Abstract
Fifty three fungi isolated from soils of different microhabitats of eastern Himalayan range (3,400~3,600 msl) were screened for mycosynthesis of silver nanaoparticles (AgNPs) and their efficacy as antimicrobials were assessed in combination with commonly used antibiotics. Three isolates Aspergillus terreus SP5, Paecilomyces lilacinus SF1 and Fusarium sp. MP5 identified based on morphological and 18S rRNA gene sequences were found to synthesize AgNPs. These nanoparticles were characterized by visual observation followed by UV-visible spectrophotometric analysis. The AgNPs synthesized by Aspergillus terreus SP5, Paecilomyces lilacinus SF1 and Fusarium sp. MP5 showed absorbance maxima at 412, 419, and 421 nm respectively in the visible region. Transmission electron microscopy micrograph showed formation of spherical AgNPs of 5~50 nm size. The antimicrobial activity of the mycosynthesized nanoparticles were investigated alone and in combination with commonly used antibiotics for analysis of growth inhibition zone against test organisms, namely, Staphylococcus aureus MTCC96, Streptococcus pyogenes MTCC1925, Salmonella enterica MTCC735 and Enterococcus faecalis MTCC2729. The mycosynthesized nanoparticles showed potent antibacterial activity and interestingly their syngergistic effect with erythromycin, methicillin, chloramphenicol and ciprofloxacin was significantly higher as compared to inhibitions by AgNPs alone. The present study indicates that silver nanoparticles synthesized using soil borne indigenous fungus of high altitudes show considerable antimicrobial activity, deserving further investigation for potential applications.
Keywords: Antibiotics, Antimicrobial activity, Fungal isolates, High altitudes, Silver nanoparticles, Synergy
Introduction
Microorganisms such as bacteria, yeast and fungi play an important role in remediation of toxic metals through reduction of metal ions and are considered as potential nanofactories [1]. Filamentous fungi are ideal candidates for environmental friendly synthesis of metal nanoparticles because these biomasses are easy to handle [2]. North-Eastern region of India in the eastern Himalayan range is one of the biodiversity hotspots [3] and is rich in diverse groups of flora and fauna which has attracted attention of researchers over the years, but the microbial groups have not been explored for their utility and applications. Therefore, there is a need to study, identify and explore the microbial groups for their application in human welfare.
The fungal systems or myconanofactories have been exploited for the synthesis of metal nanoparticles of silver, gold, zirconium, silica, titanium, iron (magnetite) and platinum [4]. Sastry et al. [5] reported the intracellular synthesis of silver nanoparticles (AgNPs) of 2~25 nm within Verticillium sp. with the deposits of the metal bound to the surface of the cytoplasmic membrane. Fungal species such as Fusarium oxysporum can readily synthesize metallic nanoparticles extracellularly using high-levels of secreted proteins and/or enzymes that not only stabilize the particles but allows for an improved yield over an intracellular one [6]. An extracellular synthesis of 60~80 nm silver nanoparticles has been achieved with the fungus Phoma sp. when the fungal cell filtrate was exposed to an aqueous silver nitrate solution at room temperature [7, 8]. Similarly, the soil borne Aspergillus sp. produced AgNPs, both extracellularly [9, 10] and intracellularly [11] after exposure to aqueous silver ions.
It has been shown that AgNPs prepared with a variety of synthetic methods have effective antimicrobial activity [12-17]. AgNPs have been applied to a wide range of healthcare products, such as burn dressings, scaffold, water purification systems, and medical devices [18, 19]. Efficient antibacterial activity of AgNPs produced by the fungus Fusarium acuminatum was observed against multidrug resistant and highly pathogenic bacteria, Staphylococcus aureus, Salmonella typhi, Staphylococcus epidermidis, and Escherichia coli [20]. In the present study, we attempted to analyse antimicrobial activity of AgNPs synthesized using soil borne fungus predominant in soil habitats at high altitudes of eastern Himalayan range and to assess their efficacy singly and in combination with commonly used antibiotics.
