nitrocellulose membrane than intact gelling materials. This was because the fluorescent reagents were in direct contact with the fungal tissues. Use of a membrane is a suitable approach for studying extra-radical mycelial networks [16-18] and observing nuclei during anastomosis [16, 19]. Recently, the occurrence of anastomoses between genetically distinct isolates was reported [20]. It would be interesting to trace the nuclear movement after exchanging nuclei through hyphal fusion between the different isolates using the membrane-added in vitro system. Additionally, long-term staining techniques to detect AM fungal nuclei need to be developed. Nuclear fluorescent stains such as SYBR Green I and DAPI are ineffective for long-term observations and they may affect fungal viability, as these fluorescent reagents bind DNA directly. Establishing stable transformants and finding suitable material that can provide better observations of the extra-radical mycelial structure as well as fluorescent nuclei will provide an opportunity to understand the extra-radical hyphal networks and nuclear behaviour in more detail.
Control of cytoplasm and nuclear distribution by septa
A high proportion of the extra-radical hyphae and fine hyphal structures such as BAS were septate and appeared empty. Septa in BAS may have formed from the hyphal apex to the BAS trunk after the BAS developed fully, as shown in Bago et al. [5] but the retraction of cytoplasm and septal formation was not clear in their study. In contrast, septa and cytoplasm blocked by septa were clearly observed in this study. There was an obvious distinction between the cytoplasmic and non-cytoplasmic hyphal regions. Cytoplasm and nuclei were only found within non-septate areas of BAS, whereas septate areas always appeared empty. This septate area in BAS has been explained as a continuous process of cytoplasm retraction by Bago et al. [5] but this suggests a very sophisticated mechanism such as completely pulling back all the cytoplasmic content followed by the formation of a septum. Otherwise, some traces of cytoplasm such as nuclei, chromatin debris or vacuoles would remain in the septate hyphal area, as shown within the old-looking runner hyphae, but none of these were not found within border regions. Furthermore, the swollen hyphal body, which may become a new spore, was always associated with non-cytoplasmic hyphae when spores began to form. It seems that cytoplasm and nuclei progressively migrate into the new forming spore following removal of the septa. Therefore, the previous suggestion that the septate and empty-looking hyphae were attributed to the process of cytoplasm retraction due to ageing and lytic events [5, 10] needs to be reconsidered. Because the distribution of the cytoplasm and nuclei appeared to be restrained by septa, formation or removal of septa may control the expansion or migration of cytoplasmic contents into new hyphal areas. The presence of septa may prevent the diffusion of damage-induced toxic materials into neighbouring hyphal areas. It may also prevent loss of intracellular organelles when hyphae are damaged [21, 22]. If distribution and migration of fungal cytoplasmic contents can be controlled by removing or forming septa, this would provide a safe manner of movement for cytoplasm and nuclei under harsh soil conditions.
Acknowledgement
I thank Peter Young for discussing this topic and Hannes Gamper who showed me fluorescently stained nuclei for the first time and provided me with the SYBR Green I fungal strain and the staining information.
References
- 1.Remy W, Taylor TN, Hass H, Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci U S A. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balestrini R, Bianciotto V, Bonfante-Fasolo P. Nuclear architecture and DNA location in two VAM fungi. Mycorrhiza. 1992;1:105–112. [Google Scholar]
- 3.Bianciotto V, Bonfante P. Quantification of the nuclear DNA content of two arbuscular mycorrhizal fungi. Mycol Res. 1992;96:1071–1076. [Google Scholar]
- 4.Bago B, Azcón-Aguilar C, Piché Y. Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia. 1998;90:52–62. [Google Scholar]
- 5.Bago B, Azcón-Aguilar C, Goulet A, Piché Y. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 1998;139:375–388. [Google Scholar]
- 6.Bécard G, Pfeffer PE. Status of nuclear division in arbuscular mycorrhizal fungi during in vitro development. Protoplasma. 1993;174:62–68. [Google Scholar]
- 7.Vierheilig H, Knoblauch M, Juergensen K, van Bel AJ, Grundler FM, Piché Y. Imaging arbuscular mycorrhizal structures in living roots of Nicotiana tabacum by light, epi-fluorescence, and confocal laser scanning microscopy. Can J Bot. 2001;79:231–237. [Google Scholar]
- 8.Dreyer B, Morte A, Pérez-Gilabert M, Honrubia M. Autofluorescence detection of arbuscular mycorrhizal fungal structures in palm roots: an underestimated experimental method. Mycol Res. 2006;110:887–897. doi: 10.1016/j.mycres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Helber N, Requena N. Expression of the fluorescence markers DsRed and GFP fused to a nuclear localization signal in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2008;177:537–548. doi: 10.1111/j.1469-8137.2007.02257.x. [DOI] [PubMed] [Google Scholar]
- 10.Bago B, Zipfel W, Williams RM, Piché Y. Nuclei of symbiotic arbuscular mycorrhizal fungi as revealed by in vivo two-photon microscopy. Protoplasma. 1999;209:77–89. doi: 10.1007/BF01415703. [DOI] [PubMed] [Google Scholar]
- 11.Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 12.St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol Res. 1996;100:328–332. [Google Scholar]
- 13.Robinow CF. Observations on cell growth, mitosis, and division in the fungus Basidiobolus ranarum. J Cell Biol. 1963;17:123–152. doi: 10.1083/jcb.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bago B, Vierheilig H, Piché Y, Azcón-Aguilar C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture. New Phytol. 1996;133:273–280. doi: 10.1111/j.1469-8137.1996.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 15.Villegas J, Williams RD, Nantais L, Archambault J, Fortin JA. Effects of N source on pH and nutrient exchange of extramatrical mycelium in a mycorrhizal Ri T-DNA transformed root system. Mycorrhiza. 1996;6:247–251. [Google Scholar]
- 16.Giovannetti M, Fortuna P, Citernesi AS, Morini S, Nuti MP. The occurrence of anastomosis formation and nuclear exchange in intact arbuscular mycorrhizal networks. New Phytol. 2001;151:717–724. doi: 10.1046/j.0028-646x.2001.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Giovannetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L. Genetic diversity of isolates of Glomus mosseae from different geographic areas detected by vegetative compatibility testing and biochemical and molecular analysis. Appl Environ Microbiol. 2003;69:616–624. doi: 10.1128/AEM.69.1.616-624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avio L, Pellegrino E, Bonari E, Giovannetti M. Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mecelial networks. New Phytol. 2006;172:347–357. doi: 10.1111/j.1469-8137.2006.01839.x. [DOI] [PubMed] [Google Scholar]
- 19.Giovannetti M, Azzolini D, Citernesi AS. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl Environ Microbiol. 1999;65:5571–5575. doi: 10.1128/aem.65.12.5571-5575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croll D, Giovannetti M, Koch AM, Sbrana C, Ehinger M, Lammers PJ, Sanders IR. Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009;181:924–937. doi: 10.1111/j.1469-8137.2008.02726.x. [DOI] [PubMed] [Google Scholar]
- 21.Trinci AP, Collinge AJ. Occlusion of the septal pores of damaged hyphae of Neurospora crassa by hexagonal crystals. Protoplasma. 1974;80:57–67. doi: 10.1007/BF01666351. [DOI] [PubMed] [Google Scholar]
- 22.Aylmore RC, Wakley GE, Todd NK. Septal sealing in the basidiomycete Coriolus versicolor. J Gen Microbiol. 1984;130:2975–2982. [Google Scholar]