Abstract
The purpose of the present study was to investigate the influence of cultural and environmental parameters affecting the growth and bioactive metabolite production of the rare strain VUK-10 of actinomycete Pseudonocardia, which exhibits a broad spectrum of in vitro antimicrobial activity against bacteria and fungi. Production of bioactive metabolites by the strain was high the in modified yeast extract-malt extract-dextrose (ISP-2) broth, as compared to other tested media. Glucose (1%) and tryptone (0.25%) were found to be the most suitable carbon and nitrogen sources, respectively, for optimum production of growth and bioactive metabolites. Maximum production of bioactive metabolites was found in the culture medium with initial pH 7 incubated with the strain for four days at 30℃, under shaking conditions. This is the first report on the optimization of bioactive metabolites by Pseudonocardia sp. VUK-10.
Keywords: Antimicrobial activity, Bioactive metabolites, Optimization, Pseudonocardia
Most of the antibiotics in use today are derivatives of natural products of actinomycetes and fungi [1]. Antibiotics produced by actinomycetes and other microbes have been evolving for one billion years [2] and their activity has been tested against microbes based on their ability to inhibit target enzymes and macromolecules. Actinomycetes can be isolated from soil and marine environments. Since collecting soil is relatively inexpensive, much is known about the distribution and abundance of terrestrial actinomycetes. Although soils have been screened by the pharmaceutical industry for the past five decades, only a small fraction of actinomycetes have been discovered [2]. Actinomycetes from unexplored habitats have gained considerable attention in recent years for the production of bioactive metabolites. The list of novel actinomycetes and products derived from poorly explored areas of the world stresses the importance of investigating new habitats [3]. Members of marine actinomycetes particularly found in mangrove habitats have been poorly studied [4].
Mangroves are unique woody plant communities of intertidal coasts in tropical and subtropical regions and are highly productive ecosystems [5]. Although surprisingly little is known about the microbial communities present there [6], there is evidence that mangrove sediments contain high populations of novel actinomycetes, as illustrated by the isolation of Asanoa iriomotensis [7] and Nonomuraea maheshkhaliensis [8]. The most promising source of future antibiotics consists of natural microbial products [9]. The requirement for discovery and isolation of novel chemical structures from unique untapped niches is undisputed. This is achieved by investigating new microbial sources for production of antimicrobial agents from non-Streptomyces actinomycetes [10].
In a screening program designed to discover antimicrobial compounds from rare actinomycetes, Pseudonocardia sp. VUK-10 was isolated from soil sediments of Nizampatnam mangrove ecosystem. The bioactive metabolite profile of Pseudonocardia spp. includes antibacterial, antifungal, enzyme inhibitors, neuroprotective and anti-Helicobacter pylori compounds. A new phenazine derivative from Pseudonocardia sp. B6273, i.e., phenazostatin D, acts as a neuroprotective substance [11]. Omura et al. [12] reported antimicrobial activity of azureomycins A and B extracted from P. azurea. Dekker et al. [13] extracted new quinolone compounds from Pseudonocardia sp. with selective and potent anti-Helicobacter pylori activity. The ability of strains to form antimicrobial substances can be significantly influenced by different conditions of nutrition and cultivation. Therefore, the medium constitution together with the metabolic capacity of the producing microorganism greatly affects synthesis of bioactive metabolites. Several environmental factors, such as temperature, pH, and incubation period, play a major role in the production of antimicrobial agents [14]. In this study, we tried to optimize the cultural conditions of the isolate Pseudonocardia sp. and facilitate improved production of biologically active compounds.
Materials and Methods
Pseudonocardia sp. VUK-10 was isolated from soil sediments of "Nizampatnam Mangrove Ecosystem" of south coastal Andhra Pradesh, India, by using the soil dilution plate technique on asparagine-glucose agar medium with pH 7.2 [15]. The pure cultures were maintained on yeast extract-malt extract-dextrose (YMD; ISP-2) agar medium at 4℃. The strain has been deposited in the NCBI GenBank with the accession No. JN087501. The test organisms used in the present study were procured from ATCC, University Boulevard, Manassas, USA and MTCC, IMTECH, Chandigarh, India and preserved at 4℃.
