Abstract
The endophytic fungal populations of different tissues of Taxus baccata grown at high altitudes in West Bengal, India were explored. These isolated fungal populations represented different genera, which were screened for taxol production using immunoassay technique. The culture AAT-TS-41 that produced taxol was identified as Gliocladium sp. based on its cultural, morphological characteristics, internal transcribed spacer, and 18S rRNA sequence analysis. Kinetics of taxol production as a function of culture growth were investigated.
Keywords: Endophytes, Internal transcribed spacer region, 18S rRNA
It has been previously shown that endophytic microbial populations can easily adapt their physiology in order to establish themselves in plant host tissues. These physiological changes can result in the production of useful metabolites, which could be exploited for human use. Taxol is one such metabolite that was first isolated from the bark of Western yew, Taxus brevifolia [1], and later from other geographically diverse Taxus species. This anti-microtubule drug is used for the treatment of a broad range of human tumors, including ovarian and metastatic breast cancers [2]. Apart from cancer treatment, its application to the treatment of rheumatoid arthritis, malaria, Alzheimer's disease, and autosomal dominant polycystic kidney disease [2] has also been reported.
Using Taxus sp. for taxol production is ecologically unsuitable, as it requires mature trees to be sacrificed. Over the past few years, other renewable sources for the commercial scale-up of taxol production have been investigated, such as isolation from needles (leaves) [3], in vitro culturing of Taxus species [4], and synthesis from readily available 10-deacetylbaccatin III (10DAB III) [5], but none could meet the high demand for taxol production. A novel method for the production of taxol by a cheaper industrial fermentation method was recently reported based on the discovery of endophytic fungi belonging to different diverse genera that produce taxol. Apart from fungi, some bacteria [6] and actinomycetes [7] that produce taxol have also been discovered. As India has a large wealth of medicinal plants containing an abundance of Taxus baccata, a screening programme was initiated to isolate endophytic microorganisms from T. baccata for production of taxol. Accordingly, this study focused on the screening of endophytic fungi for taxol production and the identification of industrially important fungi based on their cultural, morphological, and molecular characteristics. Our previous studies showed that this fungus produces taxol as well as its precursor 10DAB III [8]. Based on its morphological and molecular characteristics, we identified the fungus as Gliocladium sp. Some of the species of this genus have been identified as mycoparasites, and many novel secondary metabolites have been discovered from different species [9, 10]. However, this is the first report of fungus Gliocladium sp. isolated as an endophyte of T. baccata, which produces taxol.
Materials and Methods
Isolation of endophytic fungi from T. baccata. Endophytic fungi were isolated from the bark, stem, and needles of T. baccata obtained from West Bengal, India. The samples were cut into small pieces (approximately 0.5 × 0.5 cm), surface-sterilized with 0.01% mercuric chloride (HgCl2) solution for 1 min, and washed thoroughly with sterile distilled water [11-13]. The outer bark was teased apart with the help of a sterilized sharp blade in order to obtain the inner bark (stem). Residual water on the sample surface was removed by soaking on sterile blotting paper. Small pieces of stem and needles were placed on the surface of potato dextrose agar (PDA). After 10~15 days, fungi were observed growing from the stem and needle fragments on the plates. Individual hyphal tips of the various fungi were then transferred from the PDA plates, placed again on the new PDA plates, and incubated at room temperature for at least 10~15 days. Each fungal culture was checked for purity and transferred to agar slants by the hyphal tip and single spore isolation methods [13, 14]. Of the fungal population, only slow growing and unusual fungi were considered for further study. Stock cultures were maintained by subculturing at monthly intervals. After growing at pH 7 and 25℃ for 7 days, the slants were maintained at 15℃. From an actively growing stock culture, sub-cultures were made on fresh slants. After 7 days of incubation at pH 7 and 25℃, they were used as the starting material for the fermentation experiments.
