Abstract
The ability of benzothiadiazole (BTH) and/or humic acid (HA) used as seed soaking to induce systemic resistance against a pathogenic strain of Fusarium oxysporum was examined in four soybean cultivars under greenhouse conditions. Alone and in combination the inducers were able to protect soybean plants against damping-off and wilt diseases compared with check treatment. These results were confirmed under field conditions in two different locations (Minia and New Valley governorates). The tested treatments significantly reduced damping-off and wilt diseases and increased growth parameters, except the number of branches per plant and also increased seed yield. Application of BTH (0.25 g/L) + HA (4 g/L) was the most potent in this respect. Soybean seed soaking in BTH + HA produced the highest activities of the testes of oxidative enzymes followed by BTH in the four soybean cultivars. HA treatment resulted in the lowest increases of these oxidative enzymes. Similar results were obtained with total phenol but HA increased total phenol more than did BTH in all tested cultivars.
Keywords: Benzothiadiazole, Humic acid, Soybean, Systemic induced resistance, Wilt disease
Introduction
Soybean (Glycine max L.) is one of the world's most important sources of oil and protein. It has the highest protein content among leguminous crops [1]. Soybean plants are subject to attack by several fungal, bacterial, and viral diseases that cause great losses in the yield. Wilt disease of soybean caused by Fusarium oxysporum is one of the most destructive diseases of the crop and is a very common soil-borne fungus [2]. This pathogen is difficult to control because of its persistence in soil and wide host range. Some chemicals are effective in controlling wilt disease, but these chemicals are expensive and not environmentally-friendly. Therefore, alternative measures are being tested, including induced resistance using biotic and abiotic treatments. The phenomenon of systemic induced resistance, in which resistance to disease is enhanced in tissues distant from the site of the inducing treatment conducted earlier in time, has been extensively reported for a number of plant/pathogen systems and has been the subject of recent reviews [3]. The majority of these studies have been conducted under controlled environment conditions. However, it has been demonstrated under field conditions for a limited number of plant/pathogen interactions. Induced resistance in some plants against root diseases has been was reported [4]. Benzothiadiazole (BTH), was recently identified by scientists at Novartis as a novel disease-control compound, and has been promoted as a safe, reliable, and nonphytotoxic plant protection agent. Exogenous application of BTH to wheat and Arabidopsis leaves activate a number of systemic acquired resistance (SAR)-associated genes, leading to enhanced plant protection against various pathogens [5]. These studies provided evidence that induction of SAR gene expression by BTH does not require the contribution of salicylic acid and/or jasmonate, suggesting that this compound could act as a secondary messenger analog capable of activating the SAR signal transduction pathway independently of the accumulation of other signal molecules [5]. Benhamou and Bélanger [6] demonstrated that application of BTH to cucumber leaves before challenge with the root pathogen Pythium ultimum triggers a set of plant defense reactions that result in the creation of a fungitoxic environment, which protects the roots by restricting pathogen growth to the outermost tissues. Dann et al. [7] reported that severity of white mold disease in field grown soybeans can be significantly reduced by sprays of 2,6 dichloroisonicotinic acid (INA) and BTH, leading to increased seed yield. Sarwar et al. [4] showed that exogenously applied salicylic acid and BTH can protect chickpea plants against infection with F. oxysporum ciceri similar to that of Benlate.
Humic acid (HA) suspensions based on potassium-humates have been applied successfully in many areas of plant production as a plant growth stimulant or soil conditioner for enhancing natural resistance against plant diseases [8], stimulating plant growth through increased cell division, as well as optimizing uptake of nutrients and water and stimulating soil microorganisms [9]. Several reports indicated the efficiency of HA in reducing some plant diseases [10].
The use of BTH and HA inducing compounds could reduce the use of agrochemicals such as fungicides. With the in mind, this investigation was done to evaluate the effectiveness of BTH and HA treatments on control of wilt disease of soybean under greenhouse and field conditions, as well as seed yield. Also, biochemical changes associated with the application of the two inducers were assessed.
Materials and Methods
Isolation and identification of the causal pathogen
Naturally diseased soybean plants showing wilt disease symptoms were collected from different locations of Minia and New Valley governorates in the summer 2009 growing season. The plants were thoroughly washed in tap water, cut into small (0.5 cm) pieces, surface sterilized for 2 min using 2% sodium hypochlorite solution, rewashed several times in sterile distilled water, and dried between folded sterile filter papers. The surface sterilized and dried samples were plated onto potato dextrose agar supplemented with penicillin (20 IU/mL) and incubated at 25 ± 1℃ for 6 days. The developed fungal colonies were purified by single spore techniques then identified as described previously [11].
Pathogenicity test
Pathogenicity of eight F. oxysporum isolates obtained from diseased soybean plants were tested on the Giza 21 cultivar. This experiment was carried out at New Valley Agricultural Research Station. Soil pot infestation was done using a previously-developed homogenized culture technique [12].
Preparation of fungal inoculum
Disks taken from 1-wk-old culture of isolate of F. oxysporum were inoculated in 75 mL of potato dextrose broth in a 250 mL flask and incubated at 25 ± 1℃. The obtained fungal cells were collected on Whatman No. 1 filter paper, rinsed with sterile distilled water, placed in a Waring blender with a small amount of sterile water, and blended for 2 min at high speed. Sterile distilled water was then added to each inoculum suspension to give a final concentration of 106 colony forming units (CFU/mL) that was used for soil infestation 5 days before sowing. Five seeds were sown in each 30 cm pot. The percentage of damping-off was recorded 30 days after seeding. Plants were examined 3 mon after seeding. The severity of wilt was determined after 90 days [13] using a rating scale of 0~5 on the basis of root discoloration or leaf yellowing: 0: no root discoloration or leaf yellowing; 1: 1~25% root discoloration or one leaf yellowed; 2: 26~50% root discoloration or more than one leaf yellowed; 3: 51~75% root discoloration plus one leaf wilted; 4: up to 76% root discoloration or more than one leaf wilted; and 5: completely dead plants. For each replicate a disease severity index (DSI) similar to that described previously [14] was calculated as follows:
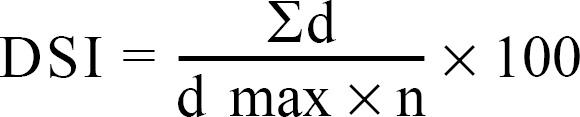
where DSI is the disease rating possible, d max is the maximum disease rating and n is the total number of plants examined in each replicate. Re-isolation of the pathogen from the infected plants was also done to confirm the causal agent of wilting.