Growth pattern and antimicrobial profile
The growth pattern and antimicrobial profile of Pseudonocardia sp. was studied at regular intervals for up to 7 days. The strain was cultured for one wk and then transferred aseptically into the seed medium (asparagine-glucose broth) and incubated for 48 hr. 10% of the 24-hr-old seed culture was inoculated into the production medium of the same composition. Fermentation was carried out at 28 ± 2℃ for 7 days, under centrifugation at 120 rpm. The culture broth was harvested at 24 hr intervals and biomass was expressed as mg/100 mL. The culture filtrate thus collected was extracted with ethyl acetate and evaporated to dryness in a water bath at 80℃ [16]. The production of bioactive metabolites was assessed by measuring the diameter of the inhibition zone against Gram positive bacteria - Staphylococcus aureus (MTCC 497), Bacillus subtilis (MTCC 3160) and Streptococcus mutans (MTCC 497) and Gram negative bacteria - Escherichia coli (ATCC 35218) and Pseudomonas aeruginosa (ATCC 9027), as well as Candida albicans (ATCC 10231).
Selection of the culture media on biomass and bioactive metabolite production
To select the suitable growth medium, the isolate actynomicete was grown in 11 different culture media, such as starch-casein broth, tyrosine broth (ISP-7), glycerol-asparagine broth, starch inorganic salts broth (ISP-4), yeast-starch broth, malt extract broth, modified YMD broth (ISP-2), tryptone-yeast extract broth (ISP-1), maltose tryptone broth, soya bean meal broth and Czapek-Dox broth [17]. The biomass accumulation and bioactive metabolite production for the media were determined after 4th day of incubation. The medium in which the strain exhibited maximum antibiotic production expressed in terms of zone of inhibition was used as the optimized medium for further study.
Effects of supplementary carbon and nitrogen sources on biomass and bioactive metabolite production
Carbon sources such as maltose, lactose, fructose, galactose, sucrose, glucose, starch, mannitol, arabinose, xylose, glycerol, and raffinose 1% were supplemented separately into the fermentation medium. The effect of varying concentrations (0.5~5%) of the best carbon source on the growth and bioactive metabolite production was also investigated. Similarly, various nitrogen sources, such as sodium nitrate, ammonium sulfate, ammonium oxalate, peptone, tryptone, casein, lysine, asparagine, arginine, glutamine, and tyrosine 0.5% were individually supplemented into the fermentation medium [18]. Further, the impact of different levels (0.1~2%) of optimized nitrogen source was studied to enhance antimicrobial metabolite production [19].
Effects of minerals on biomass and bioactive metabolite production
To evaluate the effects of minerals on the growth and bioactive metabolite production, the optimized medium with superior carbon and nitrogen sources was treated separately with different minerals, such as KH2PO4, MgSO4, FeSO4, K2HPO4, and ZnSO4, each at a concentration of 0.05% (w/v) [20].
Effects of initial pH and incubation temperature on biomass and bioactive metabolite production
The initial pH levels of the growth media were adjusted from 4 to 10, to study the impact of pH. The biomass and bioactive metabolite production were estimated and the optimum pH achieved for maximum bioactive metabolite production was used for subsequent study [21]. Similarly, the optimum temperature for cell growth and bioactive metabolite yield was measured by incubating the production medium at temperatures ranging from 20℃ to 60℃, while maintaining all other conditions at optimum levels [22].
Test organisms
The antimicrobial metabolites of the strain produced under optimized conditions were tested by the agar-diffusion assay against several bacteria, including Streptococcus mutans (MTCC 497), Lactobacillus casei (MTCC 1423), Lactobacillus acidophilus (MTCC 495). Enterococcus faecalis (MTCC 439), Staphylococcus aureus (MTCC 3160), Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 35218), Pseudomonas aeruginosa (ATCC 9027), Proteus vulgaris (MTCC 7299), Shigella flexneri (MTCC 1457) and Xanthomonas campestris (MTCC 2286), and fungi, such as Candida albicans (ATCC 10231), Aspergillus niger, Aspergillus flavus, Fusarium oxysporum (MTCC 3075), and Penicillum citrinum.
