Abstract
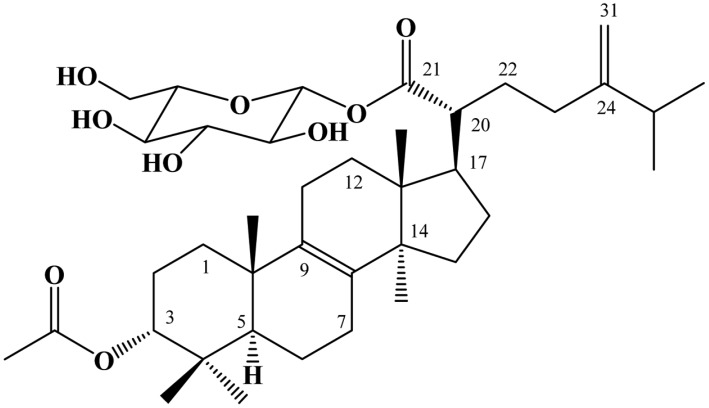
In an effort to identify the chemical constituents of fruiting bodies of Fomitopsis pinicola, a new lanostane triterpene glycoside, designated as fomitoside K, has been isolated from its methanolic extract. Its chemical structure was assigned on the basis of various spectroscopic studies.
Keywords: Fomitopsis nigra, Fomitoside K, Lanostane, Triterpene glycoside
Mushrooms produce a large variety of secondary metabolites with unique chemical structures and interesting biological activities. Fomitopsis, belonging to Polyporaceae, is known to produce various triterpenoids and triterpene glycosides [1-4]. However, metabolites from F. nigra have not yet been reported. In our ongoing effort to identify the chemical constituents of native Korean mushrooms [5-8], a new lanostane triterpene glycoside, designated as fomitoside K (Fig. 1), has been isolated from the methanolic extract of the fruiting body of F. nigra. In this paper, the isolation and structural determination of fomitoside K are described.
Fig. 1.
Structure of fomitoside K.
Following collection of fruiting bodies of F. nigra near Odae mountain, Kangwon province, Korea, they were ground and extracted with methanol at room temperature. Concentration of the methanolic extract was performed under reduced pressure, followed by consecutive partitioning of the aqueous resultant between hexane, chloroform and ethyl acetate, and water. Following concentration of the chloroform-soluble portion under reduced pressure, it was subjected to silica gel column chromatography, and stepwise elution with chloroform : methanol (100 : 1~20 : 1, v/v). Chromatography of a fraction containing high levels of triterpene-class compounds was performed on a column of Sephadex LH-20 eluted with chloroform : methanol (1 : 1, v/v), followed by preparative high performance liquid chromatography (HPLC) equipped with a reversed-phase column and elution with 70% aqueous methanol to afford fomitoside K.
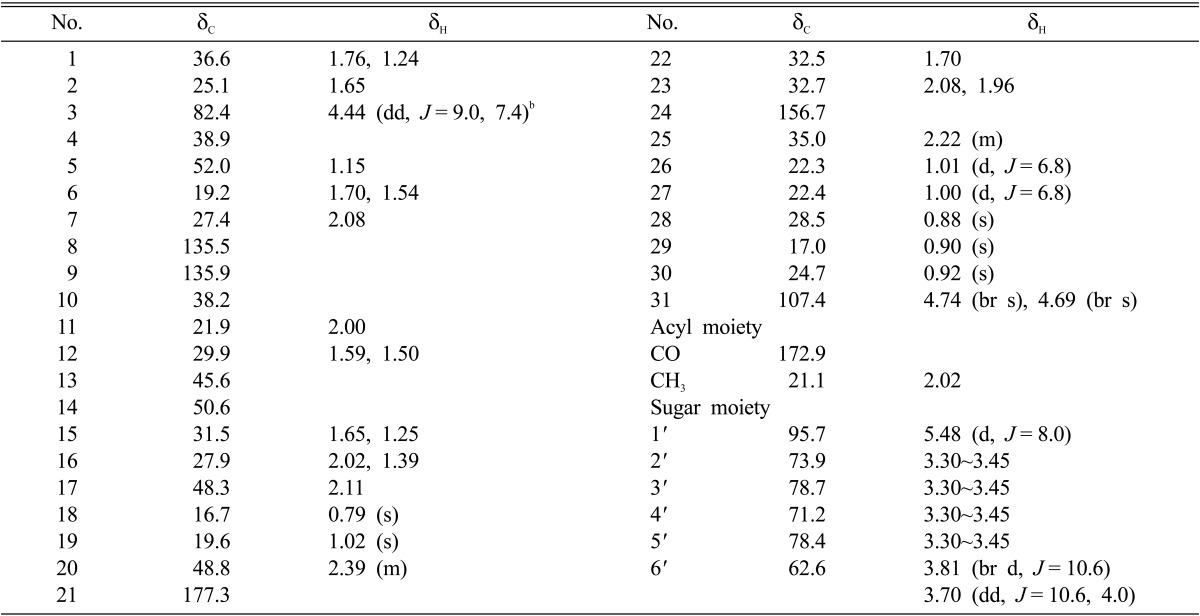
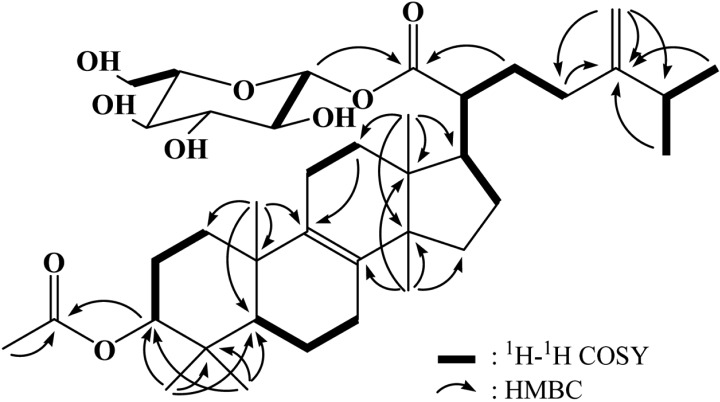
Fast-atom bombardment (FAB)-mass measurement determined that the molecular weight of fomitoside K was 674 and its molecular formula was established as C39H62O9 by high-resolution FAB-mass measurement (m/z 697.4291 [M+Na]+, Δ -0.1 mmu) combined with 1H and 13C NMR data. This molecular formula requires 9 degrees of unsaturation. 1H and 13C NMR spectra suggested that this compound was typical of a triterpene glycoside. In the 1H NMR spectrum, signals from an anomeric proton of sugar at δ 5.48 (d, J = 8.0 Hz), a non-equivalent terminal methylene at δ 4.74 and 4.69, five oxygenated methines at δ 3.3~4.5, an oxygenated methylene at δ 3.81 and 3.70 and six methyls at δ 1.02, 1.01, 1.00, 0.92, 0.90, 0.88 and 0.79 suggested that this compound was a triterpene glycoside of the lanostane skeleton. A total of 39 carbon signals were observed in the 13C NMR spectrum, supporting fomitoside K as a triterpene glycoside having two ester carbonyl carbons, four sp2 carbons, one anomeric carbon, six oxygenated carbons, and 26 other sp3 carbons. Two-dimensional NMR studies, including 1H-1H COSY, heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond coherence (HMBC) experiments, were performed for further elucidation of structure. As shown in Table 1, the proton-bearing carbons were established by the HMQC spectrum, and, as shown in Fig. 2, the 1H-1H