Abstract
While observational studies have suggested that vitamin D deficiency increases risk of depression, few clinical trials have tested whether vitamin D supplementation affects the occurrence of depression symptoms. The authors evaluated the impact of daily supplementation with 400 IU of vitamin D3 combined with 1,000 mg of elemental calcium on measures of depression in a randomized, double-blinded US trial comprising 36,282 postmenopausal women. The Burnam scale and current use of antidepressant medication were used to assess depressive symptoms at randomization (1995–2000). Two years later, women again reported on their antidepressant use, and 2,263 completed a second Burnam scale. After 2 years, women randomized to receive vitamin D and calcium had an odds ratio for experiencing depressive symptoms (Burnam score ≥0.06) of 1.16 (95% confidence interval: 0.86, 1.56) compared with women in the placebo group. Supplementation was not associated with antidepressant use (odds ratio = 1.01, 95% confidence interval: 0.92, 1.12) or continuous depressive symptom score. Results stratified by baseline vitamin D and calcium intake, solar irradiance, and other factors were similar. The findings do not support a relation between supplementation with 400 IU/day of vitamin D3 along with calcium and depression in older women. Additional trials testing higher doses of vitamin D are needed to determine whether this nutrient may help prevent or treat depression.
Keywords: antidepressive agents, calcium, clinical trial, depression, dietary supplements, postmenopause, vitamin D, women
Ample evidence suggests that vitamin D has important functions in the human brain and may play a role in depression (1–3). Vitamin D receptors are present in multiple brain regions associated with depressive disorders, including the prefrontal cortex and hippocampus (2), and cells in many of these regions are capable of metabolizing 25-hydroxyvitamin D to the biologically active metabolite 1,25-dihydroxyvitamin D (2, 4). Animal studies have suggested that vitamin D may increase the synthesis and/or metabolism of neurotransmitters, including dopamine and norepinephrine, though results have been inconsistent (1, 5, 6).
Relatively few epidemiologic studies have assessed whether vitamin D plays a role in depression (7). In most (but not all) cross-sectional (8–16) and prospective (17, 18) analyses, investigators have observed an inverse relation between blood 25-hydroxyvitamin D levels and prevalent or incident depression. However, these studies were limited by potential confounding by physical activity, body mass index, and other factors that influence 25-hydroxyvitamin D levels (19) and that may be independently associated with depression (20–22).
Randomized trials of vitamin D supplementation and depression are needed to determine whether vitamin D may hold promise for preventing or treating depression. To date, few trials have been conducted (23–30); they have had mixed results, and many have focused on seasonal depression alone. Thus, we evaluated the association between daily supplementation with 400 IU of vitamin D3, along with 1,000 mg of elemental calcium, and the occurrence of depression and antidepressant use in a randomized, double-blinded trial.
MATERIALS AND METHODS
Study design
The Women’s Health Initiative (WHI) Calcium and Vitamin D (CaD) Trial included women previously enrolled in the WHI Dietary Modification (DM) Trial and/or Hormone Therapy (HT) Trial. Establishment of the DM and HT trials has been described previously (31–33). Briefly, between 1993 and 1998, postmenopausal women aged 50–79 years were recruited through direct mailing campaigns and media awareness programs. Recruitment was conducted at 40 clinical centers throughout the United States. Major ineligibility criteria included a history of cancer (other than nonmelanoma skin cancer) within the previous 10 years, medical conditions likely to result in death within 3 years, and conditions likely to interfere with study retention. Women were also excluded if they had a history of hypercalcemia, kidney stones, or corticosteroid or calcitriol use.
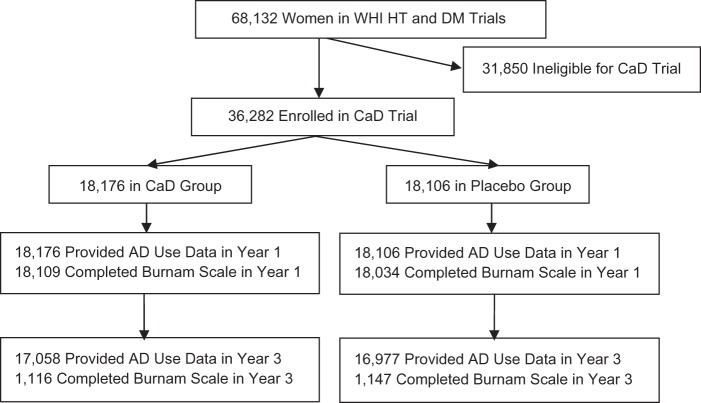
DM and HT trial participants were invited to join the CaD Trial at their first or second annual follow-up clinic visit (Figure 1). The CaD Trial was designed to test calcium and vitamin D supplementation for the prevention of fracture and colorectal cancer, and has been described in detail previously (34). Of 68,132 women enrolled in the HT Trial or the DM Trial, 36,282 women were eligible and willing to participate in the CaD Trial. More than 95% of participants joining the CaD Trial did so at their first follow-up visit (hereafter referred to as “year 1”).
Figure 1.
Derivation of the cohort for analysis of the relation between vitamin D and calcium supplementation and depressive symptoms at year 3, Women’s Health Initiative (WHI) Calcium and Vitamin D (CaD) Trial, 1995–2000. While all participants were invited to complete the Burnam scale (37) at year 1, only a subset were invited to complete the Burnam scale at year 3. (AD, antidepressant; DM, Dietary Modification; HT, Hormone Therapy).
Women were randomized to receive a calcium-and-vitamin D supplement or an identical-appearing placebo using a permuted block algorithm. The total daily dose was 1,000 mg of elemental calcium (as calcium carbonate) and 400 IU of vitamin D3, given in 2 divided doses. Women were allowed to continue personal use of calcium and vitamin D supplements, with an initial cutoff for the latter of 600 IU/day, which later was increased to 1,000 IU/day. Supplementation was terminated if women reported kidney stones, kidney dialysis, hypercalcemia, calcitriol use, or personal use of vitamin D supplements at dosages higher than 600 IU/day (later 1,000 IU/day).
Participants were contacted after 4 weeks and then twice per year for assessment of safety, adherence, and clinical outcomes. Adherence was defined as taking 80% or more of study medication. In the first 3 years of follow-up, adherence ranged from 60% to 63% (35). An additional 13%–21% of participants took at least half of their medications. At baseline, the mean 25-hydroxyvitamin D level in a subset of 898 CaD Trial participants was 52.0 nmol/L (standard deviation, 21.1) (35). In a substudy of 448 trial participants, after 2 years 25-hydroxyvitamin D levels were 28% higher in women assigned to receive supplements than in women assigned to receive placebo (36).