Results and Discussion
Growth pattern and antimicrobial profile
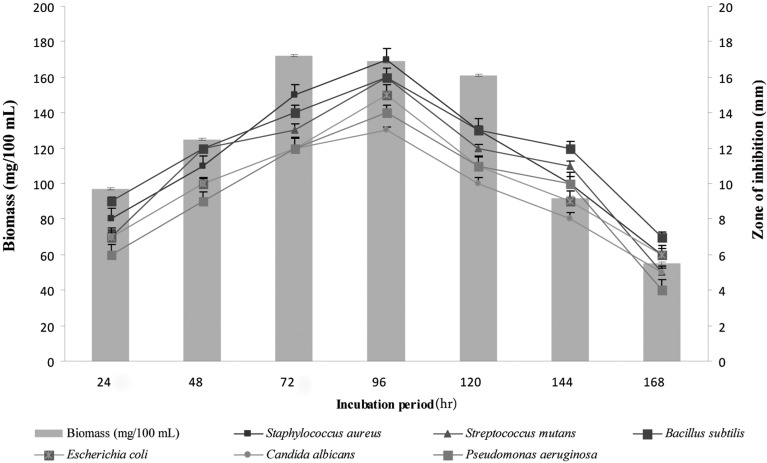
The stationary phase of Pseudonocardia sp. VUK-10 extended from 72 hr to 120 hr of incubation. The bioactive metabolites obtained from 4-day-old culture exhibited high antimicrobial activity against the test microorganisms (Fig. 1). Sujatha et al. [23] reported that metabolites elaborated from 4-day-old culture of Streptomyces psammoticus showed good antimicrobial activity against methicillin resistant Staphylococcus aureus. Similarly, metabolites collected from 4-day-old culture of Nocardia levis MK_VL113 also exhibited good antimicrobial activity [24].
Fig. 1.
Time course of biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
Selection of culture media suitable for biomass and bioactive metabolite production
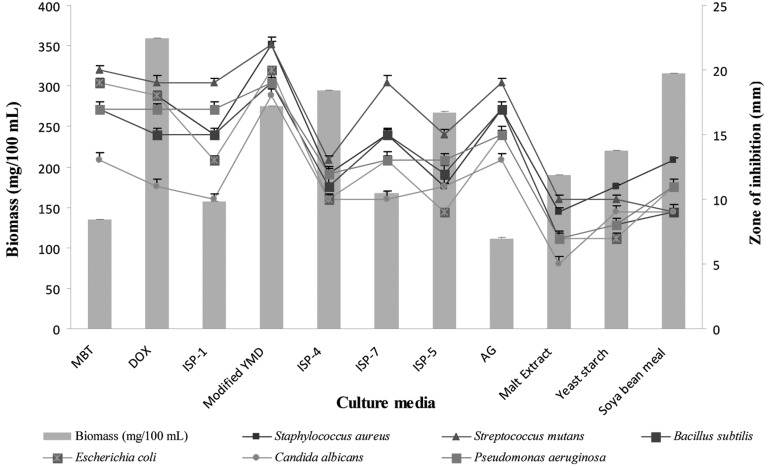
The influence of different media on the production of biomass and bioactive metabolites was recorded (Fig. 2). Among the tested media, modified YMD broth increased bioactive metabolites, followed by maltose-tryptone broth and Czapek-Dox broth, whereas the production of biomass was high in Soya bean meal broth, followed by Starch inorganic salts broth and modified YMD (ISP-2) broth. Oskay [21] showed that the activity of actinomycete isolates could be increased or decreased remarkably under different cultural conditions. Similarly, Thakur et al. [17] reported that Thornton's medium increased biomass and bioactive metabolite production of Streptomyces sp. 201.
Fig. 2.
Effects of different growth media on cell growth and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%. YMD, yeast extract-malt extract-dextrose.
Effects of supplementary carbon and nitrogen sources on biomass and bioactive metabolite production
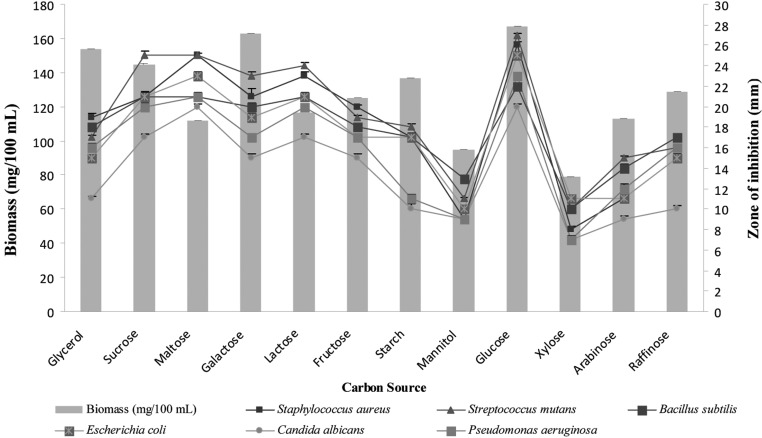
In order to design the effective medium, the roles of different carbon and nitrogen sources were evaluated for their impact on growth and bioactive metabolite production (Figs. 3 and 4). Among the various carbon sources tested, glucose was the best carbon source for both biomass and bioactive metabolite production. This result is comparable with that from a previous study [25], showing that Streptomyces sannanensis strain RJT-1 utilized glucose as the sole carbon source for antibiotic production. Similarly, Cruz et al. [26] reported that the antibiotic production of S. griseocarneus was increased by glucose.
