Abstract
Trisomy of chromosome 8 is frequently reported in myeloid lineage disorders and also detected in lymphoid neoplasms as well as solid tumors suggesting its role in neoplastic progression in general. It is likely to be a disease-modulating secondary event with underlying cryptic aberrations as it has been frequently reported in addition to known abnormalities contributing to clinical heterogeneity and modifying prognosis. Here, we share our findings of trisomy 8 in leukemia patients referred for diagnostic and prognostic cytogenetic assessment. Total 60 cases of trisomy 8, as a sole anomaly or in addition to other chromosomal aberrations, were reported (January 2005–September 2008). Unstimulated bone marrow or blood samples were cultured, followed by GTG banding and karyotyping as per the ISCN 2005. Patients with +8 were chronic myeloid leukemia (CML) (36), acute myeloid leukemia (AML) (17), and acute lymphoblastic leukemia (ALL) (7). In 7 patients, trisomy 8 was the sole anomaly, whereas in 6 patients +8 was in addition to normal clone, in 47 patients, the +8 was in addition to t(9;22), t(15;17), and others, including 3 with tetrasomy 8. Only one patient showed constitutional +8. The present study will form the basis of further cumulative studies to correlate potential differential effects of various karyotypic anomalies on disease progression and survival following a therapeutic regime. To unravel the role of extra 8 chromosome, constitutional chromosomal analysis and uniparental disomy will be considered.
Keywords: Acute myeloid leukemia, cytogenetics, trisomy 8
Introduction
Clinical impact of trisomy 8 on carcinogenesis progression[1] and treatment response have been focus of a research interest. Trisomy 8 is one of the most frequent numerical aberrations in acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), chronic myeloproliferative disorders (MPD), and acute lymphoblastic leukemia (ALL),[2] in solid tumors including colon, breast, and head and neck cancers,[3] and rarely reported in chronic lymphocytic leukemia (CLL).[4] It may be present as a sole aberration or along with other chromosomal aberrations. The constitutional presence of trisomy 8, i.e., Warkany syndrome (mosaic trisomy 8), is manifested by moderate mental retardation, bone and joint abnormalities, cardiovascular and urogenital malformations, deep palmar and plantar grooves, and agenesis of the corpus callosum. The occurrence of trisomy 8 in malignant conditions raises a possibility of underlying constitutional mosaic trisomy 8.
Here, we would like to share our findings of trisomy 8 in leukemia patients referred for diagnostic and prognostic cytogenetic assessment.
Materials and Methods
Patients
The leukemia cases studied from January 2005 to September 2008 were screened for presence of trisomy 8, either as a sole anomaly or in addition to other chromosomal aberrations. Out of 60 cases of trisomy 8 by cytogenetics, 37 (62%) were males and 23 (38%) females with age range 1–74 and median age 31 years. The morphological diagnosis was according to the FAB classification. These patients have been followed up for clinical progression; the data are available from 1 to 48 months.
Cytogenetic analysis
Bone marrow aspirate or blood samples were cultured for 24 and 48 h without a mitogen. For constitutional study, a mitogen-stimulated blood culture was set for 72 h using phytohemagglutinin as a mitogenic agent. Metaphase chromosomes were banded by GTG-banding technique. Karyotypes were analyzed according to ISCN 2005.[5]
A minimum of 20 metaphases were analyzed in each patient. The cytogenetic data were classified into three groups, i.e., chronic myeloid leukemia (CML), AML, and ALL. CML patients had t(9;22) with or without other complex aberrations. AML category included inv (16), t(15;17), and t(8;21), whereas ALL category showed various structural as well as numerical changes.
Results
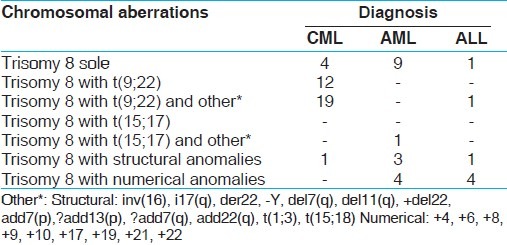
The patients included CML (n = 36 (60%), M:26/F:10), AML (n = 17 (28.0%), M:7/F:10), and acute leukemia (n = 7 (12.0%), M:4/F:3). Among 60 patients, 18 were untreated and 42 were treated (22: glivec, 8: daunorubicin, and 12: others). In seven (12.0%) patients, trisomy 8 was observed as the sole karyotypic aberration in six AML and one ALL patients. There were 6 (10%) patients with +8 as a sole anomaly in addition to normal clone, which might suggest disease progression, in 47 (78%) patients, the +8 was in addition to t(9;22), t(15;17), and others, including 3 with tetrasomy 8. One of the patients showed presence of constitutional +8, a rare but reported finding.[1,6,7] Three patients with tetrasomy 8 were classified as trisomy 8 for purposes of this analysis. There are some reports of trisomy 8 evolving into tetrasomy.[8] In contrast, cases have been reported implying that tetrasomy 8 clone is unrelated to trisomy 8;[9] these results need further study.