The study protocol was approved by institutional review boards at all participating institutions and registered at clinicaltrials.gov. An independent data and safety monitoring board reviewed all clinical outcomes. Supplementation and clinic visits continued until final planned visits were conducted from October 1, 2004, to March 31, 2005. The average duration of participant follow-up was 7.0 years (36).
Study outcome
Among all CaD participants, we assessed the prevalence of depressive symptoms at year 1 with the Burnam 8-item scale for depressive disorders (37). A subset of study participants (n = 2,263) also completed the Burnam scale at their year 3 follow-up visit, which took place approximately 2 years after CaD randomization for most women. The Burnam scale includes 6 items from the Center for Epidemiologic Studies Depression Scale and 2 items from the Diagnostic Interview Schedule. Questions from the Center for Epidemiologic Studies Depression Scale asked women to report how often, in the past week, they had felt depressed (“blue or down”); their sleep had been restless; they had enjoyed life; they had had crying spells; they had felt sad; and they had felt that people disliked them. Response options were: “rarely or none of the time (<1 day)”; “some or a little of the time (1–2 days)”; “occasionally or a moderate amount of time (3–4 days)”; and “most or all of the time (5–7 days).” Questions from the Diagnostic Interview Schedule were: “In the past year, have you had 2 weeks or more during which you felt sad, blue, or depressed, or lost pleasure in things that you usually cared about or enjoyed?”; “Have you had 2 years or more in your life when you felt depressed or sad most days, even if you felt okay sometimes?”; and “If yes, have you felt depressed or sad much of the time in the past year?”.
We calculated the Burnam score using questionnaire responses and a logistic regression-based algorithm (37). Values for this scale range from 0 to 0.99, with higher scores indicating greater depressive symptomatology. For our main analysis, we dichotomized continuous Burnam scores at the standard threshold of 0.06 to identify women experiencing symptoms consistent with depressive disorders, including major depression and dysthymia, as in previous studies in the WHI (38–40). In analyses testing the validity of the Burnam scale in relation to the gold standard of psychiatric interview using the Diagnostic Interview Schedule, the cutoff value of 0.06 was determined to maximize sensitivity, specificity, and positive predictive value in both primary care and general populations (37). As discussed previously (41), this threshold score is not itself a measure of clinically diagnosed depression but is well correlated with clinical depression. An ancillary study evaluating the reliability of the Burnam algorithm versus clinical diagnosis in the WHI found a sensitivity of 74% and a specificity of 87% (42). Furthermore, depressive symptoms assessed with the Burnam scale have been predictive of cardiovascular disease and heart rate variability in the WHI (38, 41).
Among all CaD participants, we assessed use of antidepressant medications as a proxy for previously clinically diagnosed depression at year 1 and again at year 3. We included this outcome to enhance our statistical power, since only a subset of WHI participants were invited to complete the Burnam scale at year 3. Participants were asked to bring all current medications to their clinic visits, including selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, modified cyclic agents, tricyclic agents, and other medications classified as antidepressants. The names of medications used regularly (i.e., for more than 2 weeks) were recorded, along with information on dose and duration of use.
Dietary vitamin D intake and other factors
At an HT or DM enrollment clinic visit (i.e., in “year 0”), participants were asked to complete a semiquantitative food frequency questionnaire designed and validated for use in the WHI (43). Participants reported their usual intake and portion size of 122 foods or food groups in the previous 3 months. Intakes of vitamin D and calcium from food sources were calculated by multiplying the nutrient content of the specified portion size of each food (University of Minnesota Nutrition Coordinating Center nutrient database) by its frequency of consumption and summing the contributions of all foods. At clinic visits in year 0 and year 1, intakes of vitamin D and calcium from supplemental sources were assessed by trained interviewers using a standard questionnaire that measured dose, frequency (pills per week), and duration (months and years) of use of multivitamins, multivitamin-mineral supplements, and single-nutrient supplements (44). Total nutrient intakes were determined by summing intakes from food sources and supplemental sources. In a validation study in the WHI, vitamin D and calcium intakes measured by food frequency questionnaire correlated well with intakes measured with 4 days of diet recalls plus 4 days of food records (for total vitamin D, deattenuated r = 0.73; for total calcium, deattenuated r = 0.78) (43).
At year 0, women completed questionnaires assessing demographic, behavioral, and health-related factors, including age, race/ethnicity, education, previous use of hormone therapy and oral contraceptives, alcohol intake, history of smoking, and participation in physical activity. Weight and height were measured directly and used to calculate body mass index (weight (kg)/height (m)2). Annual level of solar irradiance in Langleys (gram-calories) per cm2 at each clinical center was estimated using measurements from the National Weather Service and adapted for use in the WHI (45). The extent to which health conditions limited physical function was measured with the RAND Short Form 36 (46).
Statistical analysis
We followed intention-to-treat principles for our main analyses. Descriptive statistics are presented with frequencies and percentages. Categorical variables were compared across supplement groups using chi-square statistics, while for continuous variables we used t tests. All P values are 2-sided.
Continuous change in Burnam score (year 3 – year 1) was modeled using continuous linear regression, with change as a function of assigned supplementation group (vitamin D + calcium or placebo). We used logistic regression to calculate odds ratios and 95% confidence intervals for the relation between supplementation and depressive symptoms above the threshold (Burnam score ≥0.06) at year 3 and antidepressant use at year 3. To assess whether vitamin D and calcium supplementation differently affected risk of “new” depression at year 3 among women without evidence of depression at year 1, we repeated these analyses excluding women who at year 1 had had a Burnam score greater than or equal to 0.06 or had reported current antidepressant use.
To determine whether the effect of supplements varied by background level of vitamin D or calcium intake, we conducted a series of logistic regression analyses modeling each outcome as a function of randomized supplementation group, background nutrient intake, and the interaction of the two. Contrasts were used to calculate odds ratios within each subgroup of total vitamin D or calcium intake, and P values for interaction were calculated. In this analysis, we evaluated interactions with vitamin D and calcium intakes from all sources (i.e., total intake), from food sources only, and from supplemental sources only. We used this same approach to evaluate whether other factors such as age, body mass index, or race/ethnicity modified the effect of supplementation on antidepressant use at year 3.
In all models, results were adjusted for age and HT or DM trial treatment assignment. Furthermore, analyses using the dichotomized Burnam scale endpoint were adjusted for this variable at year 1, while analyses of antidepressant use at the year 3 endpoint were adjusted for year 1 antidepressant use.
RESULTS
Characteristics of CaD Trial participants by randomization group are presented in Table 1. The intervention groups did not differ significantly by any factor evaluated except antidepressant use at year 1, which was reported by 6.5% of women assigned to receive supplements and 7.1% of women assigned to receive the placebo (P = 0.03). A depressive symptom score above the threshold value (Burnam score ≥0.06) was reported by 9.4% of women randomized to supplement use and 9.9% randomized to placebo (P = 0.25). Women assigned to supplement use did not differ from those assigned to placebo in terms of total vitamin D or calcium intake or intake of these nutrients from foods or self-administered supplements. Overall, total vitamin D intake was low (mean = 366 IU/day), with 42% of study participants consuming at least 400 IU of vitamin D per day and fewer than 6% consuming at least 800 IU/day.