Fig. 3.
Effects of different carbon sources supplemented in modified yeast extract-malt extract-dextrose broth on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
Fig. 4.
Effects of different nitrogen sources supplemented in modified yeast extract-malt extract-dextrose broth on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
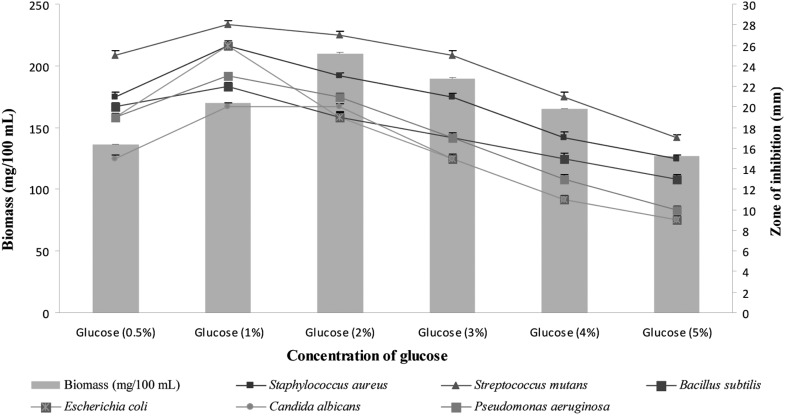
As glucose was the most preferred carbon source for biomass and bioactive metabolite production by the strain, different levels of glucose (0.5~5%) were tested to determine optimal concentration for bioactive metabolite production (Fig. 5). Two percentage glucose supplemented in the medium promoted the highest biomass production, whereas 1% glucose was found to be the best for bioactive metabolite production. Earlier reports also suggested that antibiotic production was observed when glucose at 1% concentration was used as sole source of carbon [27, 28].
Fig. 5.
Effects of different concentrations of glucose supplemented in modified yeast extract-malt extract-dextrose broth on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
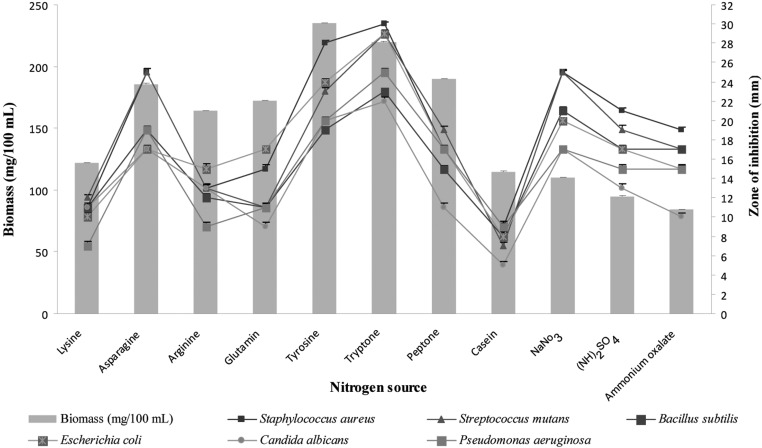
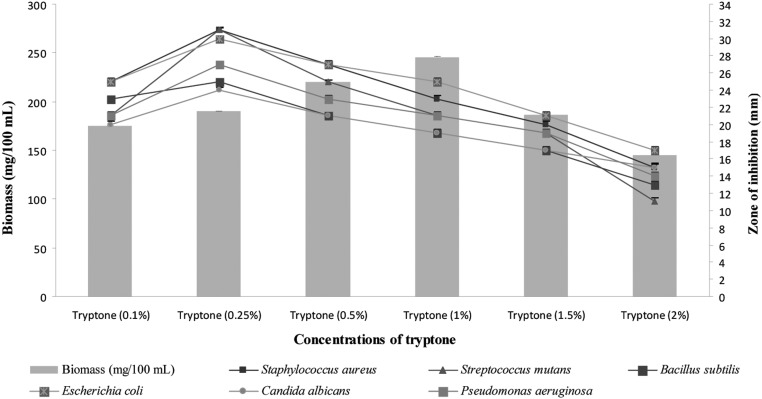
Different nitrogen sources were found to have significant effects on growth and secondary metabolite production by Pseudonocardia sp. VUK-10. Maximum antimicrobial activity was obtained in culture filtrates supplemented with tryptone, followed by tyrosine and asparagine, whereas the biomass production was found to be increased with tyrosine (Fig. 6). Similar results were obtained with Nocardia levis MK_VL113, as reported by Kavitha and Vijayalakshmi [24]. Atta et al. [29] showed that optimal antimicrobial activity was obtained with 0.25% sodium nitrate in the culture medium of Streptomyces albidoflavus 143.