Screening of endophytic fungi for taxol production
Production of taxol by the 40 endophytic fungi isolated from different plant parts of T. baccata was studied by a two-stage fermentation procedure. In the first stage, these fungi were grown in submerged culture, whereas in the second stage, they were grown as a stationary culture. These fungi were grown in 500 mL Erlenmeyer flasks containing 100 mL of modified mycological medium [15]. The flasks were inoculated with agar blocks containing mycelium from 7-day-old slants. The inoculated flasks were incubated at 25~27℃ on a rotary shaker (240 rpm) for 5 days. These cultures were then used as seed cultures (first stage). For taxol production, 10 mL seed cultures were transferred to 500 mL flasks containing 100 mL of modified S7 medium [15]. The flasks were incubated at 25~27℃ for 21 days as a stationary culture (second stage). The culture was then harvested and passed through four layers of muslin cloth to separate the mycelial mat from the culture filtrate 3 wk after the inoculations. Both culture filtrate and mycelia were lyophilized to dryness, followed by extraction three times with equal volumes of chloroform: methanol (9 : 1) each time. These extracts were then pooled and dried with anhydrous sodium sulphate and concentrated at 40℃ in vacuo to yield crude extract. A small amount of crude extract was dissolved in methanol, diluted in sample diluting buffer (0.25% bovine serum albumin, phosphate buffered saline), and then used for screening of taxol-positive cultures using immunoassay technique competitive inhibition enzyme linked immunoassay (Hawaii biotechnology group, Hawaii), according to the procedure descried by the manufacturers [16]. It was observed that most of the chloroform extracts were not completely soluble in methanol.
Production of taxol by fungal strain AAT-TS-41 at different time intervals.
The flasks were incubated at 27℃ for different (2, 5, 10, 15, and 21 days) time intervals as a stationary culture (second stage). After 2, 5, 10, 15, and 21 days of incubation, the culture was harvested and processed. Fermentation, extraction, and quantification were carried out as described above.
Cultural and morphological characters of the fungus
To study cultural and morphological characteristics, the fungus was grown on PDA. Cultural characteristics such as color and colony surface texture were determined by visual observation. Other characteristics of the fungus like mycelia, conidiophores, and conidia were studied microscopically (Axiovert 25 Inverted microscope; Carl Ziess, Jena, Germany; Nikon Eclipse E200; Nikon, Tokyo, Japan).
Nutritional studies and factors affecting growth and sporulation of the fungus
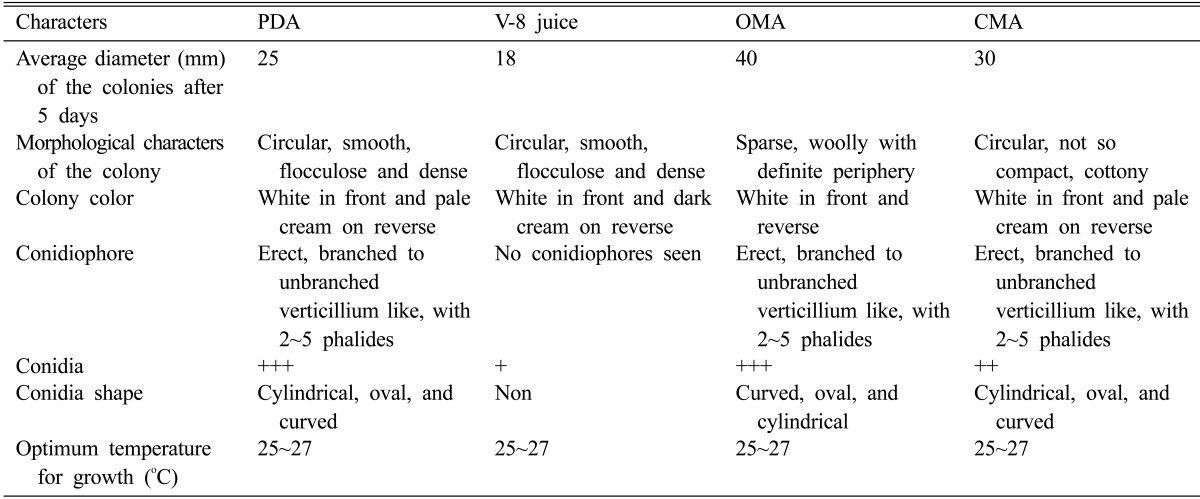
Fungus was grown on the following natural and semisynthetic media: PDA, oat meal agar (OMA), corn meal agar (CMA), and V-8 juice agar prepared as described by the manufacturer (Himedia, Mumbai, India). To study growth of the fungus on solid media, a mycelial disc (8 mm) cut from a sporulating 20-day-old culture grown on PDA was inoculated in the centre of the Petri dish (10 cm diameter) and incubated at 25~27℃ in a B.O.D incubator. Observations of the growth pattern and sporulation were recorded when the culture was 5 days old. Sporulation of the fungus on solid media (PDA, CMA, OMA, and V-8 juice) was determined based on the number of spores present per field under uniform magnification and categorized in the following grades: no sporulation, good, and excellent. Data set comprising the morphological characteristics based on the previously published description is in Table 1. Information regarding shape, temperature, conidiophores, morphological characteristics of the colony, and average diameter of the colony is also listed in Table 1.