Table 1.
Characteristics of Participants in the Women’s Health Initiative (WHI) Calcium and Vitamin D Trial at the Time of WHI Screening (i.e., Year 0), by Supplementation Group, 1995–2000
| Characteristic | Supplementation Group | Placebo Group | P Value | ||
| No. | %a | No. | %a | ||
| Age, years | >0.99 | ||||
| 50–59 | 6,726 | 37.0 | 6,696 | 37.0 | |
| 60–69 | 8,276 | 45.5 | 8,243 | 45.5 | |
| 70–79 | 3,174 | 17.5 | 3,167 | 17.5 | |
| Race/ethnicity | 0.45 | ||||
| White | 15,051 | 82.8 | 15,104 | 83.4 | |
| Black | 1,680 | 9.2 | 1,635 | 9.0 | |
| Hispanic | 785 | 4.3 | 717 | 4.0 | |
| American Indian | 77 | 0.4 | 72 | 0.4 | |
| Asian/Pacific Islander | 370 | 2.0 | 351 | 1.9 | |
| Annual income, dollars | 0.68 | ||||
| <20,000 | 2,942 | 16.2 | 2,876 | 15.9 | |
| 20,000–49,999 | 8,122 | 44.7 | 8,092 | 44.7 | |
| ≥50,000 | 6,205 | 34.1 | 6,192 | 34.2 | |
| Marital status | 0.96 | ||||
| Never married | 725 | 4.0 | 712 | 3.9 | |
| Divorced/separated | 2,850 | 15.7 | 2,875 | 15.9 | |
| Widowed | 3,004 | 16.5 | 3,008 | 16.6 | |
| Married/living as married | 11,522 | 63.4 | 11,442 | 63.2 | |
| Regional solar irradiance, Langleys | >0.99 | ||||
| 475–500 | 3,861 | 21.2 | 3,852 | 21.3 | |
| 400–430 | 3,016 | 16.6 | 3,012 | 16.7 | |
| 75–380 | 2,009 | 11.1 | 2,009 | 11.1 | |
| 350 | 3,924 | 21.6 | 3,879 | 21.4 | |
| 300–325 | 5,366 | 29.5 | 5,354 | 29.6 | |
| Smoking status | 0.50 | ||||
| Never smoker | 9,325 | 51.3 | 9,428 | 52.1 | |
| Past smoker | 7,255 | 39.9 | 7,133 | 39.4 | |
| Current smoker | 1,405 | 7.7 | 1,356 | 7.5 | |
| Alcohol intake, drinks/day | 0.63 | ||||
| 0 | 5,055 | 27.8 | 5,100 | 28.2 | |
| >0–<1 | 11,065 | 60.9 | 10,977 | 60.6 | |
| ≥1 | 1,908 | 10.5 | 1,900 | 10.5 | |
| Hormone useb | 0.24 | ||||
| Never use | 5,810 | 32.0 | 5,685 | 31.4 | |
| Past use | 3,007 | 16.5 | 2,937 | 16.2 | |
| Current use | 9,359 | 51.5 | 9,484 | 52.4 | |
| Body mass indexc | 0.17 | ||||
| <25 | 4,745 | 26.1 | 4,834 | 26.7 | |
| 25–<30 | 6,476 | 35.6 | 6,487 | 35.8 | |
| ≥30 | 6,870 | 37.8 | 6,692 | 37.0 | |
| Physical activity, MET-hours/week | 0.60 | ||||
| <3.00 | 5,405 | 29.7 | 5,340 | 29.5 | |
| 3.00–9.99 | 4,642 | 25.5 | 4,648 | 25.7 | |
| 10.00–19.99 | 3,647 | 20.1 | 3,569 | 19.7 | |
| ≥20.00 | 2,852 | 15.7 | 2,891 | 16.0 | |
| Total vitamin D intake, IU/day | 0.32 | ||||
| <100 | 2,750 | 15.1 | 2,653 | 14.7 | |
| 100–<200 | 4,092 | 22.5 | 4,025 | 22.2 | |
| 200–<400 | 3,373 | 18.6 | 3,419 | 18.9 | |
| 400–<600 | 4,180 | 23.0 | 4,293 | 23.7 | |
| ≥600 | 3,426 | 18.8 | 3,363 | 18.6 | |
| Dietary vitamin D intake, IU/day | 0.47 | ||||
| <100 | 4,893 | 26.9 | 4,747 | 26.2 | |
| 100–<200 | 7,434 | 40.9 | 7,496 | 41.4 | |
| 200–<400 | 4,651 | 25.6 | 4,651 | 25.7 | |
| ≥400 | 843 | 4.6 | 859 | 4.7 | |
| Supplemental vitamin D intake, IU/day | 0.41 | ||||
| None | 9,639 | 53.0 | 9,507 | 52.5 | |
| <400 | 1,795 | 9.9 | 1,780 | 9.8 | |
| 400–<800 | 6,339 | 34.9 | 6,401 | 35.4 | |
| ≥800 | 403 | 2.2 | 418 | 2.3 | |
| Meeting IOM guidelines for vitamin D intaked | 0.99 | ||||
| No | 15,379 | 84.6 | 15,430 | 85.2 | |
| Yes | 2,374 | 13.1 | 2,391 | 13.2 | |
| Total calcium intake, mg/day | 0.44 | ||||
| <400 | 1,318 | 7.3 | 1,273 | 7.0 | |
| 400–<800 | 4,790 | 26.4 | 4,732 | 26.1 | |
| 800–<1,000 | 2,514 | 13.8 | 2,459 | 13.6 | |
| 1,000–<1,200 | 2,198 | 12.1 | 2,195 | 12.1 | |
| ≥1,200 | 7,001 | 38.5 | 7,094 | 39.2 | |
| Dietary calcium intake, IU/day | 0.52 | ||||
| <400 | 2,406 | 13.2 | 2,378 | 13.1 | |
| 400–<800 | 7,629 | 42.0 | 7,603 | 42.0 | |
| 800–<1,000 | 2,906 | 16.0 | 2,878 | 15.9 | |
| 1,000–<1,200 | 1,868 | 10.3 | 1,853 | 10.2 | |
| ≥1,200 | 3,012 | 16.6 | 3,041 | 16.8 | |
| Supplemental calcium intake, IU/day | 0.55 | ||||
| None | 8,027 | 44.2 | 7,885 | 43.5 | |
| <400 | 4,289 | 23.6 | 4,295 | 23.7 | |
| 400–<800 | 3,358 | 18.5 | 3,406 | 18.8 | |
| ≥800 | 2,502 | 13.8 | 2,520 | 13.9 | |
| Total saturated fat intake, g/day | 0.79 | ||||
| <15.1 | 4,469 | 24.6 | 4,415 | 24.4 | |
| 15.1–21.4 | 4,463 | 24.6 | 4,479 | 24.7 | |
| 21.5–29.7 | 4,505 | 24.8 | 4,367 | 24.1 | |
| ≥29.8 | 4,384 | 24.1 | 4,492 | 24.8 | |
| Use of antidepressant medicationb | 0.03 | ||||
| No | 16,992 | 93.5 | 16,820 | 92.9 | |
| Yes | 1,184 | 6.5 | 1,286 | 7.1 | |
| Burnam depression scale (37) scoreb | 0.25 | ||||
| <0.06 | 16,401 | 90.2 | 16,244 | 89.7 | |
| ≥0.06 | 1,708 | 9.4 | 1,790 | 9.9 | |
| WHI Hormone Trial assignment | 0.74 | ||||
| Not randomized | 10,122 | 55.7 | 10,071 | 55.6 | |
| Active | 4,039 | 22.2 | 4,078 | 22.5 | |
| Placebo | 4,015 | 22.1 | 3,957 | 21.9 | |
| WHI Dietary Modification Trial assignment | 0.30 | ||||
| Not randomized | 5,582 | 30.7 | 5,490 | 30.3 | |
| Intervention | 4,767 | 26.2 | 4,878 | 26.9 | |
| Comparison | 7,827 | 43.1 | 7,738 | 42.7 | |
Abbreviations: IOM, Institute of Medicine; MET, metabolic equivalent task; WHI, Women’s Health Initiative.