Fig. 6.
Effects of different concentrations of tryptone supplemented in glucose-tryptone broth on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
Effects of minerals on biomass and bioactive metabolite production
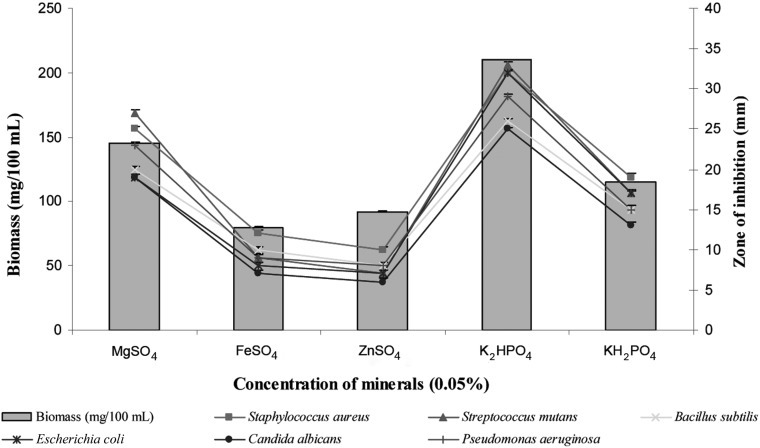
The impact of minerals on biomass and bioactive metabolite production of the investigated strain is represented in Fig. 7. K2HPO4 enhanced the production of cell mass and bioactive metabolites. In contrast, the production of bioactive metabolites was very low with ZnSO4 and FeSO4. Majumdar and Majumdar [30] reported maximum yield of neomycin by Streptomyces fradiae with K2HPO4 and least with ZnSO4. Similar results have been recorded by Ripa et al. [22] and Narayana and Vijayalakshmi [20].
Fig. 7.
Effects of different minerals on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
Effects of initial pH and incubation temperature on biomass and bioactive metabolite production
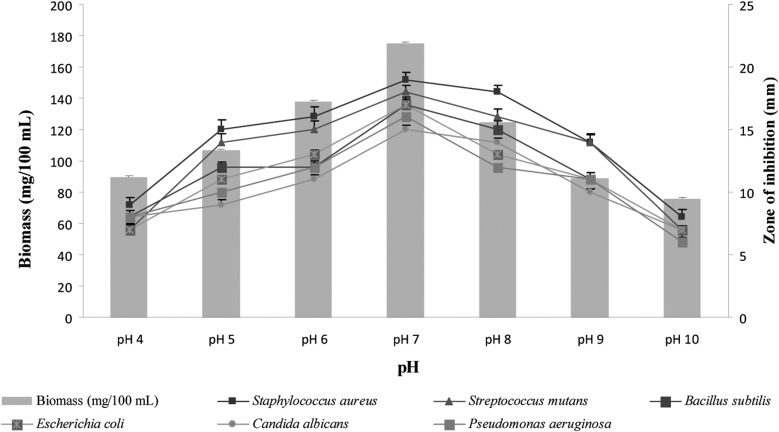
The environmental requirements and cultural conditions for growth and bioactive metabolite production have been studied. Maximum growth, as well as increased antimicrobial metabolites, were obtained at pH 7 (Fig. 8), suggesting the neutrophilic characteristics of the strain. Similar results have been reported for several Streptomyces sp. [29].
Fig. 8.
Effects of pH on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
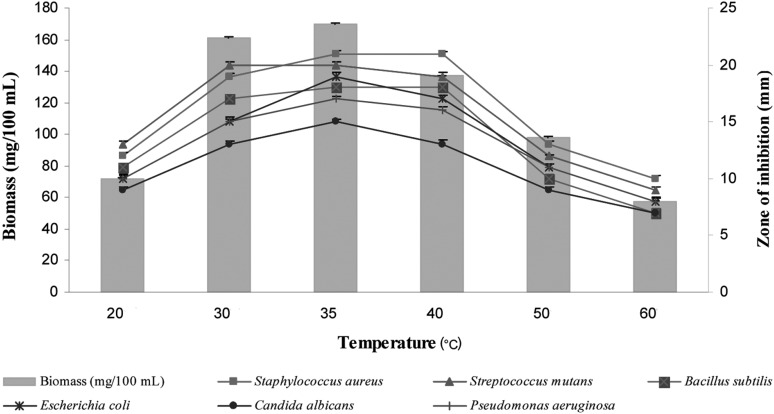
The influence of temperature on the biomass and bioactive metabolite production of the strain is presented in Fig. 9. Highest growth, as well as anti-microbial compound production, was obtained at 35℃. In terms of its optimum temperature for growth, the organism appeared to be mesophilic. These results are in complete accordance with those reported by Narayana and Vijayalakshmi [20].