Occurrence of trisomy 8 in different types of leukemia is described in Table 1.
Table 1.
Occurrence of trisomy 8 in different types of leukemia
The current study will form the basis for future correlation of various karyotypic anomalies on disease progression and survival following a therapeutic regime.
Discussion
Trisomy 8 is reported in 25% of CML cases, with or without simple or complex karyotypic changes, in 10–15% of AML patients, in 15–20% of MDS, and 5% of ALL as a part of hyper diploid karyotype or complex karyotypes with structural anomalies, exceptionally associated with non-Hodgkin lymphomas, and varies in frequency for solid tumors.[2] Trisomy 8 as a secondary chromosomal change is associated with blastic phase in CML with a relatively poor prognosis. It has been reported by cancer and leukemia group B (CALGB) that in AML +8 alone was associated with poor prognosis,[10,11] whereas according to Pedersen et al.,[12] it was an intermediate prognostic factor. The prognostic significance of extra 8 in lymphoid malignancies is not reported widely. Thus, the presence of trisomy 8 in several leukemias poses a question for its role in leukomogenesis. The trisomy 8 is likely to be a disease-modulating secondary event, with underlying cryptic translocations, deletions, or mutations as primary events.[2] The clinical reevaluation of patients to exclude Constitutional Trisomy 8 Mosaicism (CT8M) has also been indicated. The CT8M patients should be monitored for the possible risk of malignancies.
Genes with possible significance in leukomogenesis located on chromosome 8 include c-myc[13] on 8q24, c-mos[14] on 8q22, MOZ[15] on 8p11, and ETO on 8q22.[16] Trisomy 8 could represent an alternative mechanism for increasing C-MYC gene dosage to achieve amplification of C-MYC oncogene.[17] Some studies suggest that trisomy 8 associated with human hematologic neoplasia is generally not related to gross rearrangements of the c-mos or c-myc genes.[18] Mechanisms underlying the events need further study. The role of trisomy 8 in leukemogenesis may be possibly explained by imprinting effect of certain genes due to uniparental disomy caused by the acquired trisomy 8. The chromosome 8 UPD has been reported for rectal carcinoma and adenoma, pancreatic cancer, AML, and transformed B-cell lymphoma.[19]
The constitutional trisomy is associated with abnormal phenotype due to imprinting effects, reduction to homozygosity at recessive disease loci, or trisomy mosaicism, in few instances.[20] The vast majority of autosomal trisomies result from errors during maternal meiosis. Nondisjunction may occur either during meiosis or mitosis. Postzygotic mitotic errors account for some 5% of nonmosaic autosomal trisomies. The recent studies suggest occurrence of extra chromosome 8 of both, paternal as well as maternal origin, aggravating further the existing queries regarding molecular mechanisms of role of a trisomy in leukemogenesis.
It is evident that unraveling the molecular and biologic consequences of this trisomy requires further investigation. The present study will be extended by including more number of patients with longer follow-up duration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Seghezzi L, Maserati E, Minelli A, Dellavecchia C, Addis P, Pasquali F, et al. Constitutional trisomy 8 as first mutation in multistep carcinogenesis: Clinical, cytogenetic, and molecular data on three cases. Genes Chromosomes Cancer. 1996;17:94–101. doi: 10.1002/(SICI)1098-2264(199610)17:2<94::AID-GCC4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2.Huret JL. +8 or trisomy 8. Atlas Genet Cytogenet Oncol Haematol. [Last Accessed on Dec 2007]. Available from: http://www.infobiogen.fr/services/chromcancer/Anomalies/tri8ID1017.html .
- 3.Mitelman F, Johansson B, Mertens F. Mitelman database of chromosome aberrations in cancer. [Last accessed on 2010 Sep 5]. Available from: http://www.cgap.nci.nih.gov/chromosomes/Mitelman .