Materials and Methods
Isolation of fungal isolate
Surface and sub-surface soil samples were collected aseptically from different microhabitats under high altitudinal climatic zones from 3,400~3,600 msl in the eastern Himalayan range. The soil samples were kept in sterile containers and transported to the laboratory where it was stored at 4℃ until processing. Ten g of the sample was suspended in 90 mL of sterile 0.9% NaCl solution and shaken vigorously on a magnetic stirrer for 20~30 min to obtain uniform suspension. A serial dilution up to 10-5 was made and 0.1 mL aliquot from each dilution was inoculated on potato dextrose agar (PDA) plates and incubated at 25℃ for 48~72 hr. Isolates which appeared morphologically different were selected and purified, and maintained on PDA slant stored at 4℃ until further use.
Biosynthesis of AgNPs
Fifty three fungal isolates were screened for biosynthesis of AgNPs. For the synthesis of silver nanoparticles, the biomass of each fungal isolates were prepared by growing aerobically in potato dextrose broth containing infusion of 250 g potato and 20 g dextrose per litre of distilled water. The inoculated flasks were incubated on orbital shaker at 25 ± 2℃ and agitated at 120 rpm for 96 hr. The biomass was harvested after incubation by filtering followed by repeated washing with distilled water to remove any medium component from the biomass. Ten g (wet weight) was brought in contact with 100 mL of sterilized double distilled water for 48 hr at 25 ± 2℃ in a 250 mL Erlenmeyer flask and agitated again at 120 rpm. The cell filtrate was obtained by filtering it through Whatman filter paper No. 1 (GE Heallthcare, Buckinghamshire, UK). The filtrate was treated with 1 mM AgNO3 solution in an Erlenmeyer flask and incubated at room temperature in dark. Control containing cell-free filtrate without silver nitrate solution was run simultaneously as standard with the experimental flask. All experiments were done in three replicates.
Identification of the fungal isolate
Identification of the selected fungal isolate was carried out by morphological and microscopic observations followed by 18S rRNA gene sequencing. Genomic DNA was extracted using genomic DNA miniprep purification spin kit (Qiagen, Hilden, Germany). The universal 18S rRNA gene primers: forward nu-SSU-0817-5, 5'-TTAGCATGGAATAATRRAATA-3' and reverse nu-SSU-1536-3, 5'-ATTGCAATGCYCTATCCCCA-3' [21] were used for amplification of the 18S rRNA gene. Amplification of DNA was carried out with a 9700 Gold thermal cycler (Applied Biosystems, Warrington, UK) under the following conditions: initial denaturation at 94℃ for 2 min; 35 cycles of denaturation at 94℃ for 0 min, annealing at 56℃ for 10 sec, extension at 72℃ for 30 sec, and a final extension at 72℃ for 2 min. The amplified PCR product was analyzed on an agarose gel and the amplified DNA was purified using QIA quick gel extraction kit (Qiagen) which was subsequently sequenced using BigDye terminator (Applied Biosystems). The resulting sequence was analysed using the BLAST algorithm of National Centre of Biological Information (NCBI) database to obtain closely related phylogenetic sequences. The phylogenetic tree was constructed using Neighbour Joining method in MEGA ver. 4.0 software.
Characterization of AgNPs
UV-visible spectroscopy analysis
Change in colour of the cell free filtrate incubated with silver nitrate solution was visually observed over a period of time. The bio-reduction of precursor silver ions was monitored by sampling of aliquots (1 mL) at different time intervals. Absorption measurements were carried out on UV-visible spectrophotometer (CARY-100 BIO UV-Vis Spectrophotometer; Varian Inc., Palo Alto, CA, USA) at a resolution of 1 nm. UV-Visible analysis of several weeks old samples was also carried out to check the stability of synthesized AgNPs.
Transmission electron microscope (TEM)
For TEM measurements, a drop of synthesized AgNPs was placed on the carbon coated copper grids and kept overnight under vacuum desiccation before loading them onto a specimen holder. TEM micrographs of the sample were taken using the JEOL JSM 100CX TEM instrument (Jeol, Tokyo, Japan) operated at an accelerating voltage of 200 kV.
Antimicrobial activity
The antimicrobial activity of the synthesized AgNPs was assessed against four test microorganisms, viz., S. aureus MTCC96, Streptococcus pyogenes MTCC1925, Salmonella enterica MTCC735, and Enterococcus faecalis MTCC2729. The overnight grown test bacterial cultures were plated on Muller-Hinton agar (MHA). Wells were cut on the plates using cork borer and 50 µL of AgNP solution was dispensed in each well. The mycelia-free water extract alone was used as control. The plates were incubated overnight at 37℃ for 48~72 hr, and observed for the presence of zones of inhibition.
