Abstract
Pepper anthracnose caused by Colletotrichum species is one of the most important limiting factors for pepper production in Korea, its management being strongly dependent on chemicals. The aim of this work was to evaluate the possibilities of using silver nanoparticles instead of commercial fungicides. In this study, we evaluated the effect of silver nanoparticles against pepper anthracnose under different culture conditions. Silver nanoparticles (WA-PR-WB13R) were applied at various concentrations to determine antifungal activities in vitro and in the field. The application of 100 ppm concentration of silver nanoparticles produced maximum inhibition of the growth of fungal hyphae as well as conidial germination in comparison to the control in vitro. In field trials, the inhibition of fungi was significantly high when silver nanoparticles were applied before disease outbreak on the plants. Scanning electron microscopy results indicated that the silver nanoparticles caused a detrimental effect on mycelial growth of Colletotrichum species.
Keywords: Colletotrichum species, Fungicide, Inhibition effect, Silver nanoparticle
Anthracnose disease, which is caused by the fungal pathogen Colletotrichum is a devastating disease that occurs on many commercially important plants like bean, strawberry, perilla and other crop plants [1]. In order to control various phytopathogenic fungi, including Colletotrichum species, agrochemicals have been used for a long time. Widespread use of agrochemicals has certainly decreased the outbreak of fungal diseases, but at the same time has contributed to the development of resistant pathogens [2, 3]. Moreover, such chemicals can be lethal to beneficial microorganisms in the rhizosphere and useful soil insects, and they may also enter the food chain and accumulate in the human body as undesirable chemical residues [4]. With the emergence and increase of microbial organisms resistant to multiple antibiotics, and the continuing emphasis on health-care costs, many researchers have tried to develop new and effective antimicrobial reagents that do not stimulate resistance and are less expensive.
Nanoscale materials have emerged as novel antimicrobial agents owing to their high surface area to volume ratio and the unique chemical and physical properties, which increases their contact with microbes and their ability to permeate cells [5, 6]. Also, nanotechnology has amplified the effectiveness of silver particles as antimicrobial agents. Silver is known to attack a broad range of biological processes in microorganisms including cell membrane structure and functions [7-9]. Silver also inhibits the expression of proteins associated with ATP production [10], although its specific antimicrobial mechanisms are still not completely understood. The use of nano-sized silver particles as antimicrobial agents has become more common as technological advances make their production more economical. One of the potential applications of silver is to manage plant disease. Since silver displays multiple modes of inhibitory action to microorganisms [11], it may be used for controlling various plant pathogens in a relatively safer way compared to synthetic fungicides [12]. Until now, limited research has provided some evidence of the applicability of silver for controlling plant diseases [11]. Reducing the particle size of materials is an efficient and reliable tool for improving their biocompatibility. In fact, nanotechnology helps in overcoming the limitations of size and can change the outlook of the world regarding science [13]. Furthermore, nano-materials can be modified for better efficiency to facilitate their applications in different fields such as bioscience and medicine. This simple size comparison gives an idea of using nanoparticles as very small probes that would allow the elucidation of the cellular machinery without introducing too much interference [14]. Understanding of biological processes on the nanoscale level is a strong driving force behind development of nanotechnology [15].
In this study, we demonstrate the inhibition effects of silver nanoparticles against Colletotrichum species and pepper anthracnose disease in vitro and in field trial conditions, respectively. In vitro studies deal with the effect of silver nanoparticles on Colletotrichum species under different concentrations and growth medium as well as the control mechanism of silver nanoparticles against Colletotrichum in field trials.