Percentages may not sum to 100 because of missing data.
Measured at year 1.
Weight (kg)/height (m)2.
The IOM guidelines for vitamin D intake are ≥600 IU/day for women aged 50–70 years and ≥800 IU/day for women aged ≥70 years (42).
We did not find supplementation with vitamin D and calcium to be associated with a significant change in continuous Burnam score from baseline to year 3, as compared with placebo (Table 2). Among women without evidence of depression at year 1, depressive symptom score was modestly but significantly higher in women assigned to the supplementation group than in those assigned to the placebo group (for supplementation vs. placebo, multivariable-adjusted mean change = 0.009; 95% confidence interval (CI): 0.002, 0.017).
Table 2.
Association of Calcium and Vitamin D Supplementation With Change in Mean Burnam Scale Score Between Year 1 and Year 3 of Follow-up, Women’s Health Initiative Calcium and Vitamin D Trial, 1995–2003
| All Participants | Participants Without Evidence of Depression at Year 1a | |||||||
| No. of Women | Mean Changeb (SD) (Year 3 – Year 1) | Multivariable-Adjusted Mean Changec | 95% CI | No. of Women | Mean Changeb (SD) (Year 3 – Year 1) | Multivariable-Adjusted Mean Changed | 95% CI | |
| Supplementation group | 1,109 | 0.004 (0.143) | 0.007 | −0.003, 0.017 | 956 | 0.020 (0.099) | 0.009 | 0.002, 0.017 |
| Placebo group | 1,143 | −0.002 (0.113) | 0 | Referent | 1,001 | 0.010 (0.061) | 0 | Referent |
Abbreviations: CI, confidence interval; SD, standard deviation; WHI, Women’s Health Initiative.
Analysis excluded women with depressive symptoms above the threshold level (Burnam score ≥0.06) or antidepressant use at baseline.
Positive change reflects a higher Burnam score at year 3 versus baseline; negative change reflects a lower Burnam score at year 3 versus baseline.
Multivariable models adjusted for age, race/ethnicity, WHI Hormone Trial intervention, WHI Dietary Modification Trial intervention, and depressive symptoms above the threshold level (Burnam score ≥0.06) at year 1.
Multivariable models adjusted for age, race/ethnicity, WHI Hormone Trial intervention, and WHI Dietary Modification Trial intervention.
Furthermore, as shown in Table 3, compared with those randomized to placebo, women randomized to supplement use had an odds ratio of 1.16 (95% CI: 0.86, 1.56) for experiencing depressive symptoms above the threshold level. Supplementation was not associated with the likelihood of antidepressant use at year 3; compared with women assigned to placebo, women assigned to supplements had an odds ratio of 1.01 (95% CI: 0.92, 1.12) for antidepressant use. In analyses limited to women without evidence of depression at year 1, those randomized to supplement use had an odds ratio of 1.41 (95% CI: 0.97, 2.05) compared with women assigned to placebo.
Table 3.
Association of Calcium and Vitamin D Supplementation With Risk of Depressive Symptoms Above the Threshold Level at Year 3 and Risk of Antidepressant Medication Use at Year 3, Women’s Health Initiative Calcium and Vitamin D Trial, 1995–2003
| All Participants | Participants Without Evidence of Depression at Year 1a | |||||||
| No. of Cases | No. of Noncases | Multivariate ORb | 95% CI | No. of Cases | No. of Noncases | Multivariate ORc | 95% CI | |
| Depressive symptoms above threshold level (Burnam score ≥0.06) at year 3 | ||||||||
| Supplementation group | 119 | 997 | 1.16 | 0.86, 1.56 | 70 | 886 | 1.41 | 0.97, 2.05 |
| Placebo group | 108 | 1,039 | 1 | Referent | 52 | 949 | 1 | Referent |
| Use of antidepressant medication at year 3 | ||||||||
| Supplementation group | 1,326 | 15,732 | 1.01 | 0.92, 1.12 | 419 | 14,248 | 0.96 | 0.84, 1.10 |
| Placebo group | 1,380 | 15,597 | 1 | Referent | 428 | 14,001 | 1 | Referent |
Abbreviations: CI, confidence interval; OR, odds ratio; WHI, Women’s Health Initiative.
Analysis excluded women with depressive symptoms above the threshold level (Burnam score ≥0.06) or antidepressant use at baseline.
Multivariable models adjusted for age, race/ethnicity, WHI Hormone Trial intervention, and WHI Dietary Modification Trial intervention. The analysis of risk of depressive symptoms above the threshold level (Burnam score ≥0.06) included adjustment for this variable at baseline. The analysis of risk of antidepressant use included adjustment for antidepressant use at baseline.
Multivariable models adjusted for age, race/ethnicity, WHI Hormone Trial intervention, and WHI Dietary Modification Trial intervention.
Overall, we found inconclusive evidence that baseline vitamin D and calcium intakes modified the association between supplementation and risk of either depressive symptoms above the threshold level or antidepressant use (Table 4). We observed a statistically significant interaction between supplementation assignment and total vitamin D intake as related to the occurrence of depressive symptoms above the threshold level (P = 0.03) but not as related to antidepressant use. Supplementation was unrelated to risk of either outcome when we stratified the results by level of vitamin D intake from foods only, vitamin D intake from supplements only, or calcium intake.