Control of damping-off and wilt diseases caused by F. oxysporum under greenhouse condition
In this experiment, BTH [benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester wettable granule 50% WG; Bion®; Sigma Chemical, St. Louis, MO, USA] and HA (potassium humate soluble granule 85% WSG; Humus®; Broadtech Chemical International Co. Ltd., Inner Mongolia, China) were used for 20 min seed soaking to evaluate their efficiency for controlling damping-off and wilt diseases caused by F. oxysporum of pot-grown soybean plants. Five soybean seeds each from the Giza 21, Giza 22, Giza 35, and Giza 111 cultivars per pot were sown in 30 cm pots filled with sterilized F. oxysporum infested soil at the rate of 100 mL homogenized culture per pot as previously mentioned, 5 day before planting. The treatments were: BTH at 0.25 and 0.5 g a.i./L, HA at 2.5 and 5 g a.i./L as well as a combination of BTH and HA at, 0.25 g a.i./L BTH and 2.5 g a.i./L HA, 0.25 g a.i./L BTH and g a.i./L HA, 0.5 g a.i./L BTH and 2.5 g a.i./L HA, 0.5 g a.i./L BTH, and 5 g a.i./L HA. The control treatment was soil infested with F. oxysporum and sown with untreated soybean seeds at the same rate. A set of five pots for treatment were used. Each pot received equal amounts of water. Other agricultural processors were performed according to normal practice. Percentage of damping-off and wilt severity were recorded 30 and 90 days after seeding, respectively.
Field experiment
The field experiment was carried out at two localities: New Valley Agricultural Research Station and the Experimental Farm of Plant Pathology, Department of the Faculty of Agriculture, Minia University during the 2010 summer growing season. Soybean seeds (Giza 21, Giza 22, Giza 35, and Giza 111 cultivars) were soaked in the same tested treatments in a greenhouse for 20 min, then dried for 30 min before seeding. In the control treatment, seeds were soaked in distilled water as described above. Treated soybean seeds were sown in the field on May 4, 2010 in both locations. A split plot design with three replicates was used in these experiments; the main plots represented varieties, while sub-plots represented treatments. The area of each sub-plot was 10.5 m2 (3.0 × 3.5 m) containing five 3.5 m long rows separated by 60 cm. All treatments were sown in hills 20 cm apart on both sides of the row ridge, with two seeds per hill (140,000 plants/field). All recommended agricultural practices were adopted throughout the two locations. After 30 days from seeding date, damping-off was determined. Wilt severity was also recorded on a random sample of plants of the sub-plots (20 plants) 3 mon after seeding as described previously [13]. At harvest stage, plant growth parameters of plant height, number of branches and pods per plant, and seed weight (10/feddan) were recorded.
Effect of soybean seed treatment with inducer chemicals on activity of oxidative enzymes and phenol content
Activity of peroxidase (PO), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL), and total phenol contents was studied in tissue extracts of soybean plants surviving treatment with 0.5 g a.i./L BTH, 5 g a.i./L HA, at as well as a combination of 0.25 g a.i./L BTH and 4 g a.i./L HA, as well as in untreated seeds. All treatments were grown in soil infested with F. oxysporum.
One gram of plant tissue was homogenized in 10 mL of ice-cold 50 mM potassium phosphate buffer (pH 6.8) containing 1M NaCl, 1% polyvinylpyrrolidone, 1 mM EDTA, and 10 mM β-mercaptoethanol [15]. After filtration through cheesecloth, the homogenates were centrifuged at 8,000 rpm at 4℃ for 25 min. The supernatants (crude enzyme extract) were stored at -20℃ or immediately used for determination of PO, PPO, and PAL activities and total protein. For the determination of enzyme activities, each treatment consisted in four replicates (three plants/replicate) and two spectrophotometric readings were taken per replicate using a Milton Roy 1201 Spectrophotometer (PEMED®, Denver, CO, USA). The bioassay experiments were repeated twice.
PO activity
PO activity was determined directly using a spectrophotometrical method [16] using guaiacol as common substrate. The reaction mixture consisted of 0.2 mL crude enzyme extract and 1.40 mL of a solution containing guaiacol, hydrogen peroxide (H2O2) and sodium phosphate buffer (0.2 mL 1% guaiacol + 0.2 mL 1% H2O2 + 1 mL 10 mM potassium phosphate buffer). The mixture was incubated at 25℃ for 5 min and the initial rate of increase in absorbance was measured over 1 min at 470 nm. Activity was expressed as units of PO/mg protein [17].
PPO activity
The activity of PPO was determined by adding 50 µL of the crude extract to 3 mL of a solution containing 100 mM potassium phosphate buffer, pH 6.5 and 25 mM pyrocatechol. The increase of absorbance at 410 nm during 10 min at 30℃, was measured [18]. One PPO unit was expressed as the variation of absorbance at 410 nm per mg soluble protein per min.
PAL activity
PAL activity was determined following a previously-described direct spectrophotometric method [19]. Two hundred microlitres of the crude enzyme extract previously dialyzed overnight with 100 mM Tris-HCl buffer, pH 8.8, were mixed to obtain a solution containing 200 µL 40 mM phenylalanine, 20 µL 50 mM β-mercaptoethanol, and 480 µL 100 mM Tris-HCl buffer, pH 8.8. After incubation at 30℃ for 1 hr, the reaction stopped by adding 100 µL 6 N HCl. Absorbance at 290 nm was measured and the amount of trans-cinnamic acid formed was evaluated by comparison with a standard curve (0.1~2 mg/mL trans-cinnamic acid) and expressed as units of PAL/min/mg protein.