Assay to evaluate synergistic effects
Disk diffusion method, to assay the synergistic effect of extracellularly synthesized AgNPs with commonly used antibiotics, was adopted to test the bactericidal efficacy of these nanoparticles alone and in combination with antibiotics. The standard antibiotic disks were purchased from HiMedia (Mumbai, India). To determine the synergistic effects, each standard antibiotic disk was impregnated with 20 µL of freshly prepared AgNPs and was placed onto the MHA medium inoculated with test organisms. Standard antibiotic disks were used as positive control. Fungal cell-free filtrate was used as negative control. These plates were incubated at 25℃ for 24~48 hr. After incubation, the zones of inhibition for the control and treated plates were measured. All the assays were performed in triplicate.
Assessment of increase in fold area
The increase in fold area was assessed by calculating the mean surface area of the inhibition zone generated by an antibiotic alone and in combination with AgNPs. The fold increase area was calculated by the equation (b2 - a2)/a2, where a and b refer to the zones of inhibition for antibiotic alone and antibiotic with AgNPs respectively [8].
Results
Biosynthesis of AgNPs
Out of the fifty three fungal isolates screened, three isolates SP5, SF1, and MP5 were found to reduce the silver salt into the nanoparticles showing the absorbance peak at 412, 419, and 421 nm, respectively. The surface plasmon resonance was not observed for cell-free filtrates of other four fungal isolates studied during this investigation.
Identification of fungal isolates
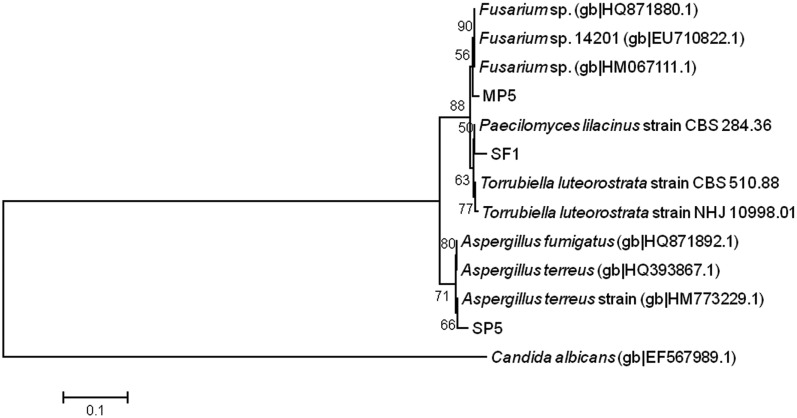
The potent fungi were tentatively identified based on the morphological and microscopical observations which were further confirmed by molecular method as Aspergillus terreus SP5, Fusarium sp. MP5 and Paecilomyces lilacinus SF1. The 18S rRNA gene was amplified and sequenced. The nucleotide sequences obtained were deposited to NCBI GenBank (accession Nos. HQ600978, JN040730, and JN860210). The closest homologues to the sequences were selected and the multiple sequence alignments were carried out using the ClustalW program in the MEGA ver. 4.0 software. Phylogenetic tree was constructed based on the obtained 18S rRNA sequences using the neighbour-joining algorithm with 1,000 bootstrap replications (Fig. 1).
Fig. 1.
Evolutionary positions of the three soil fungal isolates (MP5, SF1, and SP5) based on 18S rDNA sequence similarity.
Characterization of AgNPs
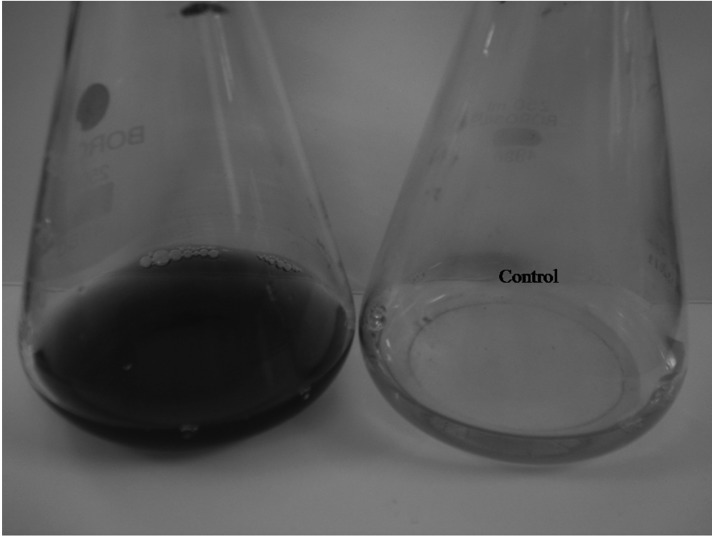
Cell-free filtrate of three fungal isolates exhibited a gradual change to brown colour when each was incubated with silver nitrate salt and maintained under dark conditions. The colour of the culture filtrate with silver nitrate salt changed to intense brown after 24 hr of incubation whereas the control (without silver nitrate salt) did not exhibit any colour change (Figs. 2~4). The colour changes observed can be atributed to the surface plasmon resonance of deposited AgNPs [22].
