Abstract
Chemical mutagenesis of basidiospores of Hypsizygus marmoreus generated new mushroom strains. The basidospores were treated with methanesulfonate methylester, an alkylating agent, to yield 400 mutant monokaryotic mycelia. Twenty fast-growing mycelia were selected and mated each other by hyphal fusion. Fifty out of the 190 matings were successful (mating rate of 26.3%), judged by the formation of clamp connections. The mutant dikaryons were cultivated to investigate their morphological and cultivation characteristics. Mutant strains No. 3 and No. 5 showed 10% and 6% increase in fruiting body production, respectively. Eight mutant strains showed delayed and reduced primordia formation, resulting in the reduced production yield with prolonged cultivation period. The number of the fruiting bodies of mutant No. 31, which displayed reduced primordial formation, was only 15, compared to the parental number of 65. Another interesting phenotype was a fruiting body with a flattened stipe and pileus. Dikaryons generated by mating with the mutant spore No. 14 produced flat fruiting bodies. Further molecular biological studies will provide details of the mechanism. This work shows that the chemical mutagenesis approach is highly utilizable in the development of mushroom strains as well as in the generation of resources for molecular genetic studies.
Keywords: Breeding, Chemical mutagenesis, Hypsizygus, Spore
Introduction
Hypsizygus marmoreus is a heterothallic bifactorial mushroom native to East Asia. It grows on the dead wood of broadleaf trees such as beech, willow, and elm trees. The fruiting body of H. marmoreus is one of the major mushroom products in East Asia. The establishment of semi-automatic mushroom cultivation plants has facilitated the commercial cultivation of this mushroom in wide-mouth polypropylene bottles with solid substrates [1]. Strains of H. marmoreus for commercial production typically originated from Japan, where it is the second most popular mushroom [2]. Although there are some local varieties in the Asian countries including Korea, Taiwan, and China [2], none of them are competent to the Japanese strains in terms of cultivation characteristics and production yield. Therefore development of new strains of H. marmoreus would be valuable in promoting further growth of the mushroom industry.
Mushroom breeding involves various methods including mycelial mating, protoplast fusion, and molecular genetic transformation. Mating of monokaryotic mycelia by hyphal fusion is a typical method to generate new dikaryotic strains. Strains of Pleurotus ostreatus adapted to warm environment were developed recently using this method [3]. Protoplast fusion is an especially efficient method to generate a novel mushroom from two different species. Patra et al. [4] created a hybrid mushroom that produces an immunoactive polysaccharide by the protoplast fusion between P. florida and Volvariella volvacea. Similarly, hybrid mushrooms producing anti-thrombin agents were developed by the protoplast fusion between Laetiporus sulphureus and H. marmoreus [5]. In this case, L. sulphureus was the producer of anti-thrombin agents, but was hard to grow. This limitation was overcome by generating fusion strains with H. marmoreus whose cultivation was well-established. Molecular breeding by gene transformation has been attempted to develop a new strain with a specific property. Particularly, the genes for biodegradative enzymes are frequent targets for genetic transformation of mushrooms [6, 7]. Restriction enzymemediated integration [8] and Agrobacterium tumefaciens-mediated transformation are some available techniques [9]. In the breeding of edible mushrooms, genetic transformation is not allowed because of safety issues. Generation of new strain largely relies on traditional mycelial mating.
Mating of a tetrapolar mushroom, which forms four kinds of haploid basidiospores, is regulated by the mating type genes in two genomic loci. The genes in the mating type locus A control the initial pairing of haploid nuclei, the synchronous division of the nuclear pair, and the development of clamp cells. The mating type locus B controls the reciprocal nuclear exchange and migration [10]. The B locus contains genes for pheromone and the pheromone receptor [10-12]. Monokaryotic mycelia developed from basidiospores mate with each other correct combinations of compatible mating type genes. Therefore, it is crucial to collect basidiospores from diverse parental strains. Chemical mutagenesis of basidiospores is a valuable tool to generate diversity in the monokaryotic mycelia without the necessity of collecting wild mushrooms. Liu et al. [13] recently generated a cold tolerant strain of V. volvacea by random mutagenesis using the alkylating mutagen ethylmethyl sulfonate (EMS). Accordingly, in this study, we report the generation of new strains of H. marmoreus by chemical mutagenesis of basidiospores and subsequent mycelial mating.