Protein concentration
Total protein content of the samples was quantified according to the method described by Bradford [20].
Determination of phenolic compounds
To assess phenolic content, 1 g fresh plant sample was homogenized in 10 mL 80% methanol and agitated for 15 min at 70℃. One milliliter of the extract was added to 5 mL of distilled water and 250 µL of 1 N Folin-Ciocalteau reagent and the solution was kept at 25℃. The absorbance was measured with a spectrophotometer at 725 nm. Catechol was used as a standard. The amount of phenolic content was expressed as phenol equivalents in mg/g fresh tissue [21].
Statistical analysis
All experiments were performed twice. Analysis of variance was carried out using MSTAT-C ver. 2.10 (Michigan State University, East Lansing, MI, USA). Least significant difference was employed to test for significant difference between treatments at p ≤ 0.05 [22].
Results
Pathogenicity test and identification of the causal organisms
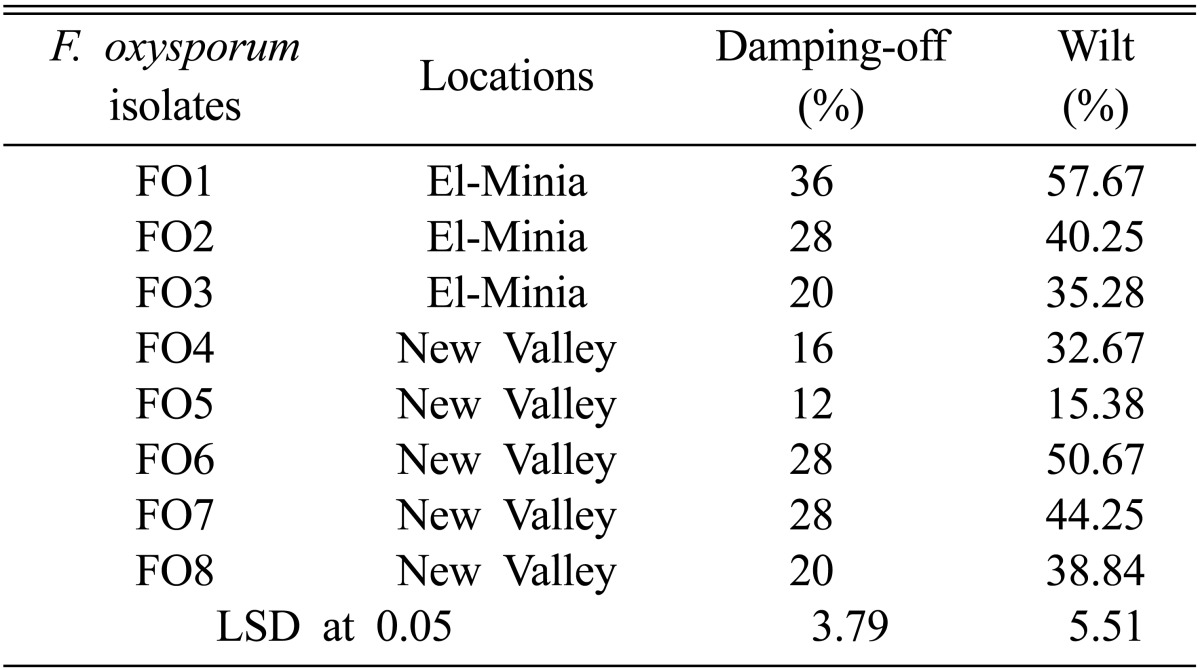
The eight fungal isolates obtained from different naturally infected soybean plants showing wilt symptoms were able to cause damping-off and wilt symptoms on artificial inoculated soybean. The highest percentage of damping-off and wilt were caused by isolate FO1 followed by isolate FO6, while isolate FO5 was least harmful (Table 1). All the obtained isolates were identified as F. oxysporum according to the descriptions of Booth [11]. The identities were confirmed by tests conducted at the Assuit University Mycological Center.
Table 1.
Pathogenicity of Fusarium oxysporum isolates on soybean plants
LSD, least significant difference.
Effect of BTH and HA on damping-off and wilt diseases caused by F. oxysporum
Green house conditions
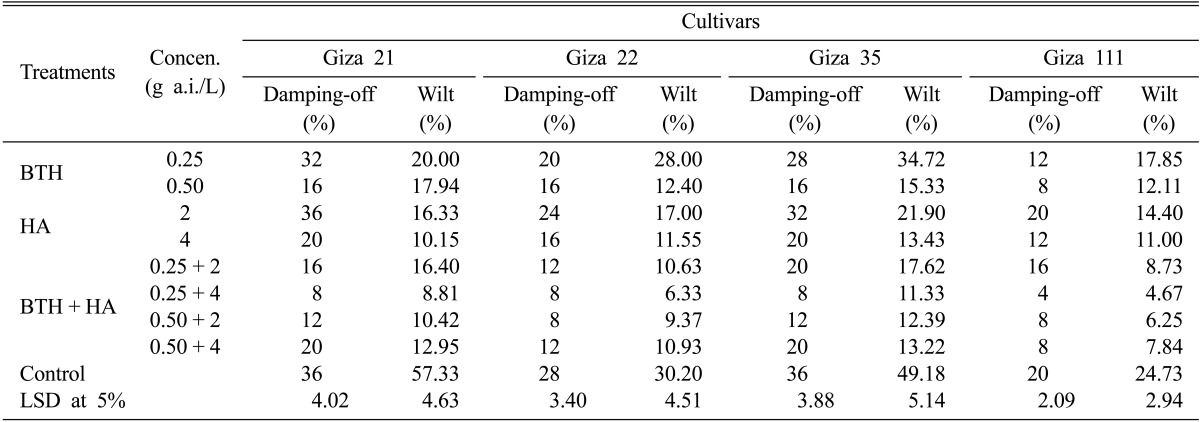
Both the tested chemical inducers applied individually or in combination, were almost always significantly effective in reducing infection with F. oxysporum under greenhouse conditions compared with the check treatment (control) (Table 2). This reduction reached its maximum when combination between BTH and HA was used at 0.25 and 4 g a.i./L followed by 0.5 and 2 g a.i./L for all the tested cultivars. BTH and HA at 0.25 and 4 g a.i./L reduced the average damping-off and wilt for the four soybean cultivars (Giza 21, Giza 22, Giza 35, and Giza 111) from 36, 28, 36, and 20% damping-off, respectively, and 57.33, 30.20, 49.18, and 24.37% wilt, respectively, in control to 8, 8, 8, and 4% damping-off and 8.81, 6.33, 11.33, and 4.67% wilt, respectively. On the other hand, soybean seeds treated with HA at 2 g/L recorded the lowest reduction of damping-off for all the tested cultivars, while seeds treated with BTH at 0.25 g a.i./L recorded the lowest wilt for the all tested cultivars. Also, considerable differences were evident in the response of different soybean cultivars to infection with F. oxysporum. Generally, soybean Giza 21 cultivar was more susceptible to F. oxysporum followed by Giza 35 and Giza 22 cultivars, whereas Giza 111 cultivar was the least affected for both damping-off and wilt.