Fig. 2.
Colour change observed in cell free fungal extract of Aspergillus terreus SP5 after exposure to silver nitrate solution.
Fig. 4.
Colour change observed in cell free fungal extract of Fusarium sp. MP5 after exposure to silver nitrate solution.
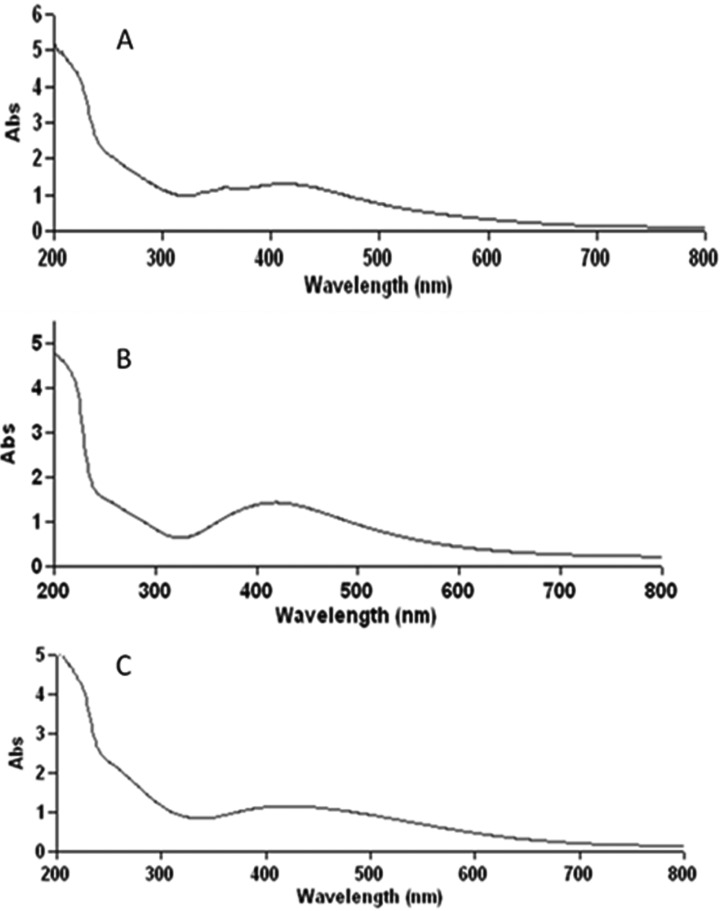
The UV-visible spectra of fungal supernatants (A. terreus SP5, P. lilacinus SF1 and Fusarium sp. MP5) treated with the silver nitrate solutions showed a characteristic surface plasmon absorption band at 412, 419, and 421 nm respectively, and the maximum colour intensity was attained after 72 hr (Fig. 5). Beyond 72 hr of incubation, no further increase in intensity was recorded indicating complete reduction of silver ions by the fungal culture filtrate. Apart from this, an absorption peak was also observed in the UV region corresponding to 280 nm indicating presence of amino acid residues.
Fig. 5.
UV-visible absorption spectra obtained for silver nanoparticles synthesized by Aspergillus terreus SP5 (A), Paecilomyces lilacinus SF1 (B), and Fusarium sp. MP5 (C).
Stability of synthesized AgNPs was monitored regularly for about 3 months. It was observed that the nanoparticles solution was extremely stable at room temperature, with no evidence of flocculation of particles as determined by UV-visible spectroscopy measurements. This indicated that the nanoparticles were well dispersed in the solution without aggregation.