Materials and Methods
Strains and culture conditions
Basidiospores of H. marmoreus Hm3-10, a wild strain collected from Deog-Yu mountain, Korea, were from Greenpeace Mushroom Research Institute. The parental and the mutant mycelia were maintained on mushroom complete media by periodic transfer. For liquid culture, five agar blocks (1 cm in diameter) from a potato dextrose agar (PDA) plate were inoculated into a potato dextrose broth (PDB; Ventech Bio, Seoul, Korea) containing potato starch (4 g/L) and glucose (20 g/L) and incubated for 3 wk at 25℃.
Chemical mutagenesis of basidiospores
Basidiospores were suspended in 1 mL PDB. To loosen the coat structure of spore, the suspension was incubated at 25℃ for 12 hr and the spores were collected by centrifugation (5 min, 10,000 rpm). The collected spores were washed with deionized water and suspended in 1 mL of deionized water. The concentration of spores was adjusted to 1.0 × 107 spores/mL. For the chemical mutagenesis, the alkylating agent methyl methanesulfonic acid (MMS, Sigma-Aldrich, St. Louis, MO, USA) was applied at various concentrations to the spore suspensions for 1 hr at 25℃. MMS-treated spores were collected and washed three times with phosphate buffered saline. The spores were resuspended in deionized water and the suspension was spread on PDA. The agar plate was incubated at 25℃ for 10 days for mycelial growth.
Screening of mutant monokaryotic mycelia and mating
Mutant monokaryotic mycelia were selected based on the mycelia growth rate. The growth rate was determined by the radial growth length of monokaryotic mycelia on PDA. Mating was conducted by placing mycelial blocks (3 × 3 mm) from opposite strains on the same PDA plate 1 cm apart. Mating was confirmed by the formation of clamp connections under the light microscope after incubation at 30℃ for 7 days.
Cultivation of the mutant dikaryons
For the cultivation, the mycelia spawn from liquid culture was inoculated onto a solid substrate consisting of pine sawdust (23%), corncob (32%), rice bran (32%), and soybean hull (22%) in a wide-mouth polypropylene bottle at 20℃. The mycelia were developed in the substrate bottle for 70 days at 18℃. The fruiting was induced by shifting the cultivation temperature to 15℃ in an incubating room with 3,000~4,000 ppm CO2 and 95% relative humidity.
Results and Discussion
Effect of MMS on the survival of basidiospore
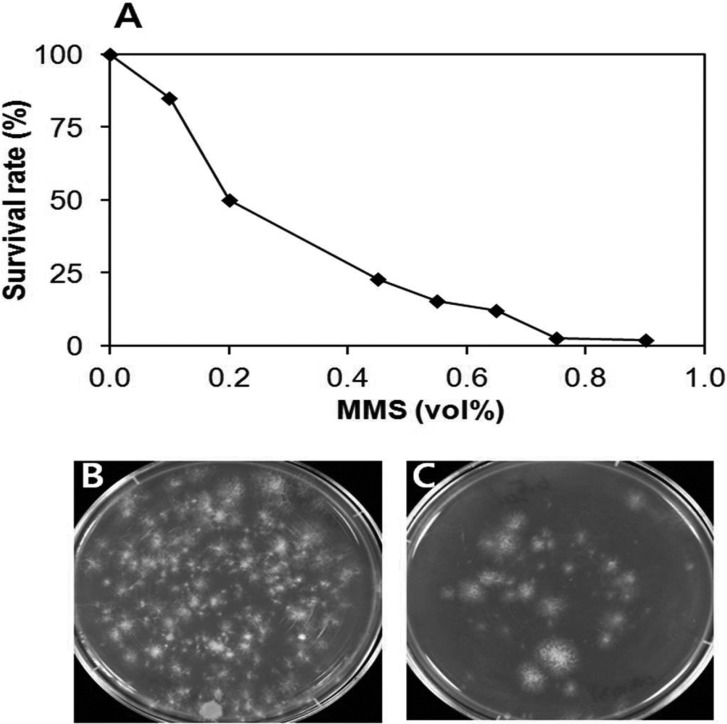
MMS is an alkylating agent that methylates the N7 and N3 positions of guanine and adenine bases, respectively. It causes mispairing of bases and stalls the replication forks to induce the base excision repair system and the expression of DNA alkyltransferase [14, 15]. To generate mutant monokaryotic mycelia, basidiospores of H. marmoreus Hm3-10 were treated with different concentrations of MMS. The survival rate decreased with the increase of MMS concentration (Fig. 1). The rate reached 12% at 0.65 vol% of MMS. This rate was less than half of the survival rate (33%) produced by the similar alkylating agent EMS at 0.75 vol% on basidiospores of V. volvacea [13]. The difference may come from the differing susceptibility of spores to the alkylating agents. The MMS concentration was set to 0.65% for the generation of mutant spores.