Table 1.
Differences in morphological characteristics of fungus AAT-TS-41 growing on different media
+++, excellent; ++, good; +, no sporulation. Excellent, more than 100 spores; Good, more than 50 spores.
PDA, potato dextrose agar; OMA, oat meal agar; CMA, corn meal agar.
Genomic DNA isolation
Genomic DNA of the fungal culture was extracted by the salting out method [17]. For this, 5 g of fungal mycelia was ground into fine powder using liquid nitrogen and suspended in 5 mL of SET buffer (75 mM NaCl, 25 mM EDTA [pH 8.0], 20 mM Tris [pH 7.5]). About 100 µL of lysozyme (final concentration [conc.] 1 mg/mL) was added to the above suspension, which was incubated at 37℃ for 1 hr, followed by addition of 140 µL of proteinase K (final conc. 0.5 mg/mL) and 600 µL of 10% SDS and incubation again for 2 hr at 55℃ with occasional mixing. Sample was precipitated by the addition of 2 mL of 5M NaCl (final conc. 1.25 M), and the mixture was cooled to 37℃. Then, 5 mL of chloroform was added and mixed for about 30 min at room temperature, followed by centrifugation for 20 min at 4,500 ×g. The supernatant was then transferred into a fresh tube, and 0.6 vol of isopropanol was added to precipitate the DNA. The precipitated DNA was pelleted and washed twice with 70% ethanol, air dried, and dissolved in 1~2 mL of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at 55℃. The quality of the DNA was checked on 0.8% agarose gel stained with ethidium bromide.
PCR amplification of internal transcribed spacer (ITS) regions and 18S rRNA
Universal primers were used for amplification of the ITS and 18S rRNA regions from the fungal strain using a Robocycler Gradient 96 (Stratagene, Cedar Greek, TX, USA). PCR reaction was set up in 15 µL volume consisting of 40 ng of fungal DNA (0.4 µL), 1.5 µL of 10× buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, 1.5 mM MgCl2, 0.1% [w/v] gelatin, 0.8% Triton X-100), 4 µL of (0.2 mM) dNTPs (Sigma, St. Louis, MO, USA), 1 µL of (1 µM) each primer set (for ITS [forward] ITS1-5'TCCGTAGGTGAACCTGCGG3'/[reverse] ITS2-5'GCTGCGTTCTTCATCGATGC3', [forward] ITS3-5'GCATCGATGAAGAACGCAGC3'/[reverse] ITS4-5'TCCTCCGCTTATTGATATGC3' [18] and for rRNA [forward] NS1-5'GTAGTCATATGCTTGTCTC3'/ [reverse] NS4-5'CTTCCGTCAATTCCTTTAAG3' [19]), and 0.5 U/µL of Taq polymerase (Bangalore Gene, Bangalore, India). PCR was carried out under the following conditions: for ITS regions, initial denaturation at 95℃ for 3 min, 36 cycles: 95℃ for 30 sec, 52℃ for 30 sec, 72℃ for 30 sec; final extension was at 72℃ for 3 min; for 18S rRNA: initial denaturation at 95℃ for 3 min, 36 cycles: 95℃ for 30 sec, 52℃ for 30 sec, 72℃ for 1.30 min; final extension was at 72℃ for 10 min. The resulting PCR products were analyzed on 1% agarose gel containing ethidium bromide. PCR fragments were eluted from gel with the help of a gel elution kit according to the manufacturer's instructions (Bangalore Gene). The purified PCR products were cloned into pGEMT vector (Promega, Madison, WI, USA) and transformed into DH5α competent cells [20]. Plasmid containing the insert was isolated using the alkali lysis method [20]. The amplified DNA fragments were visualized on 1.2% agarose gel containing ethidium bromide.