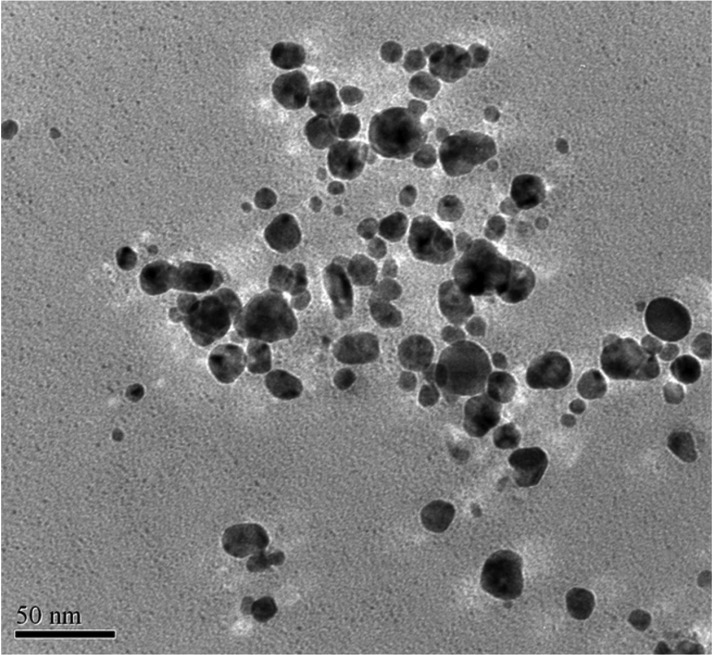
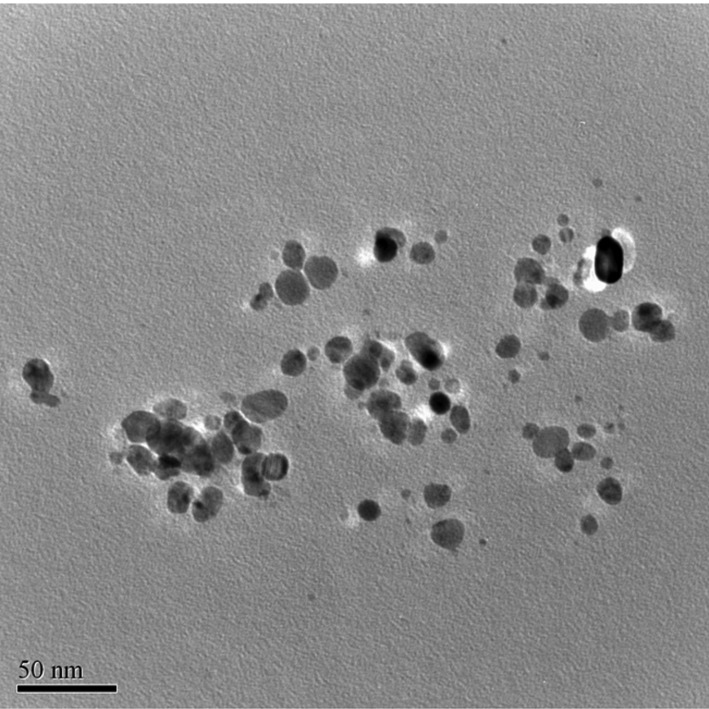
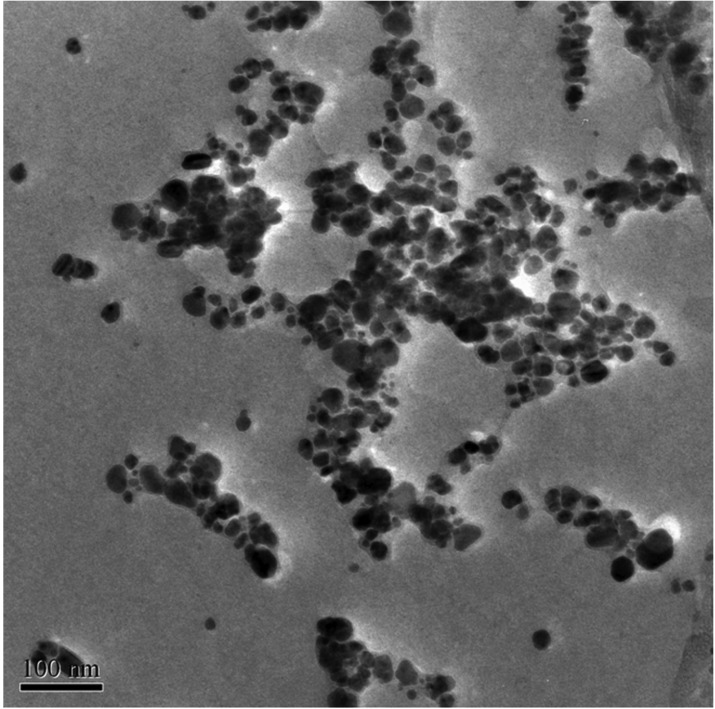
TEM provided further insight into the morphology and particle size distribution profile of the AgNPs and revealed pattern similar to the biosynthesized AgNPs characterized using TEM [23, 24]. The data obtained from transmission electron-micrograph showed distinct shape and size of nanoparticles. The particles were spherical in shape in the range of 5~50 nm and uniformly distributed without significant agglomeration (Figs. 6~8).