Table 2.
Effect of BTH and HA on damping-off and wilt diseases caused by Fusarium oxysporum isolate FO1 of the four soybean cultivars under greenhouse conditions
BTH, benzothiadiazole; HA, humic acid; LSD, least significant difference.
Field conditions
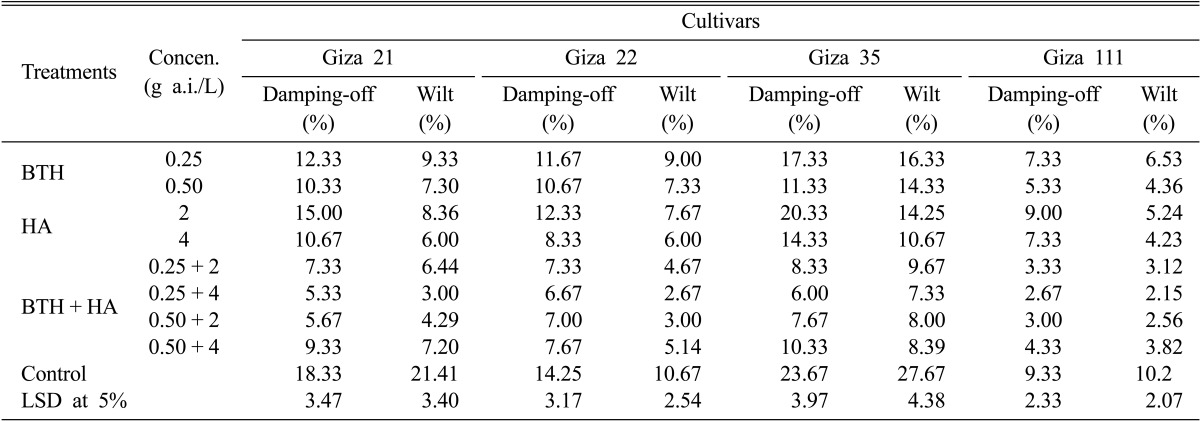
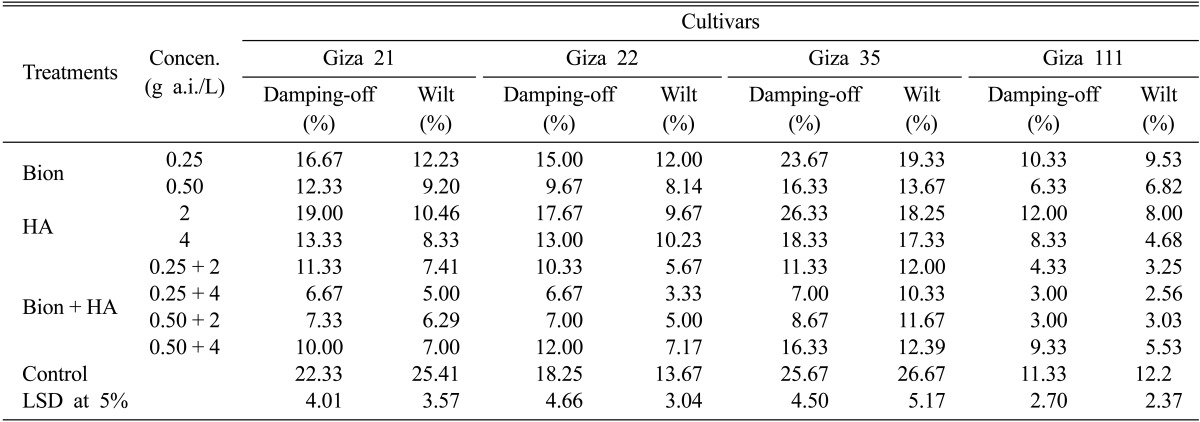
The effects of the resistance inducers HA and BTH individually or combination at different concentrations on damping-off and wilt of four soybean cultivars under field conditions are shown in Tables 3 and 4. All treatments, in most cases significantly reduced the percentage of damping-off and wilt severity compared with control in both locations. Also, the BTH + HA combination decreased the percentage of damping-off and wilt severity in both field locations more so than the individual use of the inducers for the tested soybean cultivars. Soybeans treated with BTH + HA combination at respective concentrations of 0.25 g a.i./L and 4 g a.i./L were most protected from infection-related damping-off and wilt diseases. Damping-off of the Giza 21, Giza 22, Giza 35, and Giza 111 soybean cultivars in the New Valley location was decreased on average from 18.33, 14.25, 23.67, and 9.33%, respectively, in control to 5.33, 6.67, 6, and 2.67%, respectively. In the Minia location, this treatment reduced damping-off from 22.33, 18.25, 25.67, and 11.33% in control to 6.67, 6.67, 7, and 3%, in same respective order concerning soybean cultivars. Also, for the Giza 21, Giza 22, Giza 35, and Giza 111 soybean cultivars, the wilt symptoms were reduced from 21.41, 10.67, 27.67, and 10.20%, respectively, in control to 3, 2.67, 7.33, and 2.15%, respectively, in the New Valley location, and from 25.41, 13.67, 26.67, and 12.20%, respectively, in control to 5, 3.33, 10.33, and 2.56%, respectively, in the Minia location for the tested soybean cultivars, respectively. On the other hand, soybeans treated with HA at 2 g/L were least protected, with similar results to control plants evident in both field locations, while soybeans treated with BTH at 0.25 g a.i./L displayed the lowest wilt severity for the tested cultivars in both locations compared with control.