Fig. 9.
Effects of temperature on biomass and bioactive metabolite production by Pseudonocardia sp. VUK-10. Data were statistically analyzed and found to be significant at 5%.
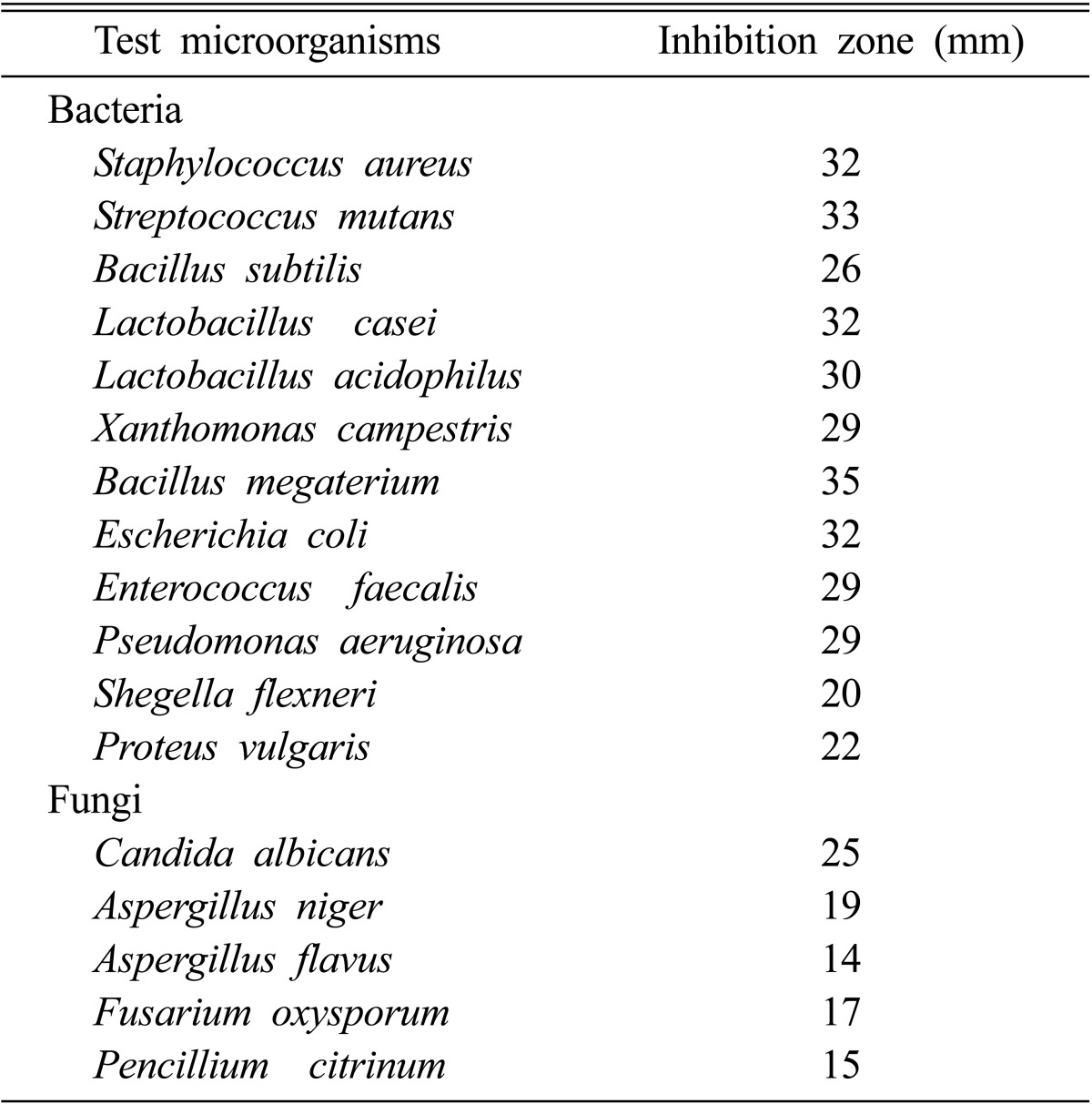
The results obtained in the present investigation revealed that both the growth and bioactive metabolite production by Pseudonocardia sp. VUK-10 were mainly influenced by several environmental and cultural conditions. The optimum conditions for in vitro production of antimicrobial metabolites could be achieved in medium supplemented with glucose at 10 g/L as carbon source, and tryptone at 2.5 g/L as nitrogen source. Four days of incubation at 35℃ and pH 7 were found to be optimum for bioactive metabolite production (Table 1).