DNA sequencing and sequence analysis
The cloned fragments were sequenced using Sanger's dideoxy method [21] using a sequencing kit (ABI Prism Big Dye Terminator Cycle seq, ABS; Applied Biosystems, Foster City, CA, USA). The most identical sequences of the strain AAT-TS-41 were identified from the NCBI database of Genbank using the BLAST algorithm [22]. The nucleotide sequences showing high sequence similarity were manually picked for further analysis. Multiple sequence alignment was performed with nucleotide sequences of other closely related species obtained from the NCBI-GenBank database using the ClustalW algorithm [23]. Phylogenetic analysis was carried out using PHYLIP ver. 3.62 suite programs [24]. Since the sequences showing maximum similarity in BLAST search were selected, DNAPARS program was used for further analysis. The bootstrap values for the parsimony tree were obtained by analysis of 100 replicates with input order jumbled 10 times using the SeqBoot program. A consensus tree was constructed from the output generated using the CONSENSUS program. DNAML, a program based on maximum-likelihood method, was also used for comparison. Both methods were used to analyze all three sequences (two ITS region sequences and one 18S rRNA sequence) of the fungus AAT-TS-41. The data generated was converted into trees using DRAWGRAM.
Results
Endophytic fungi from T. baccata
Out of 40 endophytic fungal cultures screened for taxol production, one culture assigned as AAT-TS-41 (an endophyte isolated from the stem) was confirmed to produce taxol. None of the other fungal cultures produced considerable concentrations of taxol.
Cultural and morphological characters of the fungus
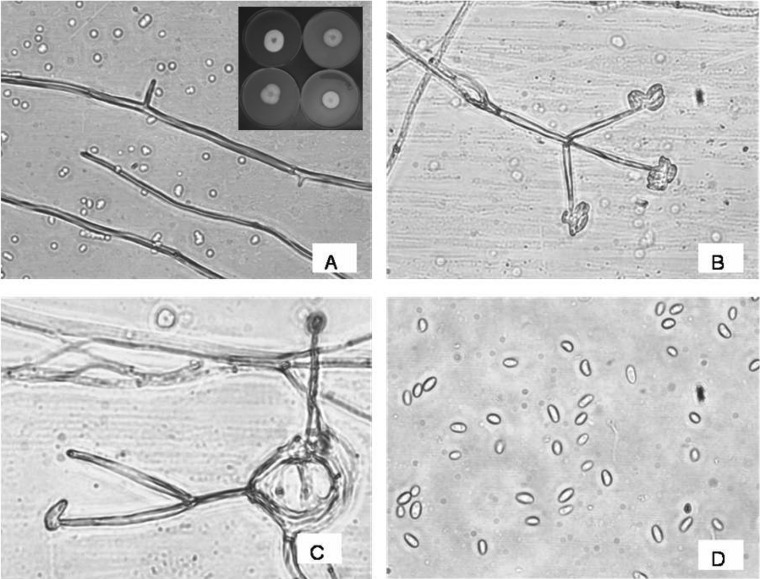
The fungus AAT-TS-41 isolated and grown on PDA medium produced slow-growing colonies (Fig. 1A). Colonies on PDA were white, flocculose, circular, compact, reverse cream, margin smooth, hyphae branched septate, smooth, and hyaline [25]. Non-stromatic conidiophores produced from superficial hyphae were erect, branched to unbranched, septate, and hyaline. Phialides solitary or produced in a group of 2~5 were straight, smooth, verticillate, and hyaline. Conidia produced singly or in a small moist clusters apically were smooth, oval to cylindrical to curved, hyaline, single-celled, and variable in shape and size [26]. Based on cultural and morphological characteristics, the strain was identified as Gliocladium sp.
Fig. 1.
Microscopic observations of morphology of fungus AAT-TS-41. A, Mycelium; B, Conidiophores bearing conidia; C, Arrangement of conidia on phialide tip; and D, Conidia. The inset in A showing the picture of Gliocladium sp. growing on potato dextrose agar, oat meal agar, corn meal agar, and V-8 juice (clockwise).
Effects of various nutrient media on growth and sporulation
The fungus showed variation in growth rate when grown on various nutrient media. The results are presented in Table 1. Among the four different media tested, it was found that OMA media was the best for growth of the fungus, followed by CMA, PDA, and V-8 juice media. Excellent sporulation was recorded on PDA and OMA media. Further, good sporulation was observed in CMA medium and no sporulation was recorded in V-8 juice medium.