Fig. 6.
Transmission electron micrograph of the silver nanoparticles synthesized by Aspergillus terreus SP5.
Fig. 8.
Transmission electron micrograph of the silver nanoparticles synthesized by Fusarium sp. MP5.
Antimicrobial activity
The mycosynthesized AgNPs were tested against S. aureus MTCC96, S. pyogenes MTCC1925, S. enterica MTCC735 and Enterococcus faecalis MTCC2729 for the antimicrobial efficacy which resulted in formation of varying zone of inhibitions. No inhibition zones were visible in the cell-free water extract alone (Table 1). Similar observations were made by Maliszewska and Puzio [25] with AgNPs synthesized by Penicillium sp.
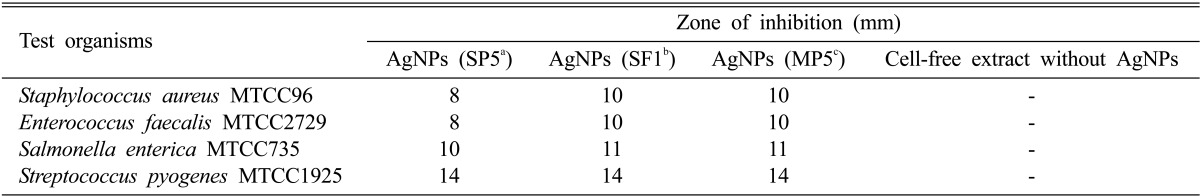
Table 1.
Antimicrobial activity observed as zones of inhibition (mm) produced by mycosynthesized silver nanoparticles (AgNPs) against the test organisms as compared to activity of fungal cell free extract
aSP5: Aspergillus terreus.
bSF1: Paecilomyces lilacinus.
cMP5: Fusarium sp.
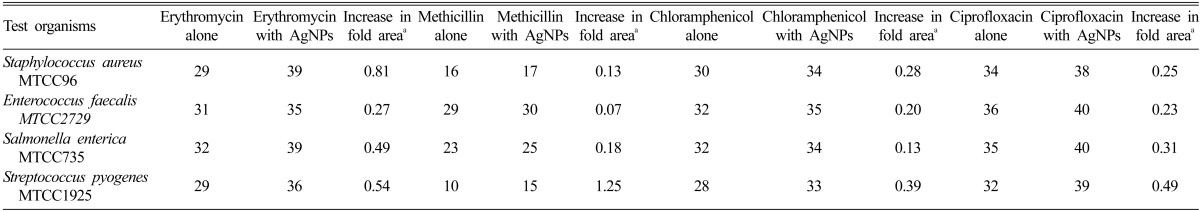
In the present study, effect of AgNPs alone and in combination with commonly used antibiotics was studied against four human pathogenic bacteria using the disc diffusion method. The diameter of inhibition zones for antibiotics alone and in combination with AgNPs showed significant increase in fold area in all the cases with erythromycin, methicillin, chloramphenicol and ciprofloxacin (Tables 2~4). The synergistic activity of AgNPs with antibiotics were found to be higher against S. aureus and S. pyogenes as compared to Enterococcus faecalis and S. enterica.