Table 1.
Antimicrobial activity of bioactive metabolite produced by Pesudonocardia sp. VUK-10 under optimized conditions against bacteria and fungi
In the present study, optimal conditions for the production of bioactive metabolites by Pseudonocardia sp. VUK-10 were determined, and metabolites showed good antimicrobial activity against Gram positive and Gram negative bacteria, as well as unicellular and filamentous fungi. Hence, further studies on purification, characterization and identification of bioactive metabolites of Pseudonocardia VUK-10 are in progress. This is the first report on the optimization of bioactive metabolites produced by Pseudonocardia sp. VUK-10.
Acknowledgements
The authors are thankful to CSIR (Council of Scientific and Industrial Research) for providing the financial support for the present study.
References
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 2.Baltz RH. Antibiotic discovery from actinomycetes: will a renaissance follow the decline and fall? SIM News. 2005;55:186–196. [Google Scholar]
- 3.Nolan R, Cross T. 1998. Isolation and screening of actinomycete. In: Goodfellow M, Williams ST, Mordarski M, editors. Actinomycetes in biotechnology. London: Academic Press; 1988. pp. 1–32. [Google Scholar]
- 4.Ratna Kala R, Chandrika V. Effect of different media for isolation, growth and maintenance of actinomycetes from mangrove sediments. Indian J Mar Sci. 1993;2:297–299. [Google Scholar]
- 5.Wang BS, Liang SC, Zhang WY, Zan QJ. Mangrove flora of the world. Acta Bot Sin. 2003;45:644–653. [Google Scholar]
- 6.Hong K, Yan B. Uncultured microorganisms in Hainan mangrove soil: diversity and functional genes. In: Liu SJ, Drake HL, editors. Microbes and the environment: perspectives and challenges. Beijing: Science Press; 2008. pp. 52–58. [Google Scholar]
- 7.Han L, Huang XS, Sattler I, Fu HZ, Grabley S, Lin WH. Two new constituents from mangrove Bruguiera gymnorrhiza. J Asian Nat Prod Res. 2007;9:327–331. doi: 10.1080/10286020600727574. [DOI] [PubMed] [Google Scholar]
- 8.Ara I, Kudo T, Matsumoto A, Takahashi Y, Omura S. Nonomuraea maheshkhaliensis sp. nov., a novel actinomycete isolated from mangrove rhizosphere mud. J Gen Appl Microbiol. 2007;53:159–166. doi: 10.2323/jgam.53.159. [DOI] [PubMed] [Google Scholar]
- 9.Thumar JT, Dhulia K, Singh SP. Isolation and partial purification of an antimicrobial agent from halotolerant alkaliphilic Streptomyces aburaviensis strain Kut-8. World J Microbiol Biotechnol. 2010;26:2081–2087. [Google Scholar]
- 10.Ismet A, Vikineswari S, Paramaswari S, Wong WH, Ward A, Seki T, Fiedler HP, Goodfellow M. Production and chemical characterization of antifungal metabolites from Micromonospora sp. M39 isolated from mangrove rhizosphere soil. World J Microbiol Biotechnol. 2004;20:523–528. [Google Scholar]
- 11.Maskey RP, Kock I, Helmke E, Laatsch H. Isolation and structure determination of phenazostatin D, a new phenazine derivative from a marine actinomycete isolate Pseudonocardia sp. B6273. Z Naturforsch B J Chem Sci. 2003;58:692–694. [Google Scholar]
- 12.Omura S, Tanaka H, Tanaka Y, Spiri-nakagawa P, Oiwa R, Takahashi Y, Matsuyama K, Iwai Y. Studies on bacterial cell wall inhibitors. VII. Azureomycins A and B New antibiotics produced by Pseudonocardia azurea nov sp. Taxonomy of the producing organism, Isolation, Characterization and biological properties. J Antibiot (Tokyo) 1997;32:985–994. doi: 10.7164/antibiotics.32.985. [DOI] [PubMed] [Google Scholar]
- 13.Dekker KA, Inagaki T, Gootz TD, Huang LH, Kozima Y, Kohlbrenner WE, Matsunaga Y, McGuirk PR, Nomura E, Sakakibara T, et al. New quinolone compounds from Pseudonocardia sp with selective and potent anti-Helicobacter pylori activity: taxonomy of producing strain, fermentation, isolation, structural elucidation and biological activities. J Antibiot (Tokyo) 1998;51:145–152. doi: 10.7164/antibiotics.51.145. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Bai L, Deng Z, Zhong JJ. Effect of ammonium in medium on ansamitocin P-3 production by Actinosynnema pretiosum. Biotechnol Bioprocess Eng. 2010;15:119–125. [Google Scholar]
