Abstract
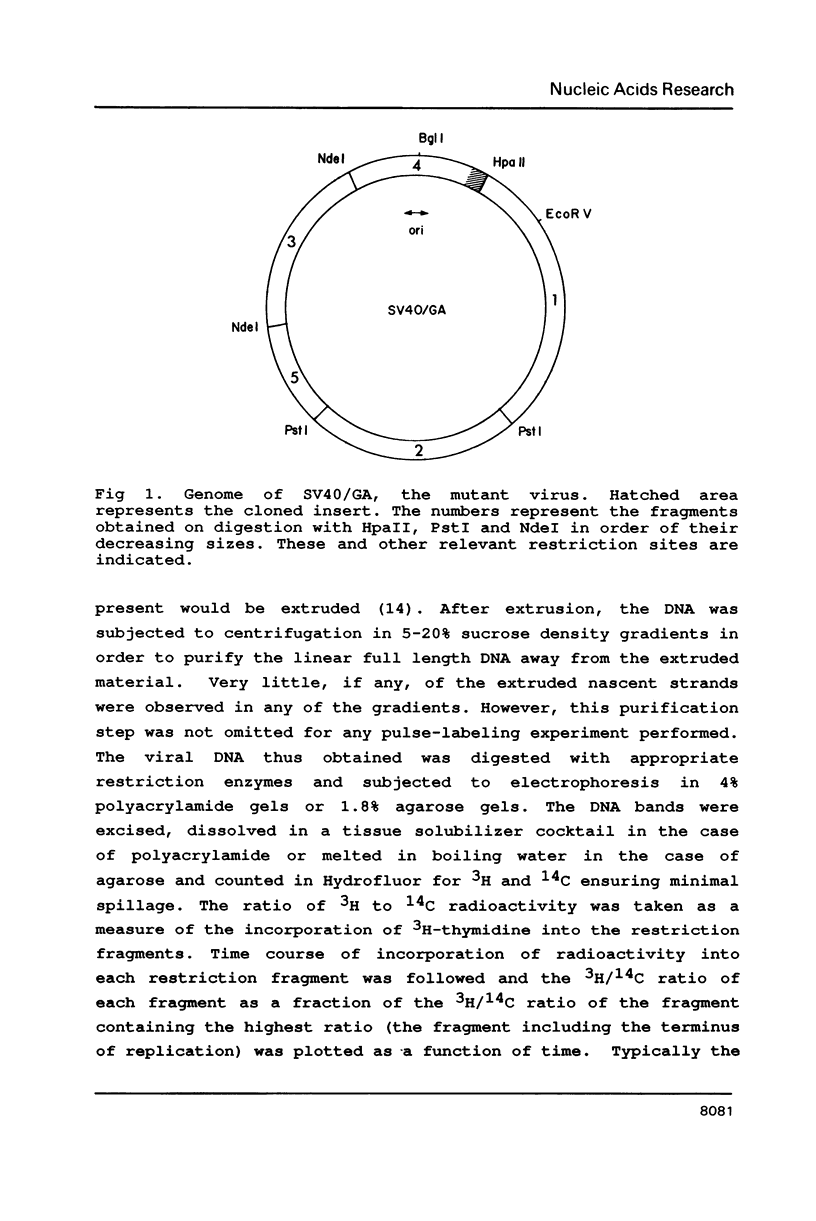
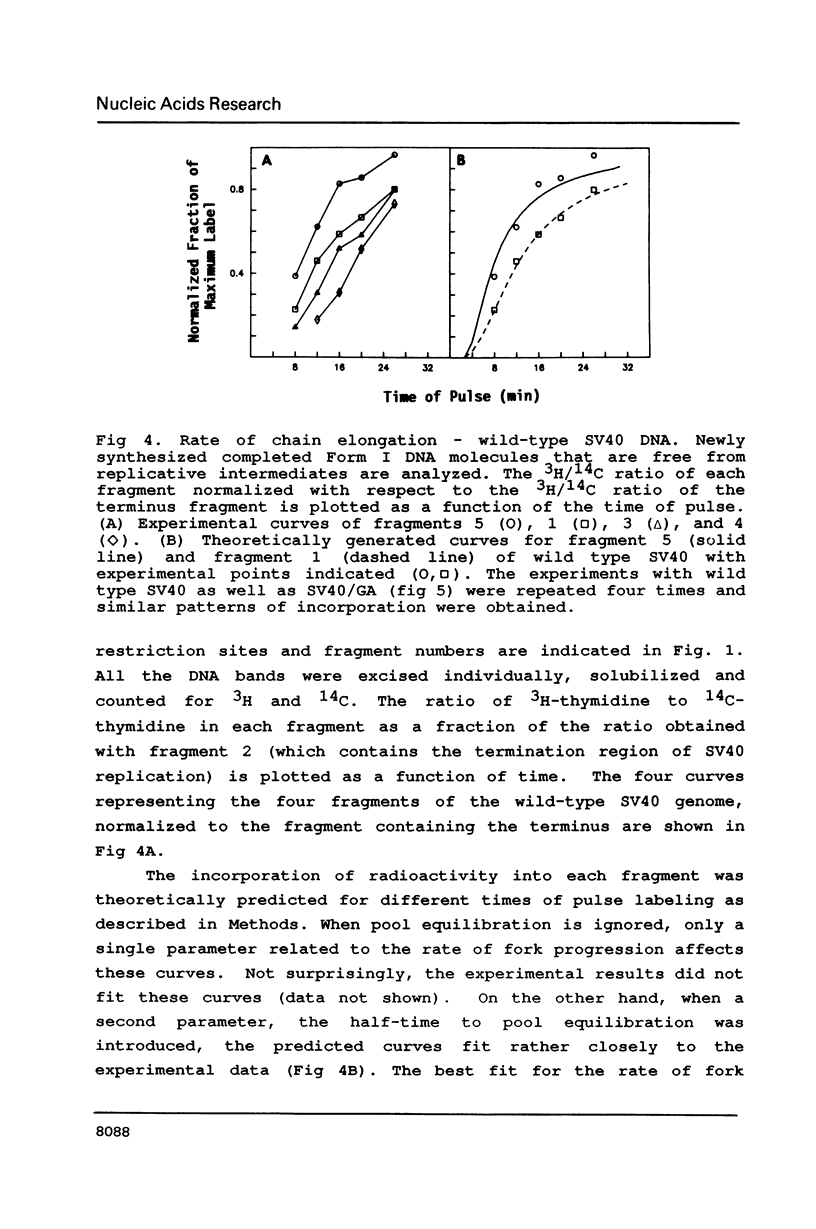
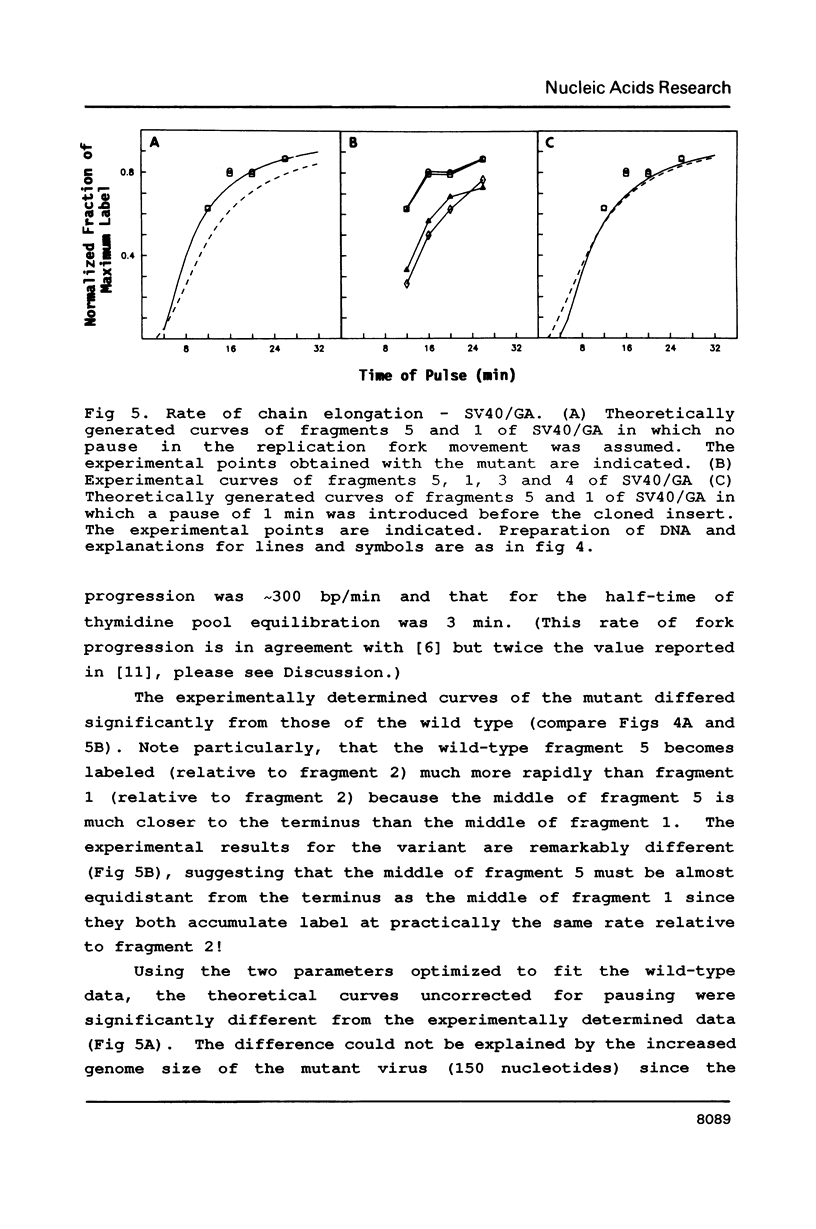
A 200 bp sequence including a stretch of 54 base pairs of alternating guanosine and adenosine nucleotide residues [(dG-dA)27.(dT-dC)27] was cloned in the simian virus 40 (SV40) genome between the KpnI and HpaII sites. This sequence was discovered earlier as part of a region limiting the amplification of sequences adjacent to an integrated polyoma virus in a transformed rat cell line. The newly constructed DNA was transfected into African Green monkey kidney CV1 cells and the variant virus was isolated by plaque-purification. The insertion was stably maintained and the variant virus grew more slowly than the wild type, had lower titers and gave smaller plaques. In mixed infection experiments, the variant was found to be stable, though the wild type replicated more rapidly. Pulse labeling experiments indicated that the unusual inserted sequence acts as a pause site for fork