Materials and Methods
Silver nanoparticles and fungicides
Silver nanoparticle (WA-PR-WB13R [PR]) was supplied by Bio-Plus Co. Ltd. (Pohang, Korea) at the initial concentration of 1,000 ppm. The colloidal silver nanoparticle solution was diluted to different concentrations of 10 ppm, 30 ppm, 50 ppm, and 100 ppm using distilled water at room temperature (25℃). These nanoparticles have colloidal shapes with an average size of 4~8 nm. All these solutions were stored at 40℃ for further use. Distilled water was used as control and two different fungicides were used as positive controls: NSS-F composed of nano silver and titanium (Dongbangagro Co., Seoul, Korea) and Fenari (Dongbu HiTek, Seoul, Korea).
Fungi and growth media
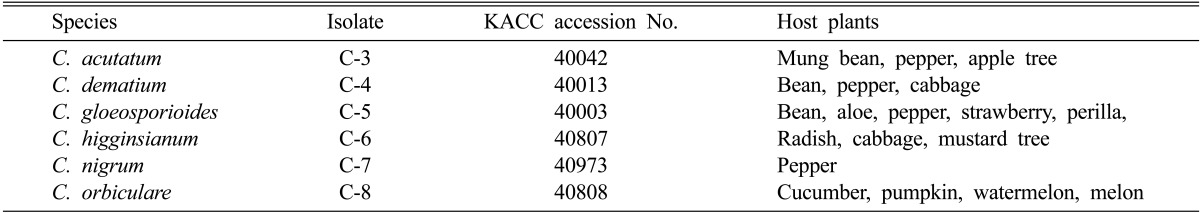
Six Colletotrichum species were obtained from Korean Agricultural Culture Collection (KACC), Suwon, Korea (Table 1). These fungi were grown on potato dextrose agar (PDA) and incubated at 28 ± 2℃. To differentiate the antifungal activities of silver nanoparticles in culture medium, three different agar media were used: PDA, malt extract agar (MEA), and corn meal agar (CMA).
Table 1.
Colletotrichum species and their major host plants used in this study
In vitro assay
In vitro assay was carried out on PDA, MEA, and CMA treated with 10, 30, 50, and 100 ppm of WA-PR-WB13R silver nanoparticles. Five mL of WA-PR-WB13R was poured into the media before plating into each 90 × 15 mm Petri dish. The media containing silver nanoparticles was incubated at room temperature. After 48 hr of incubation, an agar plug of 8 mm diameter containing fungi was inoculated simultaneously at the center of each Petri dish and incubated at 28 ± 2℃. After 2 wk of incubation, inhibition zones were measured. The test was repeated twice and each treatment replicated three times. The inhibition rate (%) was calculated by using the following formula:
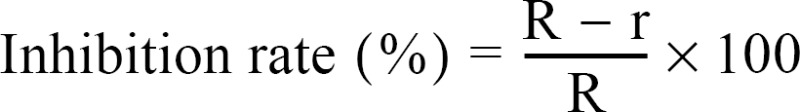
where R is radial growth of fungi in control plate and r is the radial growth of fungi in silver nanoparticle treated plates. Data from the experiments were analyzed by analysis of variance (ANOVA) using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA) and the mean values were compared using the Duncan's multiple range test (DMRT) at p = 0.05.
Field trials and data analysis
To determine the efficacy of silver nanoparticles against pepper anthracnose in the field, an experiment was carried out in Chuncheon, Kangwon-do, Korea before and after pepper was naturally infected. Silver nanoparticles were used at 10, 30, 50, and 100 ppm simultaneously. The silver nanoparticles were applied on the leaves of plants 3~4 wk before the disease outbreak, and after the disease outbreak. The silver nanoparticles were applied every week for four wk. Results were obtained four wk after the final treatment for the before-the-disease-outbreak treatment, and one wk after the final treatment for the after-the-disease-outbreak treatment. The commercial fungicide 'NSS-F,' which contains nano silver and platinum, and the chemical fungicide 'Fenari' were used as positive controls. Distilled water was used as a negative control. Disease incidence (%) was calculated by counting the numbers of infected leaves out of 150 leaves among the treated plants in field trials. Each experiment was repeated three times.