Table 4.
Association of Calcium and Vitamin D Supplementation With Measures of Depression at Year 3, According to Vitamin D and Calcium Intake at Baseline, Women’s Health Initiative Calcium and Vitamin D Trial, 1995–2003
| Depressive Symptoms (Burnam Score ≥0.06) | Use of Antidepressant Medication | |||||||
| No. of Cases | Total No. | ORa for Supplementation vs. Placebo | 95% CI | No. of Users | Total No. | ORa for Supplementation vs. Placebo | 95% CI | |
| Total vitamin D intake, IU/dayb | ||||||||
| <100 | 46 | 418 | 0.61 | 0.30, 1.22 | 350 | 4,965 | 0.89 | 0.68, 1.16 |
| 100–<200 | 49 | 487 | 2.84 | 1.38, 5.83 | 550 | 7,570 | 1.20 | 0.96, 1.49 |
| 200–<400 | 34 | 390 | 1.80 | 0.82, 3.97 | 509 | 6,382 | 0.96 | 0.76, 1.21 |
| 400–<600 | 53 | 520 | 0.91 | 0.49, 1.70 | 683 | 8,018 | 0.92 | 0.76, 1.13 |
| ≥600 | 37 | 379 | 0.99 | 0.47, 2.05 | 574 | 6,468 | 1.09 | 0.87, 1.36 |
| Pinteraction | 0.03 | 0.32 | ||||||
| Dietary vitamin D intake, IU/day | ||||||||
| <100 | 70 | 687 | 0.88 | 0.51, 1.52 | 668 | 8,989 | 0.91 | 0.75, 1.11 |
| 100–<200 | 76 | 876 | 1.46 | 0.87, 2.44 | 1,127 | 14,039 | 1.10 | 0.94, 1.28 |
| 200–<400 | 59 | 529 | 1.39 | 0.76, 2.53 | 725 | 8,782 | 0.96 | 0.79, 1.17 |
| ≥400 | 14 | 102 | 0.99 | 0.29, 3.35 | 146 | 1,593 | 1.13 | 0.73, 1.76 |
| Pinteraction | 0.55 | 0.45 | ||||||
| Supplemental vitamin D intake, IU/day | ||||||||
| None | 127 | 1,201 | 1.38 | 0.91, 2.07 | 1,296 | 17,367 | 1.02 | 0.89, 1.18 |
| <400 | 20 | 207 | 1.15 | 0.43, 3.11 | 272 | 3,383 | 1.04 | 0.76, 1.43 |
| 400–<800 | 68 | 733 | 1.04 | 0.61, 1.78 | 1,038 | 11,882 | 0.99 | 0.84, 1.17 |
| ≥800 | 4 | 53 | 0.22 | 0.02, 2.63 | 60 | 771 | 0.86 | 0.44, 1.69 |
| Pinteraction | 0.48 | 0.96 | ||||||
| Total calcium intake, mg/dayb | ||||||||
| <400 | 29 | 221 | 0.50 | 0.20, 1.24 | 146 | 2,358 | 0.87 | 0.58, 1.31 |
| 400–<800 | 65 | 655 | 1.76 | 0.98, 3.17 | 661 | 8,831 | 1.00 | 0.82, 1.22 |
| 800–<1,000 | 24 | 293 | 1.81 | 0.73, 4.48 | 403 | 4,682 | 1.24 | 0.96, 1.61 |
| 1,000–<1,200 | 19 | 244 | 1.16 | 0.43, 3.19 | 301 | 4,154 | 0.98 | 0.73, 1.32 |
| ≥1,200 | 82 | 781 | 1.05 | 0.63, 1.73 | 1,155 | 13,378 | 0.97 | 0.83, 1.14 |
| Pinteraction | 0.18 | 0.52 | ||||||
| Dietary calcium intake, mg/day | ||||||||
| <400 | 39 | 380 | 0.61 | 0.29, 1.29 | 318 | 4,429 | 0.94 | 0.71, 1.25 |
| 400–<800 | 96 | 932 | 1.28 | 0.80, 2.04 | 1,098 | 14,298 | 0.98 | 0.84, 1.14 |
| 800–<1,000 | 24 | 328 | 2.57 | 1.01, 6.55 | 450 | 5,448 | 1.15 | 0.90, 1.47 |
| 1,000–<1,200 | 24 | 231 | 1.03 | 0.41, 2.60 | 270 | 3,526 | 1.26 | 0.92, 1.73 |
| ≥1,200 | 36 | 323 | 1.27 | 0.59, 2.73 | 530 | 5,702 | 0.90 | 0.71, 1.13 |
| Pinteraction | 0.21 | 0.37 | ||||||
| Supplemental calcium intake, mg/day | ||||||||
| None | 114 | 1,024 | 1.28 | 0.83, 1.99 | 1,070 | 14,334 | 1.00 | 0.85, 1.17 |
| <400 | 47 | 528 | 1.47 | 0.77, 2.82 | 697 | 7,987 | 1.13 | 0.92, 1.38 |
| 400–<800 | 29 | 382 | 0.97 | 0.43, 2.19 | 516 | 6,342 | 0.93 | 0.74, 1.17 |
| ≥800 | 29 | 260 | 0.80 | 0.34, 1.88 | 383 | 4,740 | 0.96 | 0.74, 1.26 |
| Pinteraction | 0.66 | 0.63 | ||||||
Abbreviations: AD, antidepressant; CI, confidence interval; OR, odds ratio.
Multivariable models adjusted for age, race/ethnicity, WHI Hormone Trial intervention, and WHI Dietary Modification Trial intervention. The analysis of risk of depressive symptoms above the threshold level (Burnam score ≥0.06) included adjustment for this variable at year 1. The analysis of risk of antidepressant use included adjustment for antidepressant use at year 1.
Total nutrient intake = dietary nutrient intake (assessed at year 0) + supplemental nutrient intake (assessed at year 1).
Results did not suggest that the relation between supplementation and antidepressant use at year 3 varied by such factors as solar irradiance, physical activity, or current smoking (Table 5). The likelihood of antidepressant use was significantly modified by educational level (P = 0.007); among women with no education after high school, women randomized to supplements had an odds ratio of 0.78 (95% CI: 0.63, 0.96) compared with those randomized to placebo. Supplementation was associated with a nonsignificant reduced risk of antidepressant use in women with a normal body mass index (odds ratio = 0.82, 95% CI: 0.67, 1.00) but not in heavier women, although the test for interaction did not reach statistical significance (P = 0.06). Risk did not vary according to the presence of depression symptoms at year 1.
Table 5.