Table 3.
Effect of BTH and HA on damping-off and wilt diseases of the four soybean cultivars under field conditions in New Valley governorate during the 2010 summer season
BTH, benzothiadiazole; HA, humic acid; LSD, least significant difference.
Table 4.
Effect of BTH and HA on damping-off and wilt diseases of the four soybean cultivars under field conditions in Minia governorate during the 2010 summer season
BTH, benzothiadiazole; HA, humic acid; LSD, least significant difference.
The four tested cultivars were infected with damping-off and wilt diseases either in New Valley or in El-Minia governorates In general, BTH was highly effective in reducing the incidence of damping-off in the seedling stage of all tested cultivars in both locations. Contrary results were obtained concerning HA. The Giza 35 cultivar was the most susceptible to infection followed by Giza 21 and Giza 22 cultivars. The Giza 111 cultivar was least susceptible.
Effect of BTH and HA on growth parameters and seed yield under field conditions
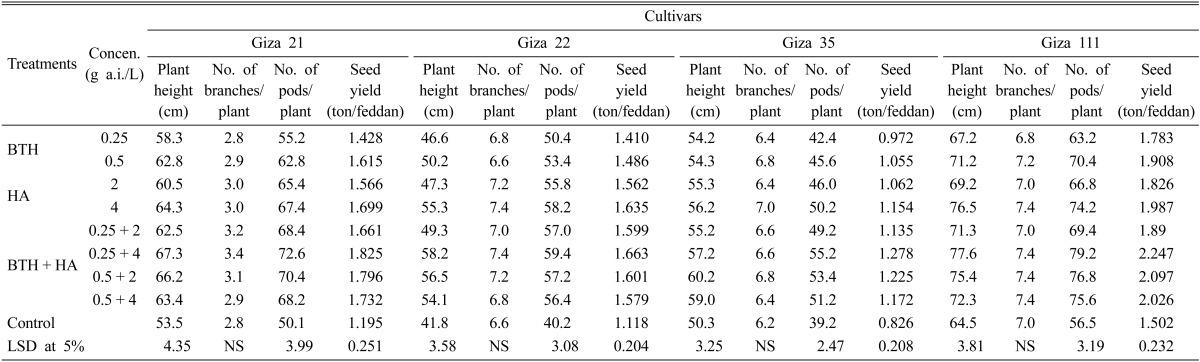
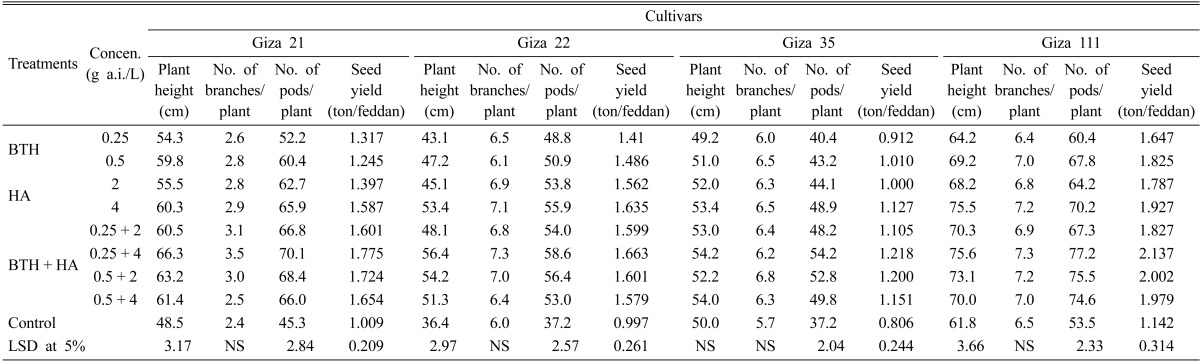
The data presented in Tables 5 and 6 summarize the various responses of the four tested soybean cultivars at their growth parameters (plant height, and number of pods/plant) and seed yield/feddan as affected by different concentrations of BTH and HA, either individually or in combination, at the Minia and New Valley governorates. All treatments significantly improved plant height and increased the number of pods/plant and seed yield/feddan, compared to check treatment for the all tested cultivars, while the increase of number of branches/plant was not significant in all tested cultivars in both locations. In this respect, the BTH + HA combination at respective concentrations of 0.25 g a.i/L and 4 g a.i./L followed by 0.5 and 2 g a.i./L were the superior treatments, while BTH when used individually at 0.25 g a.i./L were the lest effective ones. On the other hand, soybean Giza 111 cultivar gave the best results for growth parameters and seed yield in case of seed treaded or untreated in both locations.
Table 5.
Effect of BTH and HA on growth parameters of the four soybean cultivars under field conditions on New Valley governorate during the 2010 summer season
BTH, benzothiadiazole; HA, humic acid; LSD, least significant difference.
Table 6.