PCR amplification and sequence analysis
The method employed for genomic DNA isolation resulted in high quality DNA. The two different ITS regions (0.2 and 0.3 kb) and 18S rRNA regions (1.1 kb) were amplified using universal primers. BLAST analysis showed the majority of the hits from a group of fungi belonging to Ascomycetes, which included Gliocladium sp., Bionectria sp., Nectria sp., and Clonostachys sp. All three nucleotide sequences (ITS 1 & 2, ITS 3 & 4, and NS1 & NS4) showed similarity in the range of 95~98%. The nucleotide sequence analysis determined that the 0.2 kb fragment was a partial ITS region at the 5' end and partial 5.8S rRNA gene at the 3' end, the 0.3 kb fragment was a partial 5.8S rRNA gene at the 5' end and partial ITS region at the 3' end, and the 1.2 kb fragment was a 18S rRNA gene at the 5' end and partial 28S gene at the 3' end. All of the sequences were aligned with ClustalW, and the relatedness between the sequences was determined. All sequences were submitted in NCBI gene bank and are retrievable with accession numbers EU528675, EU581866, and EU581865.
Phylogenetic analysis
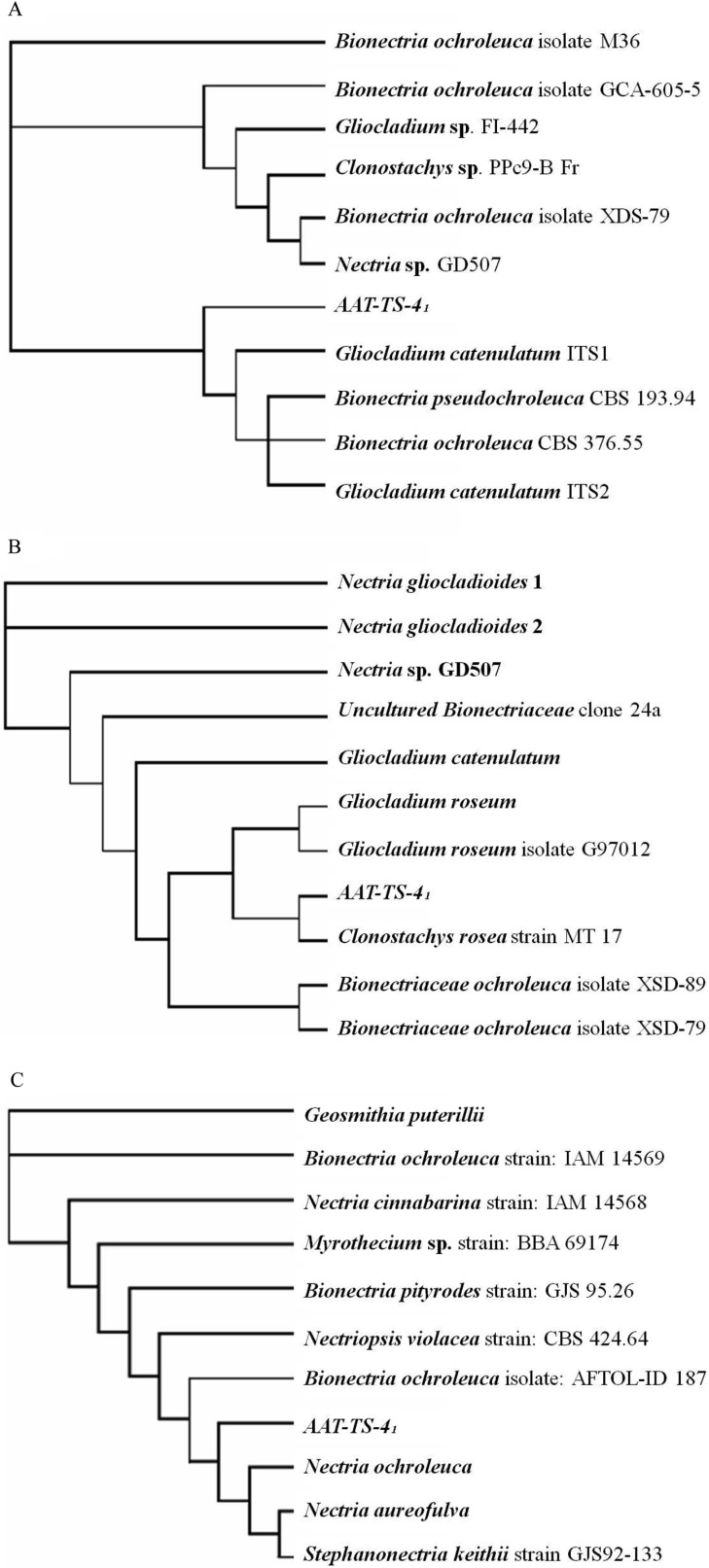
Fig. 2A and 2B shows the results of the phylogenetic analysis of the ITS 1 & 2 and ITS 3 & 4 sequences of the ITS region. The overall grouping of the fungi was the same for both methods (DNAPARS and DNAML); however, the precise ordering of the fungi was different. Sequence comparison with other closely related species confirmed that most of the sequences from the database showed higher than 95~98% sequence identity, but only Gliocladium sp. and Clonostachys sp. were grouped together with the ITS region sequence of AAT-TS-41. To be more specific, Gliocladium