Scanning electron microscopy (SEM) analysis
Approximately five mL solution of four different concentrations, 10 ppm, 30 ppm, 50 ppm, and 100 ppm, of silver nanoparticles WA-PR-WB13R was applied over fully-developed C. gloeosporioides mycelia grown on PDA using a sprayer. The application was done with the time interval of 1, 3, 5, 10, and 15 days and untreated fungal mycelium was cultivated as control. Each treatment was incubated at room temperature. The entire specimens were fixed in 4% glutaraldehyde for 3 hr and treated with 0.1M cacodylate buffer for 1 hr. After washing with distilled water, the specimen was dehydrated in a graded ethanol series up to 100%, critical-point dried, and gold-coated using an ion sputter-coater. The specimen was observed on a SEM (S-3500N; Hitachi Co., Tokyo, Japan) at an accelerating voltage of 10 kV.
Results
Inhibition of Colletotrichum species on various culture media
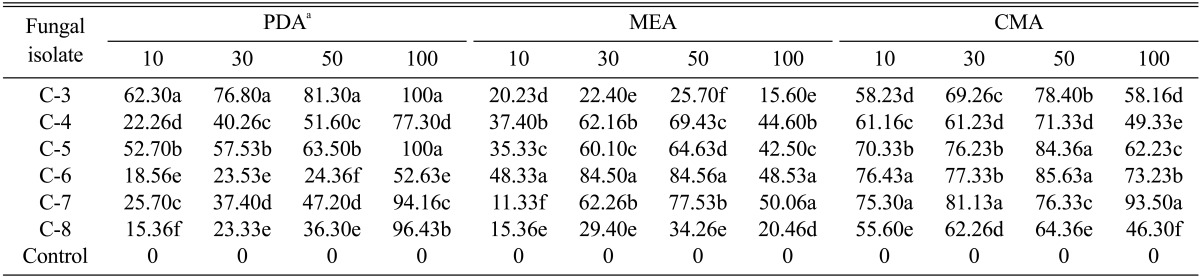
Six Colletotrichum species were selected to analyze the inhibition effect of silver nanoparticles WA-PR-WB13B on PDA, MEA, and CMA. The fungal isolates treated with distilled water were used as controls. Silver nanoparticles showed various levels of inhibition on mycelial growth of Colletotrichum species (Table 2). Significant inhibition in mycelial growth of Colletotrichum supplied with 100 ppm silver nanoparticles on PDA was evident. Complete inhibition was observed on PDA treated with 100 ppm silver nanoparticles against isolates C-3 and C-5. The C-7 and C-8 isolates also showed more than 90% inhibition on PDA treated with 100 ppm silver nanoparticles. The lowest inhibition was observed against isolate C-6 on PDA. In all 10 ppm silver nanoparticles treatments, the inhibition rate was lower as compared to other treatments.
Table 2.
Inhibition rate (%) of silver nanoparticles WA-PR-WB13R against Colletotrichum species on different growth medium and concentrations (ppm)
Data followed by the same letter(s) in the same column are not significantly different from each other according to Duncan multiple range test (DMRT) at p = 0.05.
PDA, potato dextrose agar; MEA, malt extract agar; CMA, corn meal agar.
aInhibition rate (%) was determined based on means of three replicates.
Likewise, the inhibition induced by silver nanoparticles against mycelial growth of Colletotrichum species was also observed on MEA media; however the inhibition rate was not significant as compared to PDA media. The highest inhibition rate was observed against C-6 treated with 50 ppm of silver nanoparticles (84.56%) and the treatment against C-6 on 30 ppm silver nanoparticles also showed significant inhibition on MEA (84.50%). The lowest inhibition rate was observed against C-7 treated with 10 ppm of silver nanoparticles on MEA (11.33%).