Fig. 1.
Effect of methanesulfonate methylester (MMS) concentration on the survival and germination of basidiospores. A, Survival rate of spores at different concentrations of MMS. Basidiospores (1.0 × 105) were treated with MMS for 1 hr at 25℃; B, No treatment; C, 0.65% MMS treatment.
Growth characteristics of mutant monokaryotic mycelia and mating
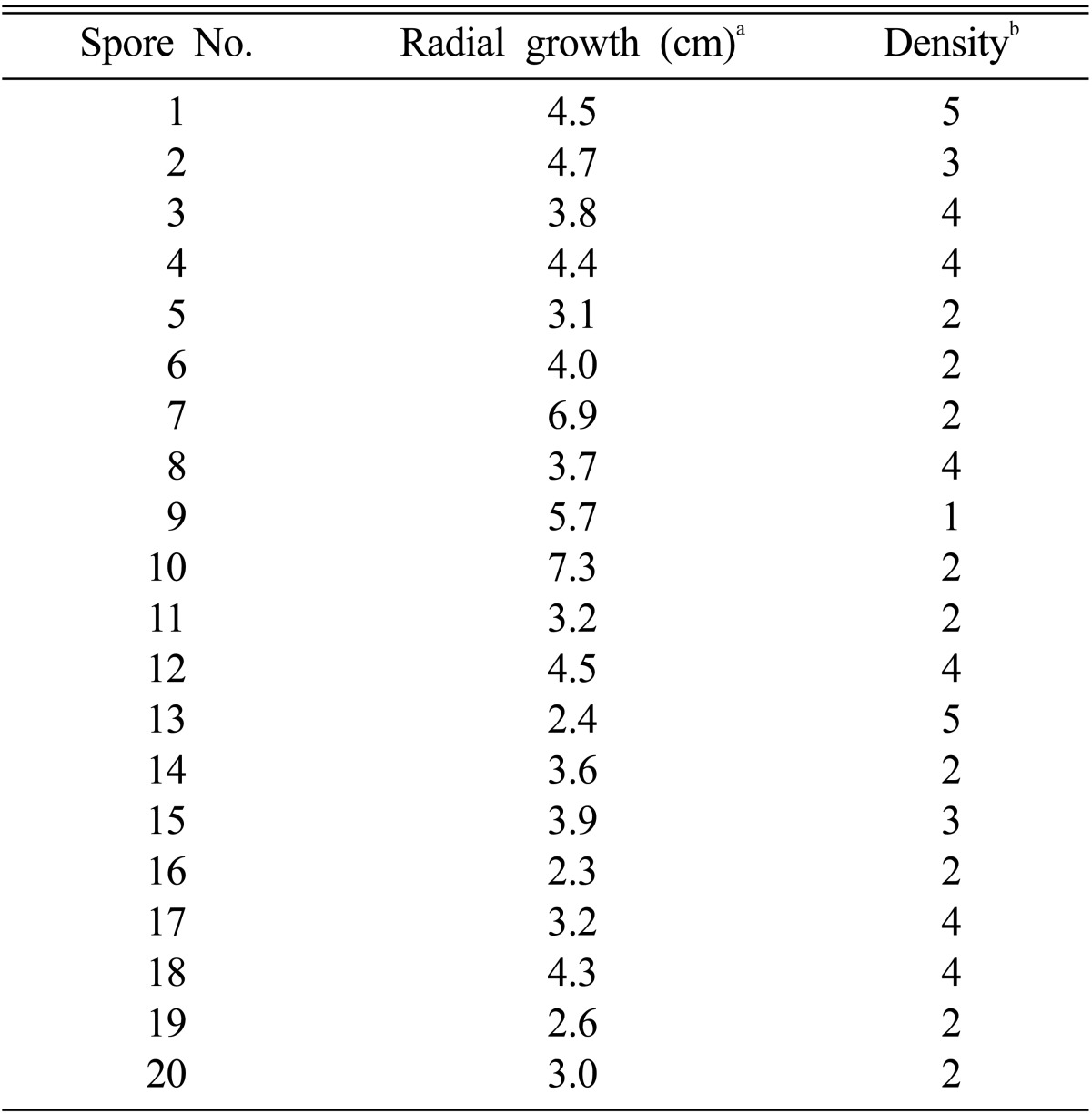
MMS mutagenesis generated a total of 400 mutant monokaryotic mycelia. Twenty fast-growing mycelia were isolated and their growth rate and hyphal density were determined (Table 1). The fastest growing monokaryotic mycelia were No. 7 and No. 10 while No. 13, No. 16, and No. 19 was the slowest growing. The hyphal density of the slow-growing mycelia was generally higher than that of the fast-growing mycelia.