sp. showed an outstanding relatedness with AAT-TS-41 and shared a common clade. Further, all Nectria sp. (teleomorph) sequences were grouped together with Gliocladium and AAT-TS-41 sequences (Fig. 2B). In the case of 18S rRNA sequence, AAT-TS-41 was always grouped with Nectria sp. in both methods of analysis (Fig. 2C), which was a teleomorph of Gliocladium sp. Even though fungi belonging to genera Myrothecium, Geosmithia, and Stephanonectria showed higher than 95% sequence similarity, none of them showed relatedness with AAT-TS-41 in phylogenetic analysis.
Fig. 2.
Phylogenetic relationships of selected members with fungi AAT-TS-41. The trees were generated by parsimony algorithm (DNAPARS). A, B, The rooted tree showing relatedness of the AAT-TS-41 ITS region sequences with the genera Gliocladium and Clonostachys; C, Phylogenetic tree showing relatedness of 18S rRNA gene with genera Nectria and Bionectria.
Production of taxol
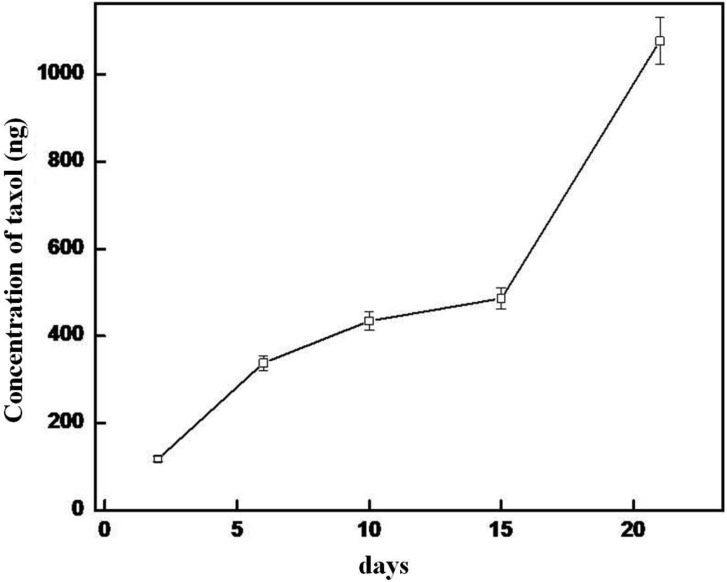
Taxol concentrations increased with an increase in culture incubation time. Low concentration of taxol was detected in the culture after as early as 2 days of incubation. Approximately 118, 338, 434, 486, and 1,076 ng/200 mL of taxol were estimated by ELISA in culture after 2, 5, 10, 15, and 21 days of incubation, respectively (Fig. 3). Taxol in mycelium and culture broth was estimated to be 481 and 1,670 ng/200 mL, respectively. Exact concentrations of the taxol in the extracts were not determined as the solubility of the chloroform extracts was very poor in methanol.
Fig. 3.
Production of taxol by the fungal strain Gliocladium sp. at different time intervals. Data represents mean values from five separate experiments ± SD.