However, on CMA media, all the concentrations showed more than 45% inhibition but the results were not as significant as on PDA media. The highest inhibition observed on CMA was against C-7 on 100 ppm concentration of silver nanoparticles (93.50%) and the lowest inhibition was observed against C-8 treated with 100 ppm silver nanoparticles. Therefore, the results showed the inhibition rate varies with the selection of growth medium and concentration of silver nanoparticles. In this experiment, PDA was the most favorable media for the suppression of pathogens in vitro. The results also showed that the inhibition rate differs within the species of Colletotrichum when used in different growth media with various concentrations of silver nanoparticles.
Effect of silver nanoparticles against anthracnose in field trial
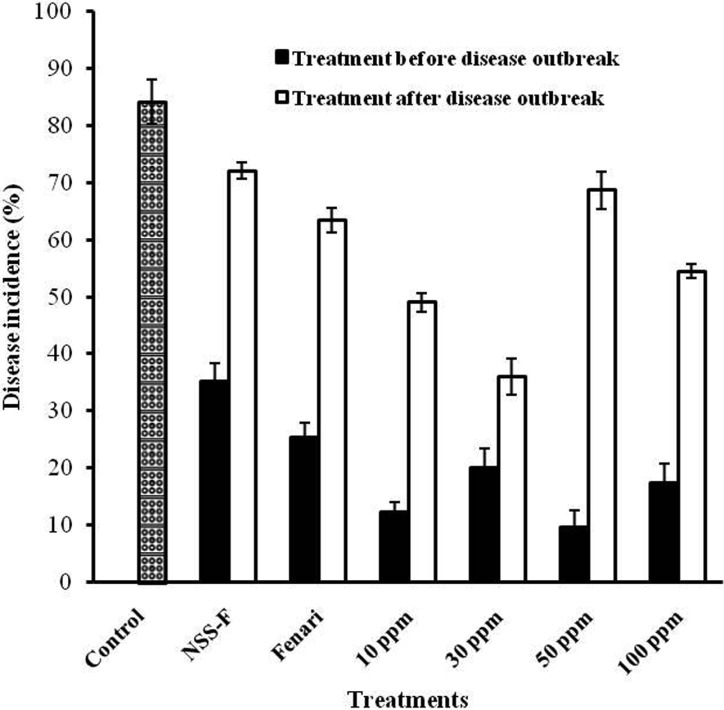
The inhibition effect of silver nanoparticles WA-PR-WB13B was analyzed against pepper anthracnose in pepper field near Chuncheon, Korea (Fig. 1). The average disease incidence was maximum (84.1%) in control plants. The results showed that, in all treatments, higher disease incidence was observed in plants treated after the disease outbreak. Commercial fungicides, NSS-F and Fenari, showed 35.2% and 25.3% disease incidence, respectively, on plants treated before-disease-outbreak. However, in after-disease-outbreak treatments, disease incidence was significantly higher as compared to before-disease-outbreak treatments with NSS-F and Fenari (72.1% and 63.4% respectively). In all treatments, the lowest disease incidence was observed on plants treated with 50 ppm silver nanoparticles before the disease outbreak (9.7%), and the highest disease incidence was observed on plants treated with NSS-F after the disease outbreak (72.1%). The results showed that silver nanoparticles treatment suppressed pathogen attack when applied before the disease outbreak on the plants. Also, the results indicated that the silver nanoparticles WA-PR-WB13B treatments were more effective than commercial fungicides NSS-F and Fenari in field trials.
Fig. 1.
Effect of silver nanoparticles WA-PR-WA13B against pepper anthracnose in the field. Results were obtained one wk after the last treatment for after-the-disease outbreak treatment (treated three times for three wk at one-wk interval) and the other results were obtained 4 wk after the last treatment for before-the-disease outbreak treatment (treated three times for 3 wk at one-wk interval). Commercial fungicides NSS-F and Fenari were used as positive controls. Distilled water was used as a negative control. Data were obtained from triplicate assays and are presented as mean ± SD.