Effect of BTH and HA on the growth parameters of the four soybean cultivars under field conditions Minia governorate during the 2010 summer season
BTH, benzothiadiazole; HA, humic acid; LSD, least significant difference.
Biochemical changes associated with inducers
PO, PPO, and PAL activities
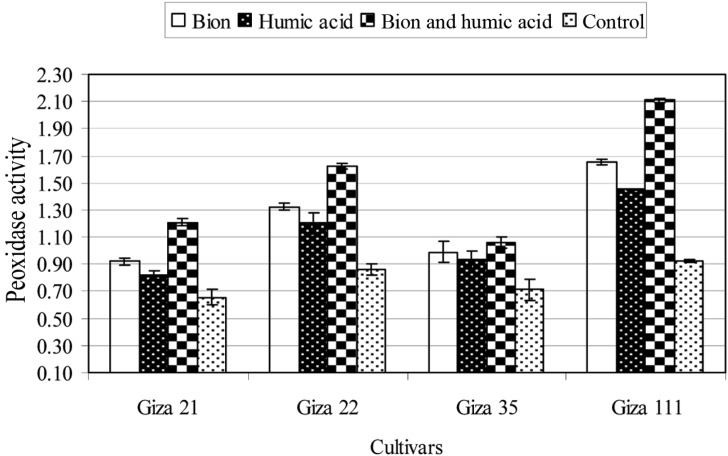
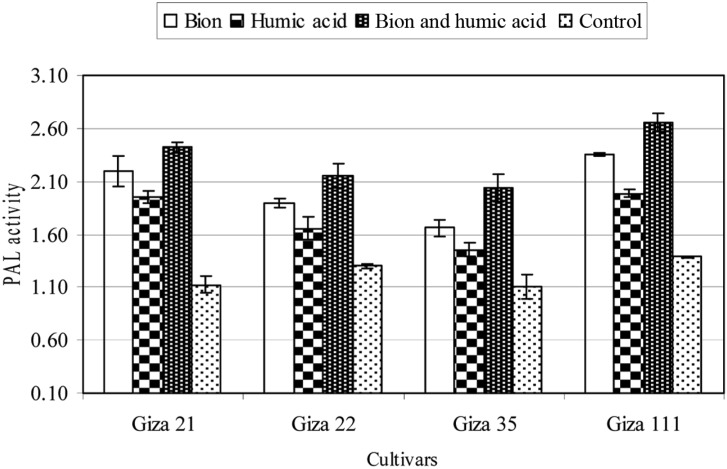
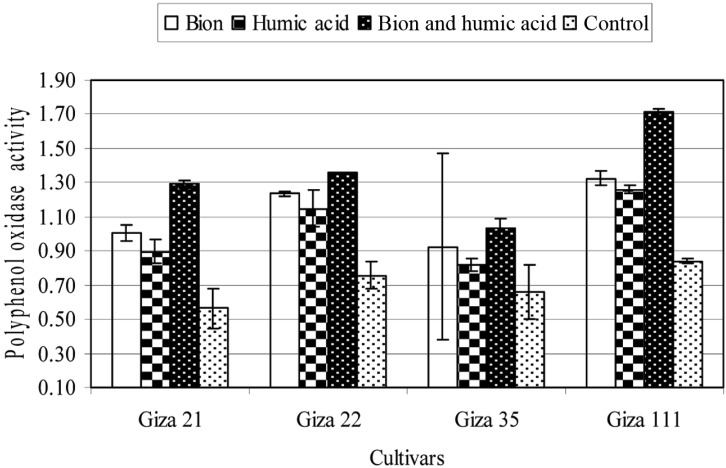
The effect of BTH and HA individually and in combination as inducer chemicals on the activities of PO, PPO, and PAL of the soybean cultivars grown in soil infested with F. oxysporum was studied. The data are summarized in Figs. 1~3. Individually and in combination, both inducers increased the activity of PO, PPO, and PAL in the four soybean tested cultivars, compared with untreated plants (control). The combination treatment produced the greatest effect, followed by BTH alone. HA treatment had the least effect. On the other hand, the susceptibility of the four soybean cultivars was positively correlated with the activity of these enzymes; the Giza 111 cultivar, which was highly resistant to F. oxysporum, presented the the highest enzyme activities, while the Giza 21 cultivar, which was highly susceptible to F. oxysporum, recorded the lowest enzyme activities, either in treated or untreated plants.
Fig. 1.
Activity of peroxidase enzyme (enzyme unit/mg protein/min) of four soybean cultivars as affected by benzothiadiazole (0.5 g a.i./L), humic acid (4 g a.i./L), or in combination (0.25 + 4 g a.i./L). Mean ± SDs for nine plants per treatment are shown.
Fig. 3.
Activity of phenylalanine ammonia lyase (PAL) enzyme (enzyme unit/mg protein/min) of four soybean cultivars as affected by benzothiadiazole (0.5 g a.i./L), humic acid (4 g a.i./L), or their combination (0.25 + 4 g a.i./L). Mean ± SDs for nine plants per treatment are shown.
Phenolic content
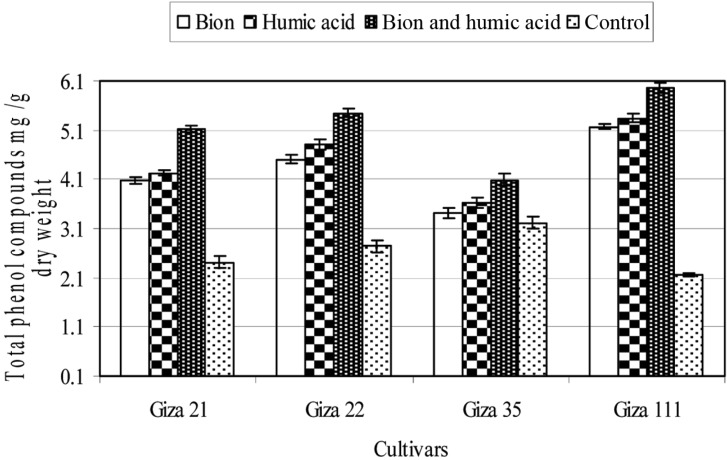
The content of total phenols was greatly increased in plants treated with either inducer, compared with untreated plants in all tested cultivars. The BTH + HA combination increased the phenolic content more than the individual use of either inducer (Fig. 4). The soybean cultivars differed in their phenolic content in treated and untreated plants; of the treated cultivars, the Giza 111 cultivar displayed the highest phenolic content, followed by the Giza 22 cultivar, while Giza 35 displayed the lowest phenolic content. Opposite results were evident for untreated plants.