Odds Ratios for Use of Antidepressant Medication at Year 3 by Randomization Group, According to Participant Characteristics, Women’s Health Initiative Calcium and Vitamin D Trial, 1995–2003
| Characteristic | Supplementation Group | Placebo Group | Multivariate Odds Ratioa for Supplementation vs. Placebo | 95% Confidence Interval | P for Interaction | ||
| No. of AD Users | Total No. | No. of AD Users | Total No. | ||||
| Age, years | 0.98 | ||||||
| 50–59 | 554 | 6,221 | 557 | 6,186 | 1.02 | 0.87, 1.19 | |
| 60–69 | 581 | 7,842 | 614 | 7,820 | 1.00 | 0.86, 1.16 | |
| 70–79 | 191 | 2,995 | 209 | 2,971 | 1.01 | 0.79, 1.29 | |
| Post-high-school education | 0.007 | ||||||
| No | 274 | 4,010 | 339 | 4,025 | 0.78 | 0.63, 0.96 | |
| Yes | 1,040 | 12,941 | 1,031 | 12,845 | 1.08 | 0.97, 1.22 | |
| Body mass indexb | 0.06 | ||||||
| <25 | 283 | 4,513 | 322 | 4,584 | 0.82 | 0.67, 1.00 | |
| 25–<30 | 431 | 6,102 | 452 | 6,120 | 1.02 | 0.86, 1.21 | |
| ≥30 | 603 | 6,360 | 597 | 6,185 | 1.12 | 0.96, 1.30 | |
| Race/ethnicity | 0.19 | ||||||
| White | 1,169 | 14,231 | 1,260 | 14,248 | 0.98 | 0.88, 1.09 | |
| Black | 78 | 1,513 | 66 | 1,474 | 1.12 | 0.76, 1.66 | |
| Hispanic | 47 | 699 | 37 | 647 | 1.31 | 0.77, 2.24 | |
| Other/unknown | 32 | 615 | 17 | 608 | 1.89 | 0.94, 3.80 | |
| Current smoking | 0.30 | ||||||
| No | 1,181 | 15,617 | 1,228 | 15,583 | 1.01 | 0.91, 1.12 | |
| Yes | 139 | 1,272 | 125 | 1,218 | 1.22 | 0.87, 1.70 | |
| Alcohol intake, drinks/day | 0.70 | ||||||
| 0 | 429 | 4,717 | 467 | 4,733 | 0.96 | 0.80, 1.15 | |
| >0–<1 | 785 | 10,407 | 778 | 10,319 | 1.04 | 0.92, 1.19 | |
| ≥1 | 105 | 1,798 | 122 | 1,805 | 0.95 | 0.68, 1.32 | |
| Physical activity, MET-hours/week | 0.47 | ||||||
| <3.00 | 474 | 5,014 | 508 | 4,935 | 0.99 | 0.84, 1.18 | |
| 3.00–9.99 | 325 | 4,349 | 354 | 4,382 | 0.99 | 0.82, 1.21 | |
| 10.00–19.99 | 266 | 3,465 | 242 | 3,379 | 1.12 | 0.89, 1.40 | |
| ≥20.0 | 157 | 2,694 | 181 | 2,734 | 0.84 | 0.64, 1.10 | |
| Physical function (RAND Short Form 36 score) | 0.88 | ||||||
| ≤50 | 251 | 1,701 | 288 | 1,725 | 0.97 | 0.75, 1.25 | |
| 51–90 | 742 | 8,701 | 775 | 8,725 | 1.00 | 0.88, 1.15 | |
| >90 | 329 | 6,614 | 316 | 6,488 | 1.05 | 0.87, 1.26 | |
| History of cardiovascular disease | 0.63 | ||||||
| No | 1,208 | 16,111 | 1,247 | 16,012 | 1.02 | 0.92, 1.13 | |
| Yes | 118 | 947 | 133 | 965 | 0.93 | 0.65, 1.32 | |
| Solar irradiance, Langleys | 0.42 | ||||||
| <350 | 432 | 5,127 | 413 | 5,082 | 1.11 | 0.93, 1.33 | |
| 350–<400 | 415 | 5,597 | 445 | 5,544 | 0.97 | 0.81, 1.15 | |
| ≥400 | 479 | 6,334 | 522 | 6,351 | 0.96 | 0.82, 1.13 | |
| Total saturated fat intake, g/day | |||||||
| <15.1 | 277 | 4,203 | 298 | 4,130 | 0.91 | 0.74, 1.12 | 0.11 |
| 15.1–<21.5 | 303 | 4,197 | 342 | 4,228 | 0.87 | 0.71, 1.07 | |
| 21.5–<29.8 | 359 | 4,240 | 334 | 4,114 | 1.10 | 0.90, 1.34 | |
| ≥29.8 | 372 | 4,101 | 381 | 4,190 | 1.17 | 0.96, 1.42 | |
Abbreviations: AD, antidepressant; MET, metabolic equivalent task; WHI, Women's Health Initiative.
Adjusted for age, WHI Hormone Trial intervention, WHI Dietary Modification Trial intervention, and use of antidepressant medication at year 1.
Weight (kg)/height (m)2.
In subanalyses limited to participants who were adherent with the treatment protocol, results were similar (results not shown).
DISCUSSION
In this randomized clinical trial, we did not find that 2 years of supplementation with 400 IU/day of vitamin D3, combined with 1,000 mg/day of elemental calcium, influenced the risk of depression. The effect of supplementation did not vary meaningfully by levels of vitamin D and calcium intake from diet and supplemental sources or by other participant characteristics.
Few intervention trials have evaluated the association of vitamin D supplementation with risk of depression (23–30). Results from these trials are difficult to compare because they differ substantially in terms of vitamin D doses tested, assessment of depression, duration of participant follow-up, use of placebo controls, sample size, and participant characteristics, including age and menopausal status. Jorde et al. (28) observed a modest but significant improvement in depressive symptom score after 1 year of supplementation in 334 Norwegians (mean age = 47 years) randomized to either 40,000 IU/week or 20,000 IU/week as compared with placebo. However, mean depressive symptom scores in this population were low, suggesting that few participants were experiencing clinically significant depression. In 3 other small trials (15–88 participants), investigators observed some improvement in mood symptoms during winter in young and middle-aged persons taking supplemental vitamin D (22–24). In these studies, supplementation doses ranged from 400 IU/day to 100,000 IU/week, and follow-up periods ranged from 5 days to 3 months. However, in 4 other clinical trials of vitamin D at doses of 800 IU/day (23), 5,000 IU/day (29), 50,000 IU/week (27), and 500,000 IU once annually (30), no effect of vitamin D on depressive symptoms was found.