Table 1.
Mutant monokaryotic mycelia selected from methyl methanesulfonic acid mutagenesis
aThe radial growth was measured by the diameter of mycelia after 10 days of incubation at 25℃ on potato dextrose agar.
bThe colony density was judged by the density of mycelial hyphae. The numbers are arbitrary unit, depicting the density of hyphal mycelia from 1 to 5. The number 5 is for the most dense mycelia.
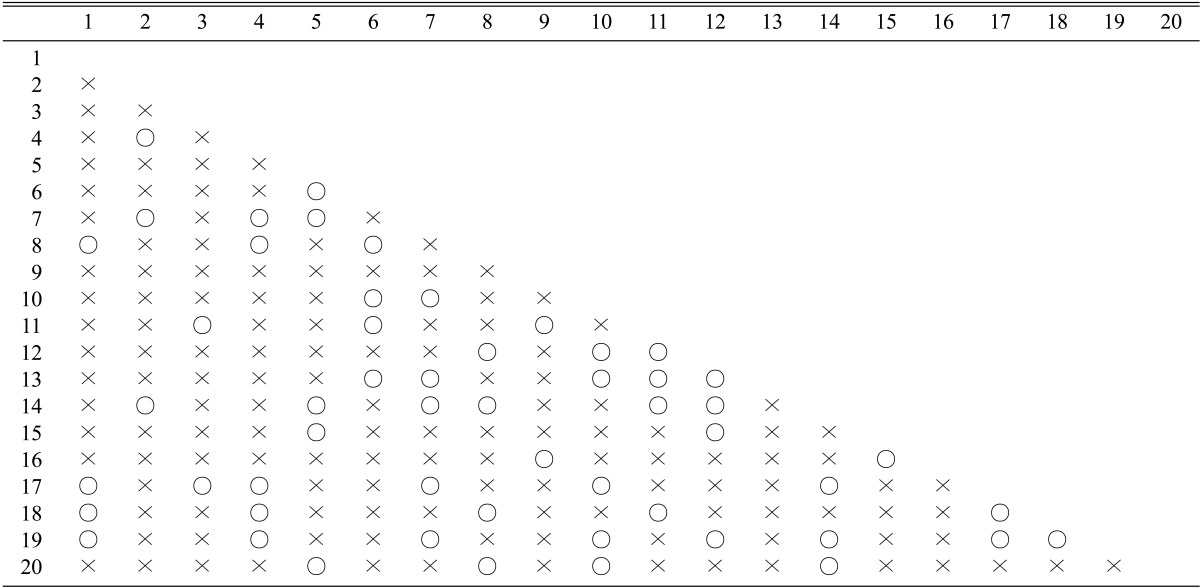
Inbreeding was conducted by the mating of the mutant monokaryons. Each of the 20 monokaryotic mycelia was mated with the remaining 19 monokaryons, resulting in a total of 190 mating. Fifty out of 190 matings were observed to make clamp connections, an indication of successful mating (Table 2). The mating frequency was 26.3%, close to the expected mating frequency in tetrapolar basidiomycetes.
Table 2.