progression during DNA replication, as evidenced by the rate of incorporation of radioactively labeled nucleotides into various regions of the SV40 genome. Statistical fit of the experimental curves with theoretically generated curves suggested the pause of fork progression to be about one minute.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran N., Lapidot A., Manor H. Unusual sequence element found at the end of an amplicon. Mol Cell Biol. 1987 Jul;7(7):2636–2640. doi: 10.1128/mcb.7.7.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran N., Neer A., Manor H. "Onion skin" replication of integrated polyoma virus DNA and flanking sequences in polyoma-transformed rat cells: termination within a specific cellular DNA segment. Proc Natl Acad Sci U S A. 1983 Jan;80(1):105–109. doi: 10.1073/pnas.80.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Gutai M. W., Nathans D. Evolutionary variants of simian virus 40: characterization of cloned complementing variants. Virology. 1975 Jul;66(1):36–52. doi: 10.1016/0042-6822(75)90177-4. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Rao B. S., Martin R. G. Abundance and degree of dispersion of genomic d(GA)n.d(TC)n sequences. J Mol Evol. 1988;27(2):96–101. doi: 10.1007/BF02138367. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. W. Rate of synthesis along replicons of different kinds of mammalian cells. J Mol Biol. 1969 Nov 14;45(3):467–479. doi: 10.1016/0022-2836(69)90306-4. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Haniford D. B., Morgan A. R. A structural basis for S1 nuclease sensitivity of double-stranded DNA. Cell. 1985 Aug;42(1):271–280. doi: 10.1016/s0092-8674(85)80122-7. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., Anderson S., DePamphilis M. L. Maturation of replicating simian virus 40 DNA molecules in isolated nuclei by continued bidirectional replication to the normal termination region. Biochim Biophys Acta. 1979 Nov 22;565(1):84–97. doi: 10.1016/0005-2787(79)90084-4. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]