- 15.Waksman SA. Classification, identification and descriptions of genera and species. Vol. 2. Baltimore: Williams and Wilkins Co; 1961. [Google Scholar]
- 16.Westley JW, Evans RH, Sello LH, Proupe N, Liu LM, Blound JF. Isolation and characterization of antibiotic X-14547A, a novel monocarboxylic acid ionophore produced by Streptomyces antibiotics NRRC 8167. J Antibiot (Tokyo) 1997;32:100–107. doi: 10.7164/antibiotics.32.100. [DOI] [PubMed] [Google Scholar]
- 17.Thakur D, Bora TC, Bordoloi GN, Maiumdar S. Influence of nutrition and culturing conditions for optimum growth and antimicrobial metabolite production by Streptomyces sp. 201. J Mycol Med. 2009;19:161–167. [Google Scholar]
- 18.Majumdar MK, Majumdar SK. Effect of minerals on neomycin production by Streptomyces fradiae. Appl Microbiol. 1967;13:190–193. doi: 10.1128/am.13.2.190-193.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kathiresan K, Balagurunathan R, Selvam MM. Fungicidal activity of marine actinomycetes against phytopathogenic fungi. Indian J Biotechnol. 2005;4:271–276. [Google Scholar]
- 20.Narayana KJ, Vijayalakshmi M. Optimization of antimicrobial metabolites production by Streptomyces albidoflavus. Res J Pharmacol. 2008;2:4–7. [Google Scholar]
- 21.Oskay M. Effects of some environmental conditions on biomass and antimicrobial metabolite production by Streptomyces sp., KGG32. Int J Agric Biol. 2011;13:317–324. [Google Scholar]
- 22.Ripa FA, Nikkon F, Zaman S, Khondkar P. Optimal conditions for antimicrobial metabolites production from a new Streptomyces sp. RUPA-08PR isolated from Bangladeshi soil. Mycobiology. 2009;37:211–214. doi: 10.4489/MYCO.2009.37.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sujatha P, Bapi Raju KV, Ramana T. Studies on a new marine Streptomycete BT-408 producing polyketide antibiotic SBR-22 effective against methicillin resistant Staphylococcus aureus. Microbiol Res. 2005;160:119–126. doi: 10.1016/j.micres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Kavitha A, Vijayalakshmi M. Cultural parameters affecting the production of bioactive metabolites by Nocardia levis MK-VL-113. J Appl Sci Res. 2009;5:2138–2147. doi: 10.1111/j.1472-765X.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- 25.Vasavada SH, Thumar JT, Singh SP. Secretion of a potent antibiotic by salt-tolerant and alkaliphilic actinomycete Streptomyces sannanensis strain RJT-1. Curr Sci. 2006;91:1393–1397. [Google Scholar]
- 26.Cruz R, Arias ME, Soliveri J. Nutritional requirements for the production of pyra zoloisoquinolinone antibiotics by Streptomyces griseocarneus NCIMB 40447. Appl Microbiol Biotechnol. 1999;53:115–119. doi: 10.1007/s002530051645. [DOI] [PubMed] [Google Scholar]
- 27.Wu JY, Huang JW, Shin HD, Lin WC, Liu YC. Optimization of cultivation conditions for fungichromin production from Streptomyces padanus PMS-702. J Chin Inst Chem Engin. 2008;39:67–73. [Google Scholar]
- 28.Singh LS, Mazumder S, Bora TC. Optimisation of process parameters for growth and bioactive metabolite produced by a salt-tolerant and alkaliphilic actinomycete, Streptomyces tanashiensis strain A2D. J Mycol Med. 2009;19:225–233. [Google Scholar]
- 29.Atta HM, Bahobail AS, El-Sehrawi MH. Studies on isolation, classification and phylogenetic characterization of atifungal sbatanes produced by Streptomyces albidoflavus-143. New York Sci J. 2011:40–53. [Google Scholar]
- 30.Majumdar MK, Majumdar SK. Effect of minerals on neomycin production by Streptomyces fradiae. Appl Environ Microbiol. 1965;13:190–193. doi: 10.1128/am.13.2.190-193.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]