Mating of the mutant monokaryons
Cultivation characteristics of the mutant dikaryon strains
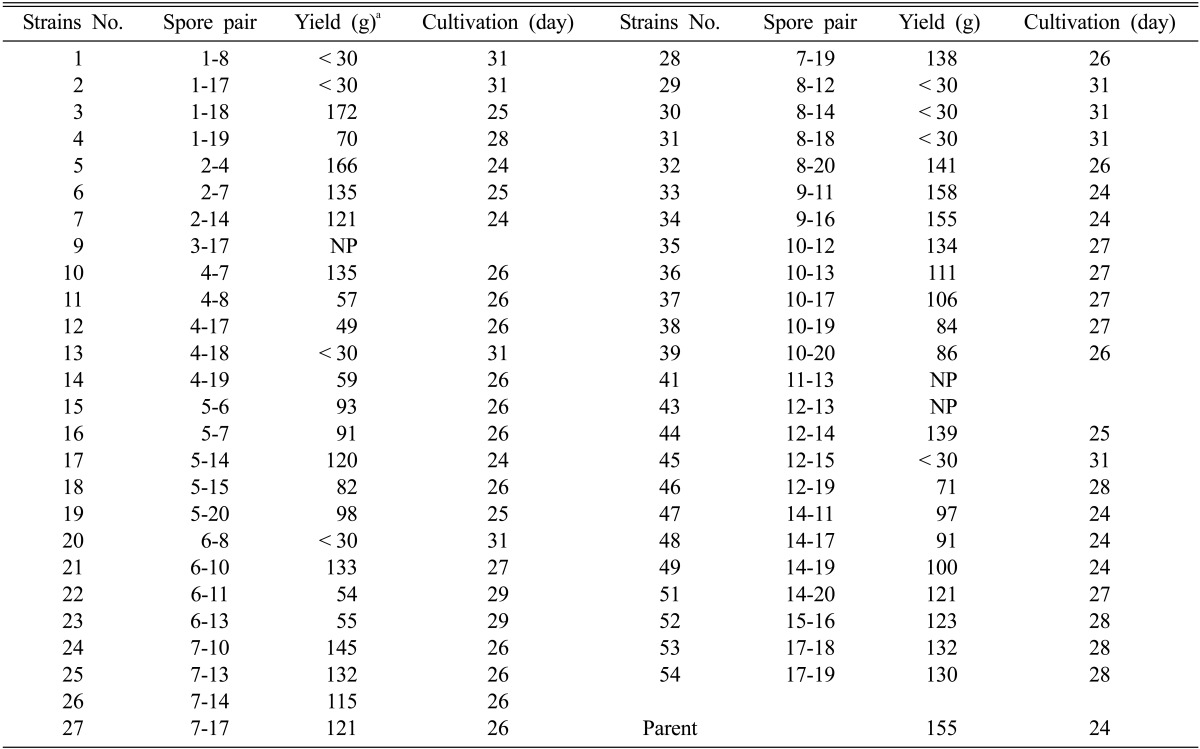
The mated dikaryotic mycelia were cultivated on the solid substrate in polypropylene bottles. For the cultivation of the mutant strains, the liquid spawns grown in PDB were inoculated into the solid substrate. The solid media were incubated for 70 days at 18℃ for the development of mycelia. Some slow growing mycelia including strains Nos. 2, 11, 12, 14, 15, and many others did not fully grow in this condition (data not shown). After 70 days, the formation of primodia was induced by removing the mycelia on top of the bottle. The bottles were transferred to the cultivation room and incubated for an additional 24~28 days for fruiting body formation. The results are summarized in Table 3. Many of the mutant dikaryons failed to form primordia (Nos. 9, 41, and 43) or exhibited delayed and reduced primordia formation (Nos. 1, 2, 13, 20, 29~31, and 45). The cultivation period for the parental strain Hm3-10 untill harvest took 24 days in the cultivation room. The fruiting body had an umbonate pileus with a light brown color. The production yield was 155 g per bottle. The mutant dikaryon strains Nos. 3, 5, 33, and 34 yielded comparable results to the parental strain with respect to the production yield. The pileus of strain No. 3 was dark brown and convex with a production yield of 172 g/bottle. Strains 5, 33, and 34 were dark brown or light grey flats (data not shown) with slightly reduced production yields than the parental strain.
Table 3.
Cultivation characteristics of the mutant dikaryons
NP: no primordia.
aAverage weight of mushroom fruiting bodies from 4 independent experiments with the standard error of ± 3 g.