The dose of vitamin D we tested (400 IU/day) may not have been sufficient to affect the occurrence of depression, although this dosage (combined with personal intake) is consistent with recent recommendations from the Institute of Medicine (47). Vitamin D intake among our participants was comparable to that among women in the WHI Observational Study, which found that intake of vitamin D from food sources at the start of follow-up was inversely associated with risk of depression 3 years later (for ≥400 IU/day vs. <100 IU/day, odds ratio = 0.80, 95% CI: 0.67, 0.96; P for trend = 0.002) (40). We did not find evidence that supplementation with 400 IU/day affected risk of depressive symptoms either among women likely to be vitamin D-deficient due to low dietary intake, high body mass index, or high latitude of residence or among women reporting high dietary intake and/or vitamin D supplement use at baseline.
It is possible that vitamin D is truly unrelated to depression and that the beneficial effects reported in observational studies are largely due to residual confounding by lifestyle and dietary behaviors. For example, fatty fish are major sources of both marine omega-3 fatty acids and vitamin D, and omega-3 fatty acid intake has been inversely associated with risk of depression in multiple studies (48, 49); most observational studies of vitamin D status and depression have not accounted for omega-3 fatty acid intake.
Alternatively, if vitamin D is truly related to depression, results from our study may have been confounded by calcium supplementation. Some evidence suggests that high long-term calcium intake may increase the risk of arterial calcification (50), which has been associated with depression, and may increase the number and size of brain lesions (51–54). We did not find supplementation to be associated with higher risk of depression in women with high background calcium intakes in our study. However, it is possible that supplemental vitamin D combined with calcium is related to depression differently than vitamin D alone, especially in older women with some degree of existing arterial calcification. Further evaluation of vitamin D alone or of this relation in younger persons without preclinical cardiovascular disease may shed light on this issue.
A limitation of our study was our reliance on self-reported symptoms to assess depression, instead of the use of psychiatric interviews. The Burnam scale primarily measures depressive symptoms experienced in the year prior to completion, as opposed to clinical diagnoses of depression made during the entire follow-up period. Thus, women who developed depression prior to their year 3 clinic visit but whose symptoms remitted either naturally or after treatment would not have been classified as cases. Only a subset of WHI participants were asked to complete the Burnam scale at year 3, thus limiting our statistical power for this analysis. For this reason, we also considered antidepressant use at year 3 to be a proxy for depression. However, women may be prescribed antidepressants for reasons other than depression, such as fibromyalgia, migraine headache, or panic disorder. This misclassification could have attenuated our findings to some extent.
To our knowledge, our study is among the first large randomized trials to have evaluated the relation of vitamin D supplementation with depressive symptoms. Additional strengths of our study include the diversity of our population, allowing us to evaluate effect modification of a possible vitamin D-depression association by a variety of characteristics, including background vitamin D and calcium intakes, race/ethnicity, body mass index, smoking status, and physical activity. Supplementation was associated with a lower risk of antidepressant use in year 3 among women with a high school education or less and was marginally significantly associated with lower risk in normal-weight women. We also observed significant interaction with total vitamin D intake, but odds ratios for the effect of supplementation did not vary across intake categories in a meaningful pattern. Given the large number of subgroups examined, these findings were probably attributable to chance. The randomized trial design of our study should have precluded the likelihood of confounding by sun exposure and 25-hydroxyvitamin D status, which may be a concern in observational studies.
In summary, we did not observe a relation between 2 years of daily supplementation with 400 IU of vitamin D3 and 1,000 mg of elemental calcium and measures of depression status. Additional trials testing higher supplemental doses of vitamin D are needed to determine whether this nutrient may be beneficial in preventing or treating depression among specific populations.
Acknowledgments
Author affiliations: Division of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts, Amherst, Massachusetts (Elizabeth R. Bertone-Johnson); Department of Psychology, College of Natural Sciences, University of Massachusetts, Amherst, Massachusetts (Sally I. Powers); Group Health Research Institute, Seattle, Washington (Leslie Spangler); Department of Family and Community Medicine, School of Medicine, University of Nevada, Reno, Nevada (Robert L. Brunner); Department of Epidemiology and Biostatistics, School of Public Health, Drexel University, Philadelphia, Pennsylvania (Yvonne L. Michael); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Joseph Larson); Department of Social and Preventive Medicine, School of Public Health and Health Professions, State University of New York at Buffalo, Buffalo, New York (Amy E. Millen); Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts (Maria N. Bueche, JoAnn E. Manson); Division of Cardiovascular Medicine, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts (Elena Salmoirago-Blotcher, Ira Ockene); Division of Preventive and Behavioral Medicine, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts (Judith K. Ockene); Department of Medicine, School of Public Health, University of California, Los Angeles, Los Angeles, California (Simin Liu); and Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Yeshiva University, Bronx, New York (Sylvia Wassertheil-Smoller).
The Women’s Health Initiative (WHI) is funded by the National Heart, Lung, and Blood Institute through contracts N01WH22110, N01WH24152, N01WH 32100-2, N01WH 32105-6, N01WH 32108-9, N01WH 32111-13, 32115, N01WH 32118-32119, N01WH 32122, N01WH 42107-26, N01WH 42129-32, and N01WH 44221.
The WHI Investigators—Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, Washington) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, Kentucky) Evan Stein; (University of California, San Francisco, San Francisco, California) Steven Cummings; Clinical Centers: (Albert Einstein College of Medicine, Bronx, New York) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, Texas) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts) JoAnn E. Manson; (Brown University, Providence, Rhode Island) Charles B. Eaton; (Emory University, Atlanta, Georgia) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, Washington) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, Oregon) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, California) Bette Caan; (Medical College of Wisconsin, Milwaukee, Wisconsin) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, Illinois) Linda Van Horn; (Rush Medical Center, Chicago, Illinois) Henry Black; (Stanford Prevention Research Center, Stanford, California) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, New York) Dorothy Lane; (Ohio State University, Columbus, Ohio) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, Alabama) Cora E. Lewis; (University of Arizona, Phoenix, Arizona) Cynthia A. Thomson; (State University of New York at Buffalo, Buffalo, New York) Jean Wactawski-Wende; (University of California, Davis, Sacramento, California) John Robbins; (University of California, Irvine, Irvine, California) F. Allan Hubbell; (University of California, Los Angeles, Los Angeles, California) Lauren Nathan; (University of California, San Diego, La Jolla, California) Robert D. Langer; (University of Cincinnati, Cincinnati, Ohio) Margery Gass; (University of Florida, Gainesville, Florida) Marian Limacher; (University of Hawaii, Honolulu, Hawaii) J. David Curb; (University of Iowa, Iowa City, Iowa) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, Massachusetts) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, New Jersey) Norman Lasser; (University of Miami, Miami, Florida) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, Minnesota) Karen Margolis; (University of Nevada, Reno, Nevada) Robert Brunner; (University of North Carolina at Chapel Hill, Chapel Hill, North Carolina) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, Pennsylvania) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, Tennessee) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, Texas) Robert Brzyski; (University of Wisconsin, Madison, Wisconsin) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, Michigan) Michael S. Simon; WHI Memory Study: (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker.