SEM observation of C. gloeosporioides mycelia
Different concentration of silver nanoparticles and its inhibition of mycelial germination of C. gloeosporioides was analyzed via SEM. In order to elucidate the effect of silver nanoparticles on fungal growth, healthy fungal hyphae grown on PDA plates were sprayed with 30, 50, or 100 ppm silver nanoparticle solution, and observed by SEM. This microscopic observation revealed that silver nanoparticles clearly damaged the hyphae (Fig. 2B~2D), while hyphae treated with water (control) appeared to remain intact (Fig. 2A). The damage on the hyphae increased with the increment of concentration and time. Fungal mycelia were sunken and damaged due to the effect of silver nanoparticles (Fig. 2B~2D). The fungal hyphae observed 3 days after the treatment of 100 ppm silver nanoparticles showed deformities in mycelial growth and the shape of hyphal walls. The layers of hyphal walls were also tore off on damaged hyphae at 3 days (Fig. 2D) and many hyphae were collapsed at 5 days. Similar results were also observed on spores of C. gloeosporioides (not shown). Therefore, the results suggested that concentration and treatment time plays a pivotal role in inhibition of pathogen.
Fig. 2.
Scanning electron microscopy of hyphae of Colletotrichum gloeosporioides treated with silver nanoparticles. Fungal hyphae grown on potato dextrose agar plates were sprayed with either water as a control (A) or equal volume of 30, 50, and 100 ppm silver nanoparticle solution (B~D, respectively). Photos were taken three days after the treatment (scale bars = 5 µm).
Discussion
The obtained results of the antifungal activity clearly reveal that the growth of Colletotrichum species including C. gloeosporioides is inhibited at different concentrations of silver nanoparticles. Based on the comparison of results obtained from before and after the disease outbreak treatments, the results indicate that the inhibition of fungus can be achieved when it is applied before disease symptoms occur on plants. The highest antifungal properties were observed in the case of treatment with 50 ppm silver nanoparticles in field trials and 100 ppm silver nanoparticles in vitro. Therefore, the results clearly demonstrate that the silver nanoparticles have the potential to inhibit species of the fungal pathogen Colletotrichum in field conditions as well as in a controlled environment.
Nanometer-sized silvers possess different properties, which might come from morphological, structural, and physiological changes [16]. Indeed, several lines of evidence support the enhanced efficiency of silver nanoparticles on antimicrobial activity. Silver nanoparticles are highly reactive as they generate Ag+ ions while metallic silver is relatively unreactive [5]. It was also shown that the nanoparticles efficiently penetrate into microbial cells, which implies lower concentrations of nano-sized silver would be sufficient for microbial control. This would be efficient, especially for some organisms that are less sensitive to antibiotics due to the poor penetration of some antibiotics into cells [17]. A previous study observed that silver nanoparticles disrupt transport systems including ion efflux [5]. The dysfunction of ion efflux can cause rapid accumulation of silver ions, interrupting cellular processes at their lower concentrations such as metabolism and respiration by reacting with molecules. Also, silver ions are known to produce reactive oxygen species via their reaction with oxygen, which is detrimental to cells, causing damage to proteins, lipids, and nucleic acids [18, 19]. Meanwhile, a previous study suggested that silver nanoparticles can significantly delay mycelial growth of C. gloeosporioides in a dose-dependent manner in vitro [20]. However, in this study we focused on the inhibition effect of silver nanoparticles against six Colletotrichum species on three different growth media in vitro as well as in field trials. Our previous studies also suggested that the application of silver nanoparticles is effective against sclerotium forming fungi, powdery mildews, and Raffaelea sp. [21-24]. Similarly, the present study also suggests that silver nanoparticles are effective for the control of pepper anthracnose pathogen Colletotrichum species including C. gloeosporioides. Thus, silver nanoparticles prepared by a cost effective method have great promise as an antimicrobial agent, and the antifungal activity of silver nanoparticles has a great potential for use in controlling spore-producing fungal plant pathogens. The field study also suggests that, since the efficacy of silver is greatly influenced by application time, preventative applications of silver nanoparticles work better before fungal isolates penetrate and colonize within the plant tissue.