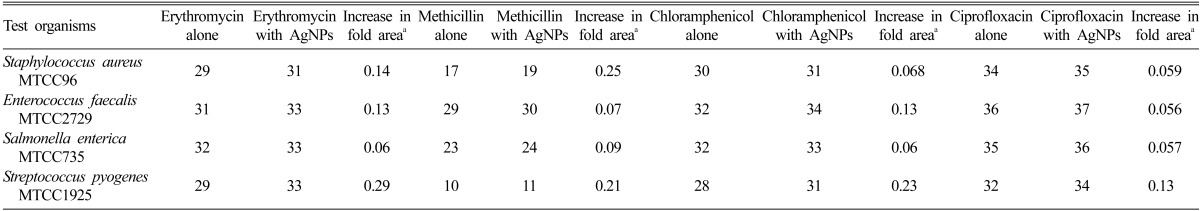
Table 2.
Mean zone of inhibition (mm) produced by different antibiotics without/with silver nanoparticles (AgNPs) synthesized using the fungal isolate SF1 against the test organisms
aIncrease in fold area of inhibition zones were calculated by considering the inhibition zone generated by antibiotics alone and inhibition zones recorded for antibiotics in combination with AgNPs synthesized using the fungal isolate.
Table 4.
Mean zone of inhibition (mm) produced by different antibiotics without/with silver nanoparticles (AgNPs) synthesized using the fungal isolate MP5 against the test organisms
aIncrease in fold area of inhibition zones were calculated by considering the inhibition zone generated by antibiotics alone and inhibition zones recorded for antibiotics in combination with AgNPs synthesized using the fungal isolate.
Discussion
Fungal cell filtrate treated with silver nitrate solution (1 mM) are known to show sharp peak at around 420 nm with high absorbance [20] which supports our finding of the peaks observed for absorbance at 412, 419, and 421 nm indicating the synthesis of nanoparticles by the selected fungal isolates characterized from soils of high altitudes. The absorption at 280 nm indicates the presence of tryptophan and tyrosine residues present in the protein [9]. This observation indicates the release of proteins into filtrate that suggests possible mechanisms for the reduction of silver ions present in the solution. The reduction of the Ag+ ions occur due to reductases released by the fungus into the solution. TEM provided further insight into the morphology and particle size distribution profile of the AgNPs and revealed pattern similar to the biosynthesized AgNPs previously characterized using TEM [23, 24].
Silver is known to have broad-spectrum antimicrobial activity against bacteria, viruses and eukaryotic microorganisms [26, 27]. Kim et al. [28] reported effective antimicrobial activity of AgNPs against E. coli and S. aureus. Our findings of synergistic effects corroborate with the report of Shahverdi et al. [29] who reported the increase in antibacterial activities of penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin in combination with the mycosyhthesized AgNPs against E. coli, Pseudomonas aeruginosa and S. aureus. Fayaz et al. [30] also reported the increase in the antibacterial activities of ampicillin, kanamycin, erythromycin, and chloramphenicol in combination with AgNPs against S. typhi, E. coli, S. aureus and Micrococcus luteus.
This study demonstrates that the fungi isolated from soil systems of high altitudinal cold climatic zones in eastern Himalayan range were capable of synthesizing stable AgNPs. The mycosynthesized AgNPs showed potent antimicrobial activity against various pathogenic bacterial strains namely, S. aureus, S. pyogenes, S. enterica and Enterococcus faecalis. However, enhanced antimicrobial activities of commonly used antibiotics were observed in combination with the mycosynthesized AgNPs. Therefore, it can be concluded that AgNPs alone or their formulations in combination with commonly used antibiotics can be used as effective bactericidal agents. There is an ongoing study on the exploration of microhabitats of Northeast India under eastern Himalayan range for novel indigenous fungal isolates which could be potent candidates for efficient and eco-friendly synthesis of stable antimicrobial AgNPs for pharmaceutical usages.
Fig. 3.
Colour change observed in cell free fungal extract of Paecilomyces lilacinus SF1 after exposure to silver nitrate solution.
Fig. 7.
Transmission electron micrograph of the silver nanoparticles synthesized by Paecilomyces lilacinus SF1.
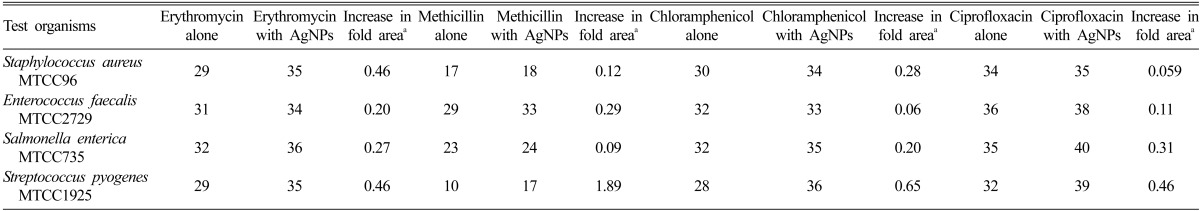
Table 3.