Fig. 4.
Total phenolic compounds content of four soybean cultivars as affected by benzothiadiazole (0.5 g a.i./L), humic acid (4 g a.i./L), or their combination (0.25 + 4 g a.i./L). Mean ± SDs for nine plants per treatment are shown.
Discussion
Wilt disease caused by F. oxysporum is the most important disease to affect soybean plants during the growing season [2]. The present study investigated the possibility of minimizing the effects of infection in terms of damping-off and wilt disease using BTH and HA as resistance inducers. BTH and HA applied individually and in combination significantly reduced damping-off and wilt disease in plants cultivated in greenhouse and field conditions, compared with the untreated control plants. In general, the combined use of BTH and HA (0.25 g a.i/L and 4 g a.i./L, respectively) was more efficient in reducing disease incidence than using these chemicals individually. Also, in the field, these chemicals significant increased growth parameters and seed yield and, in combination, resulted in the highest increased in these parameters. The present findings are consistent with previous results [6, 10, 23].
BTH and HA induce disease resistance and increase yield in a number of plants including soybean and other legumes against a broad range of pathogens [5, 7, 23, 24]. These compounds have no direct antimicrobial activity against many fungal and bacterial pathogens [23]. We suggest, that, for soybean plants, BTH and HA may stimulate inherent defense mechanisms that enable plants to respond more quickly against invading and colonizing fungi. It seems likely that the increased activity of enzymes involved in defense reactions may be one of the basic ways that BTH and HA induce resistance in soybeans against wilt disease. This view is entirely consistent with the present observations of increased activity of PO, PPO, and PAL in soybean plants treated with BTH and/or HA. On the other hand, the susceptibility of the four soybean cultivars was positively correlated with the activity of these enzymes; the Giza 111 cultivar, which was more resistant to F. oxysporum, displayed the highest activities of the enzymes, while the Giza 21 cultivar, which was highly susceptible, displayed the lowest enzyme activities in treated or untreated plants. Also, the total phenolic content was markedly increased in plants treated with the inducers compared with untreated ones in all the tested cultivars, with the BTH + HA combination producing more of an increase than their individual application.
Concerning the role of BTH as an resistance inducer, it has been reported that induction of SAR gene expression by BTH does not require the contribution of salicylic acid (SA), which suggests that this compound could act as a secondary messenger analog capable of activating SAR signal transduction pathway independent of the accumulation of other signal molecules [5]. Application of BTH to a variety of plants before pathogen challenge triggers a set of plant defense reactions that result in the creation of a fungitoxic environment, which protects them by different physical and/or chemical mechanisms [23]. Also, treatment with BTH and HA presently led to an increase in PO, PPO, and PAL activity, consistent with the previous demonstration that the expression of resistance is often accompanied by the activation of phenol-oxidizing enzymes such as PO, PPO, and PAL [25]. Increased PO and PPO activity may contribute to defense through the production of oxidized forms of quinones, which can inactivate pectinolytic enzymes produced by pathogens. This suggests that PO and PPO could play an effective role in the observed resistance. PO and PPO have been associated with induced resistance and are involved in several plant defense mechanisms, such as lignin biosynthesis, oxidative cross-linking of plant cell walls, and generation of active oxygen species [26]. PAL is a key enzyme of the phenylpropanoid pathway that leads to a variety of defense-related plant secondary metabolites such as SA, phytoalexins, and lignin-like polymers [27]. PAL has been shown to play a critical role in acibenzolar-s-methyl (ASM)-mediated resistance, as its expression was primed early by ASM in Japanese pear [26] and cucumber [27]. However, the direct role of PAL in resistance induced by ASM comes from the work of Stadnik and Buchenauer [28] who showed that chemical inhibition of PAL abolished resistance in wheat induced against Blumeria graminis f. sp. tritici.
On the other hand, data strongly suggest that HA acts directly or indirectly as a signal for inducing systemic resistance as proposed by Yigit and Dikilitas [5]. HA suspensions based on potassium humates have been applied successfully in many areas of plant production as a plant growth stimulant or soil conditioner to enhance natural resistance against plant diseases and pests [7], which consequently increases plant yield. Application of HA enhances the activity of antioxidants such as α-tocopherol, α-carotene, superoxide dismutases, and ascorbic acid concentrations in turf grass species. These antioxidants may play a role in the regulation of plant development, flowering, and chilling of disease resistance [29]. HA may increase the permeability of plant membranes and enhance the uptake of nutrients. Moreover, HA may also improve soil nitrogen uptake and facilitate the uptake of potassium, calcium, magnesium, and phosphorus, making these nutrients more mobile and available to plant root systems [30].
In conclusion, the combined treatment with BTH and HA might be used commercially for controlling soybean diseases under field conditions.
Fig. 2.
Activity of peroxidase enzyme (enzyme unit/mg protein/min) of four soybean cultivars as affected by benzothiadiazole (0.5 g a.i./L), humic acid (4 g a.i./L), or in combination (0.25 + 4 g a.i./L). Mean ± SDs for nine plants per treatment are shown.