Discussion
In the present study, slow growing and unusual endophytic fungal cultures were isolated from different tissues (stem, bark, and needles) of T. baccata. The endophyte population explored from the Taxus sp. represents different genera of fungi. Forty endophytic fungal strains were screened for taxol production using immunoassay. Among the fungal cultures, one strain designated as AAT-TS-41 was detected as taxol-producing. Taxol production increased exponentially with an increase in growth of the fungal culture (21 days), indicating that taxol was produced in maximum quantities at later log phase or early stationary phase of fungal growth. However, as taxol was observed in culture after as early as 2 days of incubation, these concentrations were much higher in comparison with other fungal strains reported to produce ng quantities of taxol after 21 days of culture [15]. Taxol production was estimated to be high in culture broth as compared to fungal mycelium, indicating that the metabolites were secreted into the medium. The culture on solid media took more than 1 wk to reach optimum growth, which suggests its identity as an endophyte [27]. Microscopic analyses showed that the mycelia were hyaline and septate, bearing verticillium-like conidiophores, a characteristic feature of Gliocladium sp. However, we did not observe any secondary penicilliate conidiophores. Phialides were solitary or produced in a group of 2~5, straight, smooth, and verticillium-like. Conidia were produced singly or clustered apically and were smooth, oval to cylindrical to curved, hyaline, single-celled, and variable in shape and size, typical of Gliocladium sp. All of the mentioned characteristics were identical to those described for the fungus Gliocladium sp. [25, 28]; hence, fungus AAT-TS-41 could be referred to as Gliocladium sp. As some of the morphological characteristics such as conidiophore structure and shape of conidia resembled or were very similar to another fungus belonging to genus Verticillium sp., we found it difficult to identify the fungus based on morphological characteristics alone. Thus, we analyzed the ITS and 18S rRNA regions to confirm the identity of the fungus.
In sequence analyses using BLAST and ClustalW, both ITS sequences (0.2 kb and 0.3 kb sequences) and 18S rRNA sequence (1.1 kb) show highest similarity with genera - Gliocladium, Bionectria, and Clonostachys. Phylogenetic studies indicated that the nearest relative of the fungus AAT-TS-41 was either from genus Gliocladium or Clonostachys. Considering morphological characteristics, we identified the fungus as Gliocladium sp., as the fungus did not show any asci formation, which is very often seen in Bionectria, and also did not produce conidia in columns as reported in Clonostachys [29]. 18S rRNA phylogenetic analysis showed that the fungus was closely related to genus Nectria sp., a teleomorph (perfect state) of Gliocladium sp. The sequence analysis and phylogenetic analysis supported the identification. However, in recent reports, many species of genus Gliocladium have been classified under Clonostachys and Bionectria based on morphological and molecular similarities [26]. To the best of our knowledge, this is the first report of the isolation of taxol from Gliocladium sp.
Stierle et al. [15] and others have isolated taxanes from different endophytic fungal species (Taxomyces andreanae, Pestalotiopsis, Pestalotia, Fusarium, Alternaria, and others) obtained from Taxus sp., which are common to Europe, Asia, and North America. The isolation of taxol from the culture of Gliocladium sp. is the first demonstration of its occurrence in fungi isolated from Indian Yew tree T. baccata. The other interesting aspect of our study is the occurrence of Gliocladium sp. as an endophyte, since some Gliocladium sp. have been isolated as mycoparasites producing novel secondary metabolites. The discovery of such a mycoparasitic fungus being associated as an endophyte might help us understand the mode of transformation of genetic material between the endophyte and the host, as many of these endophytes mimic the chemical diversity of the host. In conclusion, we isolated an endophytic fungus Gliocladium sp. producing taxol extra/intracellularly.
Acknowledgements
D. Sreekanth, G. K. Sushim, and A. Syed thank the Council of Scientific and Industrial Research (CSIR), Government of India for a Research Fellowship. Financial support from the Department of Biotechnology (DBT), New Delhi, India is gratefully acknowledged.