Mutants with abnormal morphologies
Most of the mutants exhibited some prominent characteristics in the morphology of fruiting bodies. The mutant strains with delayed and reduced primordia formation (Nos. 1, 2, 13, 20, 29~31, and 45) took more than 31 days for full growth. Moreover, these mutants showed a drastic reduction in the number of fruiting bodies. For example, the number of fruiting bodies for the strain No. 31 was only 15 while the parental strain had 65 (Fig. 2A and 2D for the parental strain and Fig. 2B and 2E for the mutants). The reasons for the delayed fruiting and the reduced number of fruiting bodies are not known. The second most frequent phenotype among the mutants was the flattened pileus and stipe morphology (Fig. 2C and 2F). Mutant strains Nos. 44, 47, 48, and 49 showed this phenotype. The abnormality was observed at the early stage of fruiting. The early stage fruiting bodies of the mutant strain No. 44 emerged as flattened, while the parental strain displayed a shape similar to a bowling pin (Fig. 3A and 3D). The flattened morphology became evident following growth to a few days before harvest (Fig. 3B and 3E). Most of the fruiting bodies had a short flat stipe with elongated pileus on top (Fig. 3C and 3F). This phenotype only came from the dikaryons mated with the monokaryotic mycelia derived from the mutant basidospore No. 14, which showed a moderate mycelial growth with low hyphal density (Table 1). The mutant dikaryons Nos. 44, 47, 48, and 49 were the mates of the spore No. 14 with the spores Nos. 11, 14, 17, and 19, respectively (Table 3). Other mates with No. 14, including the dikaryons Nos. 7, 17, 26, and 30, did not show such morphology, except for lowered production yield (Table 3). Therefore, it is conceivable that the abnormality may reside within the chromosomes of spore No. 14. But, the abnormality is expressed only when it meets with a certain partner that can induce the phenotype.
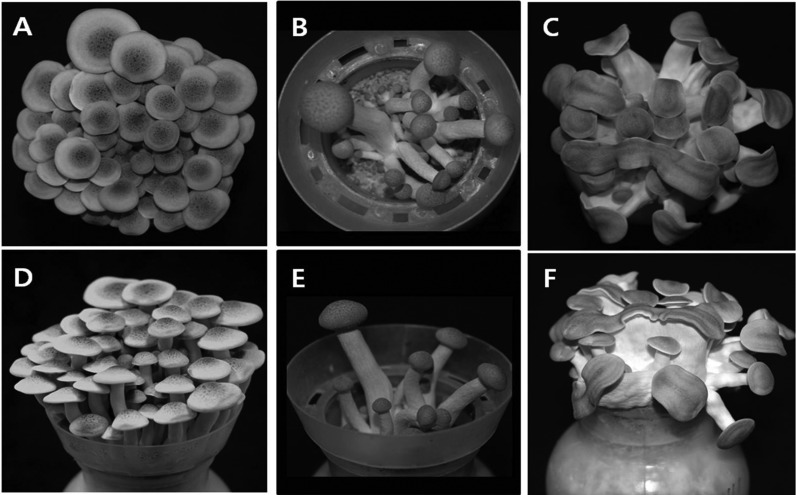
Fig. 2.
Morphologies of selected mutant fruiting bodies. A, D, Parental strain Hm3-10; B, E, Mutant strain No. 31; C, F, Mutant strain No. 44.
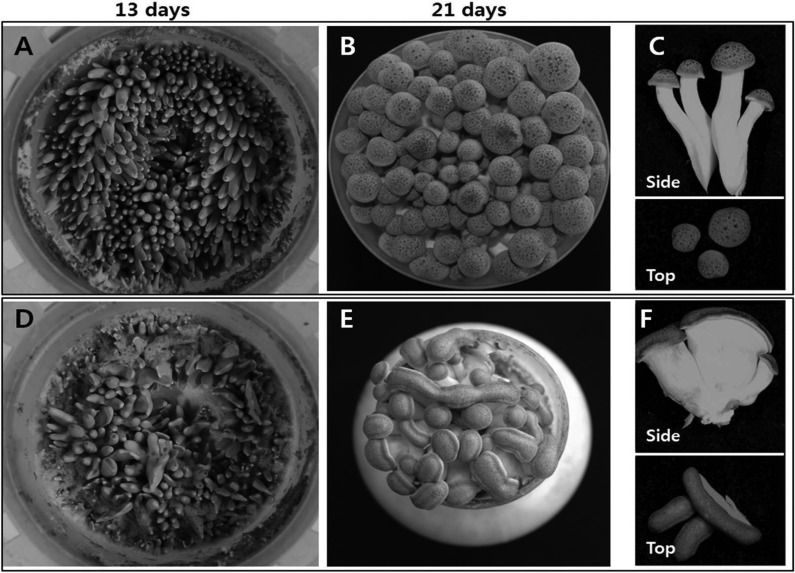
Fig. 3.