COSY spectrum showed seven partial structures. The partial structures were connected by the HMBC spectrum, which exhibited critical long-range correlations from the oxygenated methine proton at δ 4.44 to the carbonyl carbon at δ 172.9 and from the anomeric proton at δ 5.48 and the methylene protons at δ 1.70 to the carbonyl carbon at δ 177.3. As a result of other long-range correlations, we assigned the lanostane skeleton, as shown in Fig. 2. Based on comparison of its carbon chemical shifts with other triterpene glycosides, the sugar was established as glucose [4]. Therefore, the structure of fomitoside K was determined as a new lanostane triterpene glucoside. Many triterpene glycosides, named fomitosides A~J, have previously been isolated from fruiting bodies of Fomitopsis pinicola; fomitoside K was classified as an acetylated form of fomitoside J [4]. Fomitoside K induced apoptosis of human oral squamous carcinoma cells (YD-10B). Details on the biological properties of fomitoside K will be published elsewhere.
Table 1.
1H and 13C NMR spectral data of fomitoside K in CD3ODa
aNMR data were recorded at 400MHz for proton and at 100 MHz for carbon.
bProton resonance multiplicity and coupling constant (J = Hz) are in parentheses.
Fig. 2.
Two-dimensional NMR correlations for fomitoside K. HMBC, heteronuclear multiple bond coherence.
Acknowledgements
This work was supported by a grant from the Korea Forest Service and, in part, by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0005844), Republic of Korea.
References
- 1.Liu XT, Winkler AL, Schwan WR, Volk TJ, Rott M, Monte A. Antibacterial compounds from mushrooms II: lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med. 2010;76:464–466. doi: 10.1055/s-0029-1186227. [DOI] [PubMed] [Google Scholar]
- 2.Petrova A, Popov S, Gjosheva M, Bankova V. A new triterpenic alcohol from Fomitopsis pinicola. Nat Prod Res. 2007;21:401–405. doi: 10.1080/14786410500520251. [DOI] [PubMed] [Google Scholar]
- 3.Keller AC, Maillard MP, Hostettmann K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry. 1996;41:1041–1046. doi: 10.1016/0031-9422(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa K, Inoue M, Matsumoto Y, Sakakibara C, Miyataka H, Matsumoto H, Arihara S. Lanostane triterpenoids and triterpene glycosides from the fruit body of Fomitopsis pinicola and their inhibitory activity against COX-1 and COX-2. J Nat Prod. 2005;68:69–73. doi: 10.1021/np040130b. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Lee IK, Seok SJ, Yun BS. Chemical constituents of the fruiting bodies of Clitocybe nebularis and their antifungal activity. Mycobiology. 2008;36:110–113. doi: 10.4489/MYCO.2008.36.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IK, Lee JH, Yun BS. Polychlorinated compounds with PPAR-gamma agonistic effect from the medicinal fungus Phellinus ribis. Bioorg Med Chem Lett. 2008;18:4566–4568. doi: 10.1016/j.bmcl.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Jang YW, Lee IK, Kim YS, Seok SJ, Yu SH, Yun BS. Chemical constituents of the fruiting body of Xylaria polymorpha. Mycobiology. 2009;37:207–210. doi: 10.4489/MYCO.2009.37.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee IK, Kim SE, Yeom JH, Ki DW, Lee MS, Song JG, Kim YS, Seok SJ, Yun BS. Daldinan A, a novel isoindolinone antioxidant from the ascomycete Daldinia concentrica. J Antibiot (Tokyo) 2012;65:95–97. doi: 10.1038/ja.2011.109. [DOI] [PubMed] [Google Scholar]