References
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Croom EM., Jr . Taxus for taxol and taxoids. In: Suffness M, editor. Taxol: science and applications. Boca Raton: CRC Press; 1995. pp. 37–70. [Google Scholar]
- 3.Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM, Cragg GM. Taxus spp. needles contain amounts of taxol comparable to the bark of Taxus brevifolia: analysis and isolation. J Nat Prod. 1990;53:1249–1255. doi: 10.1021/np50071a017. [DOI] [PubMed] [Google Scholar]
- 4.Roberts SC, Shuler ML. Large-scale plant cell culture. Curr Opin Biotechnol. 1997;8:154–159. doi: 10.1016/s0958-1669(97)80094-8. [DOI] [PubMed] [Google Scholar]
- 5.Gebetta B, Orsini P, Peterlongo F, Appendino G. Paclitaxel analogues from Taxus baccata. Phytochemisrty. 1998;47:1325–1329. [Google Scholar]
- 6.Page M, Landry N, Boissinot M, Halie MC, Harvey H, Gagne M, inventors. Bacterial mass production of taxanes and paclitaxel. No. WO 99/ 32651. US Patent. 1999
- 7.Caruso M, Colombo AL, Crespi-Perellino N, Fedeli L, Malyszko J, Pavesi A, Quaroni S, Saracchi M, Ventrella G. Studies on a strain Kitasatospora sp. paclitaxel producer. Ann Microbiol. 2000;50:89–102. [Google Scholar]
- 8.Sreekanth D, Syed A, Sarkar S, Sarkar D, Santhakumari B, Ahmad A, Khan I. Production, purification and characterization of taxol and 10DAB III from a new endophytic fungus Gliocladium sp. isolated from the Indian yew tree, Taxus baccata. J Microbiol Biotechnol. 2009;19:1342–1347. doi: 10.4014/jmb.0904.4041. [DOI] [PubMed] [Google Scholar]
- 9.Joshi BK, Gloer JB, Wicklow DT. New verticillin and glisoprenin analogues from Gliocladium catenulatum, a mycoparasite of Aspergillus flavus sclerotia. J Nat Prod. 1999;62:730–733. doi: 10.1021/np980530x. [DOI] [PubMed] [Google Scholar]
- 10.Dong JY, He HP, Shen YM, Zhang KQ. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J Nat Prod. 2005;68:1510–1513. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 11.Bills GF. Isolation and analysis of endophytic fungal communities from woody plants. In: Redlin SC, Carris LM, editors. Endophytic fungi in grasses and woody plants: systematics, ecology, and evolution. St. Paul: American Phytopathological Society Press; 1996. pp. 31–65. [Google Scholar]
- 12.Janardhanan KK, Ahmad A, Gupta M, Husain A. Grassy-shoot, a new disease of lemongrass caused by Balansia sclerotica (Pat.) Hohnel. J Phytopathol. 1991;133:163–168. [Google Scholar]
- 13.Ahmad A. Investigations on the grassy-shoot disease of lemongrass (Cymbopogon flexuosus) and characterization of toxic metabolites produced by the causal agent Balansia sclerotic [dissertation] Lucknow: University of Lucknow; 1991. [Google Scholar]
- 14.Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X. Taxol from fungal endophytes and the issue of biodiversity. J Ind Microbiol. 1996;17:417–423. [Google Scholar]
- 15.Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, and endophytic fungus of pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 16.Grothaus PG, Bignami GS, O'Malley S, Harada KE, Byrnes JB, Waller DF, Raybould TJ, McGuire MT, Alvarado B. Taxane-specific monoclonal antibodies: measurement of taxol, baccatin III, and "total taxanes" in Taxus brevifolia extracts by enzyme immunoassay. J Nat Prod. 1995;58:1003–1014. doi: 10.1021/np50121a004. [DOI] [PubMed] [Google Scholar]
- 17.Neumann B, Pospiech A, Schairer HU. Rapid isolation of genomic DNA from gram-negative bacteria. Trends Genet. 1992;8:332–333. doi: 10.1016/0168-9525(92)90269-a. [DOI] [PubMed] [Google Scholar]
- 18.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Orlando: Academic Press; 1990. [Google Scholar]
- 19.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. 1977. Biotechnology. 1992;24:104–108. [PubMed] [Google Scholar]
- 22.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Stinson M, Ezra D, Hess WM, Sears J, Strobel G. An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 2003;165:913–922. [Google Scholar]
- 26.Schroers HJ, Samuels GJ, Seifert KA, Gams W. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia. 1999;91:365–385. [Google Scholar]
- 27.Petrini O. Ecological and physiological aspects of host-specificity in endophytic fungi. In: Redline SC, Carris LM, editors. Endophytic fungi in grasses and woody plants: systematics, ecology and evolution. St. Paul: APS Press; 1996. pp. 87–100. [Google Scholar]
- 28.Barnett HL, Lilly VG. A destructive mycoparasite, Gliocladium roseum. Mycologia. 1962;54:72–77. [Google Scholar]
- 29.Barron GL. The genera of Hyphomycetes from soil. Baltimore: The Williams and Wilkins Co.; 1968. pp. 177–178. [Google Scholar]