The funding organization was independent of the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript.
Dr. JoAnn E. Manson and colleagues at Brigham and Women’s Hospital, Harvard Medical School, are recipients of funding from the National Institutes of Health to conduct the Vitamin D and Omega-3 Trial, a large-scale randomized trial of the role of vitamin D and omega-3 fatty acids in the prevention of cancer and cardiovascular disease.
Glossary
Abbreviations
- CaD
Calcium and Vitamin D
- CI
confidence interval
- DM
Dietary Modification
- HT
Hormone Therapy
- WHI
Women’s Health Initiative
References
- 1.Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 2.Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 5.Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res. 1982;242(2):387–390. doi: 10.1016/0006-8993(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 6.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 7.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67(8):481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart R, Hirani V. Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom Med. 2010;72(7):608–612. doi: 10.1097/PSY.0b013e3181e9bf15. [DOI] [PubMed] [Google Scholar]
- 9.Schneider B, Weber B, Frensch A, et al. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm. 2000;107(7):839–842. doi: 10.1007/s007020070063. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 11.Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 12.Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29. doi: 10.1186/1755-7682-3-29. (doi:10.1186/1755-7682-3-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1–3):240–243. doi: 10.1016/j.jad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Nanri A, Mizoue T, Matsushita Y, et al. Association between serum 25-hydroxyvitamin D and depressive symptoms in Japanese: analysis by survey season. Eur J Clin Nutr. 2009;63(12):1444–1447. doi: 10.1038/ejcn.2009.96. [DOI] [PubMed] [Google Scholar]
- 15.Zhao G, Ford ES, Li C, et al. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br J Nutr. 2010;104(11):1696–1702. doi: 10.1017/S0007114510002588. [DOI] [PubMed] [Google Scholar]
- 16.Chan R, Chan D, Woo J, et al. Association between serum 25-hydroxyvitamin D and psychological health in older Chinese men in a cohort study. J Affect Disord. 2011;130(1-2):251–259. doi: 10.1016/j.jad.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Milaneschi Y, Shardell M, Corsi AM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010;95(7):3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May HT, Bair TL, Lappé DL, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. 2010;159(6):1037–1043. doi: 10.1016/j.ahj.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D: a D-lightful health perspective. Nutr Rev. 2008;66(10 suppl 2):S182–S194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 20.Pasco JA, Williams LJ, Jacka FN, et al. Habitual physical activity and the risk for depressive and anxiety disorders among older men and women. Int Psychogeriatr. 2011;23(2):292–298. doi: 10.1017/S1041610210001833. [DOI] [PubMed] [Google Scholar]
- 21.Pasco JA, Williams LJ, Jacka FN, et al. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Br J Psychiatry. 2008;193(4):322–326. doi: 10.1192/bjp.bp.107.046706. [DOI] [PubMed] [Google Scholar]
- 22.Bjerkeset O, Romundstad P, Evans J, et al. Association of adult body mass index and height with anxiety, depression, and suicide in the general population: the HUNT study. Am J Epidemiol. 2008;167(2):193–202. doi: 10.1093/aje/kwm280. [DOI] [PubMed] [Google Scholar]
- 23.Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151–153. [PubMed] [Google Scholar]
- 24.Gloth FM, III, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5–7. [PubMed] [Google Scholar]
- 25.Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl) 1998;135(4):319–323. doi: 10.1007/s002130050517. [DOI] [PubMed] [Google Scholar]
- 26.Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8. doi: 10.1186/1475-2891-3-8. (doi:10.1186/1475-2891-3-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvold DS, Odean MJ, Dornfeld MP, et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocr Pract. 2009;15(3):203–212. doi: 10.4158/EP.15.3.203. [DOI] [PubMed] [Google Scholar]
- 28.Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 29.Dean AJ, Bellgrove MA, Hall T, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—a randomised controlled trial. PLoS One. 2011;6(11):e25966. doi: 10.1371/journal.pone.0025966. (doi: 101371/journal.pone.0025966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198(5):357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 33.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 34.Jackson RD, LaCroix AZ, Cauley JA, et al. The Women’s Health Initiative Calcium-Vitamin D Trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 suppl):S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. Women’s Health Initiative Investigators. J Natl Cancer Inst. 2008;100(22):1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. Women’s Health Initiative Investigators. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 37.Burnam MA, Wells KB, Leake B, et al. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26(8):775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kim CK, McGorray SP, Bartholomew BA, et al. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165(11):1239–1244. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 39.Spangler L, Scholes D, Brunner RL, et al. Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med. 2008;23(5):567–574. doi: 10.1007/s11606-008-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertone-Johnson ER, Powers SI, Spangler L, et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am J Clin Nutr. 2011;94(4):1104–1112. doi: 10.3945/ajcn.111.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Arch Intern Med. 2004;164(3):289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 42.Tuunainen A, Langer RD, Klauber MR, et al. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103(2-3):261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 43.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 44.Patterson RE, Levy L, Tinker LF, et al. Evaluation of a simplified vitamin supplement inventory developed for the Women’s Health Initiative. Public Health Nutr. 1999;2(3):273–276. doi: 10.1017/s1368980099000361. [DOI] [PubMed] [Google Scholar]
- 45.Millen AE, Pettinger M, Freudenheim JL, et al. Incident invasive breast cancer, geographic location of residence, and reported average time spent outside. Cancer Epidemiol Biomarkers Prev. 2009;18(2):495–507. doi: 10.1158/1055-9965.EPI-08-0652. [DOI] [PubMed] [Google Scholar]
- 46.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 47.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Kraguljac NV, Montori VM, Pavuluri M, et al. Efficacy of omega-3 fatty acids in mood disorders—a systematic review and metaanalysis. Psychopharmacol Bull. 2009;42(3):39–54. [PubMed] [Google Scholar]
- 50.Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–266. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne ME, Anderson JJ, Steffens DC. Calcium and vitamin D intakes may be positively associated with brain lesions in depressed and nondepressed elders. Nutr Res. 2008;28(5):285–292. doi: 10.1016/j.nutres.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamer M, Kivimaki M, Lahiri A, et al. Persistent cognitive depressive symptoms are associated with coronary artery calcification. Atherosclerosis. 2010;210(1):209–213. doi: 10.1016/j.atherosclerosis.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agatisa PK, Matthews KA, Bromberger JT, et al. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165(11):1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 54.Matthews KA, Chang YF, Sutton-Tyrrell K, et al. Recurrent major depression predicts progression of coronary calcification in healthy women: Study of Women’s Health Across the Nation. Psychosom Med. 2010;72(8):742–747. doi: 10.1097/PSY.0b013e3181eeeb17. [DOI] [PMC free article] [PubMed] [Google Scholar]