Growth characteristics of the parental strain (A~C) and the mutant strain No. 44 (D~F). Pictures were taken after 13 days (A, D) and 21 days (B, E) post the induction of primordial. Fruiting bodies of post-harvest samples are shown in (C) for parental strain and (F) for the mutant strain.
In conclusion, this work shows that some mutant dikaryons generated by the chemical mutagenesis exceed the parental cultivation characteristics and so are worthy of further investigation. The various irregular morphologies generated by the chemical mutagenesis will be useful resources for molecular genetic studies.
Acknowledgements
This work was supported by Mushroom Export Research Program, Ministry of Agriculture and Forestry, Republic of Korea.
References
- 1.Lee CY, Park JE, Kim BB, Kim SM, Ro HS. Determination of mineral components in the cultivation substrates of edible mushrooms and their uptake into fruiting bodies. Mycobiology. 2009;37:109–113. doi: 10.4489/MYCO.2009.37.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YJ, Lee CY, Park JE, Kim SW, Lee HS, Ro HS. Molecular genetic classification of Hypsizigus marmoreus and development of strain-specific markers. Korean J Mycol. 2010;38:34–39. [Google Scholar]
- 3.Gaitán-Hernández R, Salmones D. Obtaining and characterizing Pleurotus ostreatus strains for commercial cultivation under warm environmental conditions. Sci Hortic. 2008;118:106–110. [Google Scholar]
- 4.Patra S, Maity KK, Bhunia SK, Dey B, Mandal S, Maiti TK, Sikdar SR, Islam SS. Structural characterization and study of immunoenhancing properties of heteroglycan isolated from a somatic hybrid mushroom (PfloVv1aFB) of Pleurotus florida and Volvariella volvacea. Carbohydr Res. 2011;346:1967–1972. doi: 10.1016/j.carres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Okamura T, Takeno T, Dohi M, Yasumasa I, Hayashi T, Toyoda M, Noda H, Fukuda S, Horie N, Ohsugi M. Development of mushrooms for thrombosis prevention by protoplast fusion. J Biosci Bioeng. 2000;89:474–478. doi: 10.1016/s1389-1723(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 6.Irie T, Honda Y, Watanabe T, Kuwahara M. Homologous expression of recombinant manganese peroxidase genes in ligninolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol. 2001;55:566–570. doi: 10.1007/s002530000540. [DOI] [PubMed] [Google Scholar]
- 7.Lim H, Choi HT. Enhanced expression of chitinase during the autolysis of mushroom in Coprinellus congregatus. J Microbiol. 2009;47:225–228. doi: 10.1007/s12275-008-0247-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Song J, Choi HT. Genetic transformation and mutant isolation in Ganoderma lucidum by restriction enzymemediated integration. FEMS Microbiol Lett. 2004;233:201–204. doi: 10.1016/j.femsle.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Mikosch TS, Lavrijssen B, Sonnenberg AS, van Griensven LJ. Transformation of the cultivated mushroom Agaricus bisporus (Lange) using T-DNA from Agrobacterium tumefaciens. Curr Genet. 2001;39:35–39. doi: 10.1007/s002940000178. [DOI] [PubMed] [Google Scholar]
- 10.Brown AJ, Casselton LA. Mating in mushrooms: increasing chances but prolonging the affair. Trends Genet. 2001;17:393–400. doi: 10.1016/s0168-9525(01)02343-5. [DOI] [PubMed] [Google Scholar]
- 11.Kronstad JW, Staben C. Mating type in filamentous fungi. Annu Rev Genet. 1997;31:245–276. doi: 10.1146/annurev.genet.31.1.245. [DOI] [PubMed] [Google Scholar]
- 12.van Peer AF, Park SY, Shin PG, Jang KY, Yoo YB, Park YJ, Lee BM, Sung GH, James TY, Kong WS. Comparative genomics of the mating-type loci of the mushroom Flammulina velutipes reveals widespread synteny and recent inversions. PLoS One. 2011;6:e22249. doi: 10.1371/journal.pone.0022249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Zhang K, Lin JF, Guo LQ. Breeding cold tolerance strain by chemical mutagenesis in Volvariella volvacea. Sci Hortic. 2011;130:18–24. [Google Scholar]
- 14.Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 15.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]