Mean zone of inhibition (mm) produced by different antibiotics without/with silver nanoparticles (AgNPs) synthesized using the fungal isolate SP5 against the test organisms
aIncrease in fold area of inhibition zones were calculated by considering the inhibition zone generated by antibiotics alone and inhibition zones recorded for antibiotics in combination with AgNPs synthesized using the fungal isolate.
Acknowledgements
Authors acknowledge the financial support received from Department of Information Technology (MC & IT), Govt. of India to carry out the present study.
References
- 1.Fortin D, Beveridge TJ. Mechanistic routes towards biomineral surface development. In: Bäuerlein E, editor. Biomineralisation: from biology to biotechnology and medical application. Verlag: Wiley-VCH; 2000. pp. 7–24. [Google Scholar]
- 2.Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajayakumar PV, Alam M, Kumar R, et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1:515–519. [Google Scholar]
- 3.Myers N, Mittermeier RA, Mittermeier CG, de Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 4.Gade AK, Ingle A, Whiteley C, Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. 2010;32:593–600. doi: 10.1007/s10529-009-0197-9. [DOI] [PubMed] [Google Scholar]
- 5.Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170. [Google Scholar]
- 6.Riddin TL, Gericke M, Whiteley CG. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology. 2006;17:3482–3489. doi: 10.1088/0957-4484/17/14/021. [DOI] [PubMed] [Google Scholar]
- 7.Chen JC, Lin ZH, Ma XX. Evidence of the production of silver nanoparticles via pretreatment of Phoma sp. 3.2883 with silver nitrate. Lett Appl Microbiol. 2003;37:105–108. doi: 10.1046/j.1472-765x.2003.01348.x. [DOI] [PubMed] [Google Scholar]
- 8.Birla S, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48:173–179. doi: 10.1111/j.1472-765X.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- 9.Bhainsa KC, D'Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces. 2006;47:160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Gade AK, Bonde P, Ingle AP, Marcato PD, Durán N, Rai MK. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2008;2:243–247. [Google Scholar]
- 11.Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418. [Google Scholar]
- 12.Aymonier C, Schlotterbeck U, Antonietti L, Zacharias P, Thomann R, Tiller JC, Mecking S. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem Commun (Camb) 2002;24:3018–3019. doi: 10.1039/b208575e. [DOI] [PubMed] [Google Scholar]
- 13.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5:244–249. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 15.Melaiye A, Sun Z, Hindi K, Milsted A, Ely D, Reneker DH, Tessier CA, Youngs WJ. Silver(I)-imidazole cyclophane gemdiol complexes encapsulated by electrospun tecophilic nanofibers: formation of nanosilver particles and antimicrobial activity. J Am Chem Soc. 2005;127:2285–2291. doi: 10.1021/ja040226s. [DOI] [PubMed] [Google Scholar]
- 16.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38:2199–2204. [Google Scholar]
- 18.Kim S, Kim HJ. Anti-bacterial performance of colloidal silver-treated laminate wood flooring. Int Biodeterior Biodegrad. 2006;57:155–162. [Google Scholar]
- 19.Thomas V, Yallapu MM, Sreedhar B, Bajpai SK. A versatile strategy to fabricate hydrogel-silver nanocomposites and investigation of their antimicrobial activity. J Colloid Interface Sci. 2007;315:389–395. doi: 10.1016/j.jcis.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 20.Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci. 2008;4:141–144. [Google Scholar]
- 21.Borneman J, Hartin RJ. PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol. 2000;66:4356–4360. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788–800. [Google Scholar]
- 23.Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces. 2009;71:133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Jain N, Bhargava A, Majumdar S, Tarafdar JC, Panwar J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale. 2011;3:635–641. doi: 10.1039/c0nr00656d. [DOI] [PubMed] [Google Scholar]
- 25.Maliszewska I, Puzio M. Extracellular biosynthesis and antimicrobial activity of silver nanoparticles. Acta Phys Pol A. 2009;116(Suppl):S160–S162. [Google Scholar]
- 26.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]