- 4.Lau L, Kee S, Tien S, Mickey B. Trisomy 8 as sole cytogenetic abnormality in a case of chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2007;172:66–9. doi: 10.1016/j.cancergencyto.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 5.An International System for Human Cytogenetic Nomenclature, ISCN. Basel, Switzerland: S. Karger; 1995. [Google Scholar]
- 6.Kapaun P, Kabisch H, Held KR, Walter TA, Hegewisch S, Zander AR. Atypical chronic myelogenous leukaemia in a patient with trisomy 8 mosaicism syndrome. Ann Hematol. 1993;66:57–8. doi: 10.1007/BF01737691. [DOI] [PubMed] [Google Scholar]
- 7.Hasle H, Clausen N, Pedersen B, Bendix-Hansen K. Myelodysplastic syndrome in a child with constitutional trisomy 8 mosaicism and normal phenotype. Cancer Genet Cytogenet. 1995;79:79–81. doi: 10.1016/0165-4608(94)00099-w. [DOI] [PubMed] [Google Scholar]
- 8.Kameoka J, Funato T, Obara Y, Kadowaki I, Yokoyama H, Kimura T, et al. Clonal evolution from trisomy into tetrasomy of chromosome 8 associated with the development of acute myeloid leukemia from myelodysplastic syndrome. Cancer Genet Cytogenet. 2001;124:159–64. doi: 10.1016/s0165-4608(00)00347-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XX, Robinson LJ, Stenzel TT, Qumsiyeh MB. Translocation (15; 17) (q22; q21) as a secondary chromosomal abnormality in a case of acute monoblastic leukemia with tetrasomy 8. Cancer Genet Cytogenet. 1999;113:9–13. doi: 10.1016/s0165-4608(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 10.Byrd J, Lawrence D, Arthur D, Pettenati M, Tantravahi R, Bloomfield C, et al. Patients with isolated trisomy 8 in acute myeloid leukemia are not cured with cytarabine-based chemotherapy: Results from cancer and leukemia group B 8461. Clin Cancer Res. 1998;4:1235–41. [PubMed] [Google Scholar]
- 11.Mayer RJ, Schiffer CA, Peterson BA, Budman DR, Silver RT, Rai KR, et al. Intensive postremission therapy in adults with acute nonlymphocytic leukemia using various dose schedules of Ara-C: A progress report from the CALGB. Semin Oncol. 1987;14(Suppl 1):25–31. [PubMed] [Google Scholar]
- 12.Pedersen B. MDS and AML with trisomy 8 as the sole chromosome aberration show different sex ratios and prognostic profiles: A study of 115 published cases. Am J Hematol. 1997;56:224–9. doi: 10.1002/(sici)1096-8652(199712)56:4<224::aid-ajh5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Koskinen PJ, Alitalo K. Role of myc amplification and over expression in cell growth, differential and death. Semin Cancer Biol. 1993;4:3–12. [PubMed] [Google Scholar]
- 14.Diaz MO, Le Beau MM, Rowley JD, Drabkin HA, Patterson D. The role of c-mos gene in the 8:21 translocation in human acute myeloblastic leukemia. Science. 1985;229:767–9. doi: 10.1126/science.3860954. [DOI] [PubMed] [Google Scholar]
- 15.Borrow J, Stanton V, Jr, Andresen M, Becher R, Behm F, Raju S, et al. The translocation t (8:16) (p11:p13) of acute myeloid leukemia fuses a putative acetyl transferase gene to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wang M, Liu J. Transformation properties of ETO gene, fusion partner in t(8;21) leukemia. Cancer Res. 1997;57:2951–5. [PubMed] [Google Scholar]
- 17.Jennings BA, Mills KI. C-myc locus amplification and the acquisition of trisomy8 in the evolution of chronic myeloid leukaemia. Leuk Res. 1998;22:899–903. doi: 10.1016/s0145-2126(98)00097-6. [DOI] [PubMed] [Google Scholar]
- 18.Diaz MO, Le Beau MM, Harden A, Rowley JD. Trisomy 8 in human hematologic neoplasia and the c-myc and c-mos oncogenes. Leuk Res. 1985;9:1437–42. doi: 10.1016/0145-2126(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 19.Tuna M, Knuutila S, Mills G. Uniparental disomy in cancer. Trends Mol Med. 2009;15:120–8. doi: 10.1016/j.molmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Karanjawala Z, Kääriäinen H, Ghosh S, Tannenbaum J, Valle T, Collins F, et al. Complete maternal isodisomy of chromosome 8 in an individual with an early-onset ileal carcinoid tumor. Am J Med Genet. 2000;93:207–10. doi: 10.1002/1096-8628(20000731)93:3<207::aid-ajmg9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