In summary, application of silver nanoparticles may lead to valuable discoveries in various fields such as pathogen control and antimicrobial systems. However, with the advent of silver nanoparticles and its major use as an antimicrobial agent, much experimental trials are needed to understand the toxicity. There are some questions still remaining to be addressed, such as the exact mechanism of interaction of silver nanoparticles with fungal cells and how the surface area of nanoparticles influences killing activity. Also animal models and clinical studies will need to be performed to get a better understanding of the antifungal efficiency of silver nanoparticles.
Acknowledgements
This research was supported by grants from the Ministry of Food, Agriculture, Forestry and Fisheries, and in part, the Agriculture and Life Sciences Research Institute (ALSRI) of Kangwon National University. We would like to thank Bio-Plus Co. Ltd. (Pohang, Korea) for providing silver nanoparticles used in this study.
References
- 1.Perfect SE, Hughes HB, O'Connell RJ, Green JR. Colletotrichum: a model genus for studies on pathology and fungal-plant interactions. Fungal Genet Biol. 1999;27:186–198. doi: 10.1006/fgbi.1999.1143. [DOI] [PubMed] [Google Scholar]
- 2.Beever RE, Laracy EP, Pak HA. Strains of Botrytis cinerea resistant to dicarboximide and benzimidazole fungicides in New Zealand vineyards. Plant Pathol. 1989;38:427–437. [Google Scholar]
- 3.Raposo R, Gomez V, Urrutia T, Melgarejo P. Fitness of Botrytis cinerea associated with dicarboximide resistance. Phytopathology. 2000;90:1246–1249. doi: 10.1094/PHYTO.2000.90.11.1246. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Manag Sci. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- 5.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement JL, Jarrett PS. Antibacterial silver. Met Based Drugs. 1994;1:467–482. doi: 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Kim SH, Kim HJ, Choi SH. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol J. 2006;22:295–302. [Google Scholar]
- 13.Mirkin CA, Taton TA. Semiconductors meet biology. Nature. 2000;405:626–627. doi: 10.1038/35015190. [DOI] [PubMed] [Google Scholar]
- 14.Taton TA. Nanostructures as tailored biological probes. Trends Biotechnol. 2002;20:277–279. doi: 10.1016/s0167-7799(02)01973-x. [DOI] [PubMed] [Google Scholar]
- 15.Whitesides GM. The 'right' size in nanobiotechnology. Nat Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 16.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 17.Samuel U, Guggenbichler JP. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents. 2004;23(Suppl 1):S75–S78. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang BI, Gu MB. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small. 2008;4:746–750. doi: 10.1002/smll.200700954. [DOI] [PubMed] [Google Scholar]
- 19.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 20.Aguilar-Méndez MA, Martín-Martinez ES, Ortega-Arroyo L, Cobián-Portillo G, Sánchez-Espíndola E. Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J Nanopart Res. 2011;13:2525–2532. [Google Scholar]
- 21.Jung JH, Kim SW, Min JS, Kim YJ, Lamsal K, Kim KS, Lee YS. The effect of nano-silver liquid against the white rot of green onion caused by Sclerotium cepivorum. Mycobiology. 2010;38:39–45. doi: 10.4489/MYCO.2010.38.1.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SW, Kim KS, Lamsal K, Kim YJ, Kim SB, Jung M, Sim SJ, Kim HS, Chang SJ, Kim JK, et al. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J Microbiol Biotechnol. 2009;19:760–764. [PubMed] [Google Scholar]
- 23.Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS. Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology. 2011;39:26–32. doi: 10.4489/MYCO.2011.39.1.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min JS, Kim KS, Kim SW, Jung JH, Lamsal K, Kim SB, Jung M, Lee YS. Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol J. 2009;25:376–380. [Google Scholar]