References
- 1.El-Abady MI, Seadh SE, Attia AN, El-Saidy Aml EA. Impact of foliar fertilization and its time of application on yield and seed quality of soybean; The 2nd Field Crops Conference, FCRI, AV; 2008 Oct 14-16; Giza, Egypt. [Google Scholar]
- 2.Hashem EA, Abdalla HE, Hussein YA, Abd-Elnabi MA. In vitro selection of soybean callus resistant to Fusarium oxysporum metabolites. Res J Agric Biol Sci. 2009;5:588–596. [Google Scholar]
- 3.Hammerschmidt R. Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol. 1999;55:77–84. [Google Scholar]
- 4.Sarwar N, Ch MH, Haq I, Jamil FF. Induction of systemic resistance in chickpea against Fusarium wilt by seed treatment with salicylic acid and Bion. Pak J Bot. 2005;37:989–995. [Google Scholar]
- 5.Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou N, Bélanger RR. Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response. Plant J. 1998;14:13–21. doi: 10.1046/j.1365-313X.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Dann E, Diers B, Byrum J, Hammerschmidt R. Effect of treating soybean with 2,6-dichloroisonicotinic acid (INA) and benzothiadiazole (BTH) on seed yields and the level of disease caused by Sclerotinia sclerotiorum in field and greenhouse studies. Eur J Plant Pathol. 1998;104:271–278. [Google Scholar]
- 8.Scheuerell SJ, Mahaffee WH. Compost tea as a container medium drench for suppressing seedling damping-off caused by Pythium ultimum. Phytopathology. 2004;94:1156–1163. doi: 10.1094/PHYTO.2004.94.11.1156. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, De Nobili M, Aviad T. Stimulatory effects of humic substances on plant growth. In: Magdoff F, Weil RR, editors. Soil organic matter in sustainable agriculture. Boca Raton: CRC Press; 2004. pp. 103–130. [Google Scholar]
- 10.Yigit F, Dikilitas M. Effect of humic acid applications on the root-rot diseases caused by Fusarium spp. on tomato plants. Plant Pathol J. 2008;7:179–182. [Google Scholar]
- 11.Booth C. The genus Fusarium. Surrey: Commonwealth Mycological Institute; 1985. [Google Scholar]
- 12.Muthomi JW, Otieno PE, Chemining'wa GN, Nderitu JH, Wagacha JM. Effect of legume root rot pathogens and fungicide seed treatment on nodulation and biomass accumulation. J Biol Sci. 2007;7:1163–1170. [Google Scholar]
- 13.Abdou E, Abd-Alla HM, Galal AA. Survey of sesame root rot/wilt disease in Minia and their possible control by ascorbic and salicylic acids. Assiut J Agric Sci. 2003;32:135–152. [Google Scholar]
- 14.Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology. 1995;85:695–698. [Google Scholar]
- 15.Biles CL, Martyn RD. Peroxidase, polyphenoloxidase, and shikimate dehydrogenase isozymes in relation to tissue type, maturity and pathogen induction of watermelon seedlings. Plant Physiol Biochem. 1993;31:499–506. [Google Scholar]
- 16.Hammerschmidt R, Nuckles EM, Kuć J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol. 1982;20:73–82. [Google Scholar]
- 17.Urbanek H, Kuzniak-Gebarowska E, Herka H. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol Plant. 1991;13:43–50. [Google Scholar]
- 18.Gauillard F, Richard-Forget F, Nicolas J. New spectrophotometric assay for polyphenol oxidase activity. Anal Biochem. 1993;215:59–65. doi: 10.1006/abio.1993.1554. [DOI] [PubMed] [Google Scholar]
- 19.Cavalcanti FR, Resende ML, Carvalho CP, Silveira JA, Oliveira JT. An aqueous suspension of Crinipellis perniciosa mycelium activates tomato defense responses against Xanthomonas vesicatoria. Crop Prot. 2007;6:729–738. [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Saikia R, Yadav M, Varghese S, Singh BP, Gogoi DK, Kuma R, Arora DK. Role of riboflavin in induced resistance against Fusarium wilt and charcoal rot diseases of chickpea. Plant Pathol J. 2006;22:339–347. [Google Scholar]
- 22.Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York: Wiley Interscience Publication; 1984. p. 678. [Google Scholar]
- 23.Nafie E, Mazen M. Chemical-induced resistance against brown stem rot in soybean: the effect of benzothiadiazole. J Appl Sci Res. 2008;4:2046–2064. [Google Scholar]
- 24.El-Ghamry AM, Abd El-Hai KM, Ghoneem KM. Amino and humic acids promote growth, yield and disease resistance of faba bean cultivated in clayey soil. Aust J Basic Appl Sci. 2009;3:731–739. [Google Scholar]
- 25.Goodmann RN, Novacky AJ. The bacteria-induced hypersensitive reaction. In: Goodman RN, Novacky AJ, editors. The hypersensitive reaction in plants to pathogens. St. Paul: American Phytopathological Society; 1994. pp. 117–173. [Google Scholar]
- 26.Faize M, Faize L, Koike N, Ishizaka M, Ishii H. Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology. 2004;94:604–612. doi: 10.1094/PHYTO.2004.94.6.604. [DOI] [PubMed] [Google Scholar]
- 27.Cools HJ, Ishii H. Pre-treatment of cucumber plants with acibenzolar-S-methyl systemically primes a phenylalanine ammonia lyase gene (PAL1) for enhanced expression upon attack with a pathogenic fugus. Physiol Mol Plant Pathol. 2002;61:273–280. [Google Scholar]
- 28.Stadnik MJ, Buchenauer H. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp. tritici. Physiol Mol Plant Pathol. 2000;57:25–34. [Google Scholar]
- 29.Dmitriev A, Tena M, Jorrin J. Systemic acquired resistance in sunflower (Helianthus annuus L.) Tsitol Genet. 2003;37:9–15. [PubMed] [Google Scholar]
- 30.Piccolo A, Nardi S, Concheri G. Structural characteristics of humic substances as regulated to nitrate uptake and growth regulation in plant systems. Soil Biochem. 1992;24:373–380. [Google Scholar]