Abstract
INTRODUCTION:
Increase in the instability of cellular genome with an increasing age is the result of an accumulation of cellular damage and mutations. This instability which might be observed as chromosome damage or chromosome losses can be measured by the micronucleus technique.
AIM:
The aim of this study was to investigate the effect of aging and oxidative stress induced by non-toxic levels of H2O2 on micronuclei induction and their relationship to cell proliferation in human peripheral blood lymphocytes.
MATERIALS AND METHODS:
Healthy volunteers with different ages were choosen. Spontaneous and H2O2 induced micronuclei frequencies were measured in peripheral blood lymphocytes of 30 volunteers by the micronucleus method.
RESULTS:
Spontaneous micronuclei frequencies increased first then started to decrease after 50 years of age. This biphasic response was significantly higher than micronucleus (MN) frequencies induced by H2O2 (P < 0.05), which followed the similar shape of response to increasing ages with lower frequencies. Proliferative capacity of cells either treated with H2O2 or not did not differ with an increasing age giving similar responses.
CONCLUSION:
These results indicate biphasic character of chromosome damage; first increase and decrease after 50 years with an increasing age. But this change pattern was not correlated with the steady state of proliferation capacity of cells through an increasing age. Decreases in H2O2-induced MN frequencies compared to spontaneous MN frequencies may be inducing an apoptosis by H2O2 treatment leading to underscoring damaged cells.
Keywords: Aging, cell kinetics, lymphocytes, micronucleus
Introduction
Aging, which is generally described by decreasing in the functions of tissues and organs of body, is the result of increasing instabilities in expression and structure of genome.[1–3] Although the mechanism of aging is not certainly understood, the accumulation of oxidative damage occurring on macromolecules (protein, lipids, or nucleic acids) in years is one of the important determinants in aging.[2,4] Increase in the amount of mutations as a result of accumulation of oxidative damage in time may also explain the increasing cancer incidences with aging. Unrepaired and/or misrepaired DNA double breaks that were caused by physical or chemical agents in cellular genome can be observed as chromosome damage.[5,6] In addition, chromosome losses resulting from the damage on centromers or spindle fibers and unsegregation of chromosomes during the cell division are important events in cancer and aging.
Micronucleus (MN) technique is a cytological marker of chromosome damage and chromosome losses in the binucleate cells (BN) obtained by blocking cytokinesis by cytochalasin-B (cyt-B) agent (5). Accentric chromosomes or chromosomes with damaged centromers or whole chromosome loss can be seen as micronucleus in binucleate cells. In this technique, proliferative indexes (PI) can also be obtained by calculating the ratio of a number of multinucleated cells to a number of total cells, thus providing information about cell proliferation in addition to chromosome damage. Therefore, MN technique can be used in studying toxic effects of chemical agents and radiation on genome, in identifying chromosome irregularities resulting from the lack of essential metabolites, in predicting cancer risk and degenerative diseases of aging.[7–11]
Ramsey et al.,[12] and Bolognesi et al.,[13] have shown the spontaneous chromosome aberrations that were measured in healthy human lymphocytes increased with an increasing age and defined the possible responsibility of the environmental factors in this increase in addition to the inherent genetical factors. Ganguly[14] has shown that the chromosome aberrations and MN frequencies that were measured from lymphocytes of different aged individuals gave a positive correlation with individual ages, whereas mitotic indexes that were measured for cellular proliferation gave a negative correlation with individual ages. In some another studies that were performed with MN technique applied to lymphocytes, Fenech[15] has shown an increase in the MN frequencies with an increase in age and the effect of aging on the induction of chromosome damage rates was at least 25%; Bolognesi et al.,[16] and Ganguly[14] have also shown a decrease in the proliferative capacity of lymphocytes with an increasing age.
The aim of this research was to investigate the effect of aging on both MN formations as determinants of chromosome damage in human peripheral blood lymphocytes and proliferative effectiveness of lymphocytes. The role of environmental factors and diet regimes should also be acknowledged besides the role of genetical factors of aging in chromosome damage formation.[17,18] Therefore, it was also aimed to investigate MN frequencies and proliferative indexes induced by non-toxic hydrogen peroxide (H2O2) stress in addition to in vitro measuring of spontaneous MN frequencies in this project.
Materials and Methods
Study group
This study was performed with peripheral blood lymphocytes that were collected from 30 healthy volunteers including both man and woman with different ages after approval by the Istanbul Universit.y Istanbul Medical Faculty Ethics Committee. Volunteers signed informed consent documents. Their ages ranged between 23 and 73 years. They did not take treatment for any kind of systemic disease in their histories. Twenty-one donors were man and 9 were woman. Only 5 of them were current smokers, and 3 were ex-smokers (smoked between 10 and 30 years).
Micronucleus assay
For each donor, peripheral blood was drawn into two different 4.5-ml sterilized lithium-heparin tubes. One tube was left for control to measure spontaneous MN frequencies and the other one was treated with non-toxic levels of H2O2 (500 μM for 30 mins) to measure stress-induced MN frequencies. Microculture method[19,20] was used for lymphocyte culture with small modifications; for each donor, 0.5 ml of whole blood either treated and untreated was added in culture containing 15 g/ml phytohemagglutinin (Sigma, St. Louis, MO, USA), 1 ml newborn calf serum and 4 ml of RPMI-1640 with glutamine (Sigma) supplemented with 100 μg/ml streptomycin and 100 IU/ml penicillin, and incubated at 37°. At the 44th hours of incubation, cytochalasin-B (Sigma, St. Louis, MO, USA) solution (6μg/ml) was added and cells were incubated for another 28 h in order to obtain micronuclei in binucleate cells.[5] At the end of total incubation period of 72 hours, cells were treated with ice-cold hypotonic KCI solution (0.075M). Immediately after, cells were washed three times with methanol:acetic acid (7:1) solution. Staining was performed after dropping cells on ice-cold glass slides with 5% Giemsa solution. One thousand binucleate per patient were scored.
Fenech[5] criteria for scoring micronuclei were applied. Binucleate cells with two identical nuclei were considered for evaluation. Binucleate cells either containing micronuclei or not were scored for evaluating MN frequencies. Nucleoplasmic bridges, nuclear buds (blebs and extrusions) were also included in scoring in order to see their effect on aging other than MNi. Cells with one, two, three, and four nuclei were scored for calculating proliferative index (PI) in order to evaluate proliferative status of each donor's lymphocytes according to the formulation by Eastmond and Tucker:[21] PI=(M1+(2×M2)+(3×M3)+(4×M4))/N, where M1,M2,M3, and M4 represent the number of cells with 1-4 nuclei, and N the total number of scored cells.
Statistical analyses
Comparisons between MN frequencies and proliferative indexes before and after H2O2 treatment for each donor's lymphocytes were made with paired t-test. Unpaired t-test was applied to compare scores between different gender and smoking conditions. The effects of aging on spontaneous, H2O2-induced MN frequencies and proliferative indexes were evaluated with regression analysis.
Results
The age, gender, cigarette smoking conditions, spontaneous, and H2O2-induced MN frequencies and proliferative indexes (PI) that were measured in lymphocytes were given in Table 1. The distribution of donor ages was from 23 to 73 years in the range of 50 years. Twenty-two of donors never smoked cigarettes, 5 were current users of cigarettes, and 3 were past users.
Table 1.
The age, gender, cigarette smoking status, spontaneous and H2O2-induced MN frequencies, and proliferative indexes
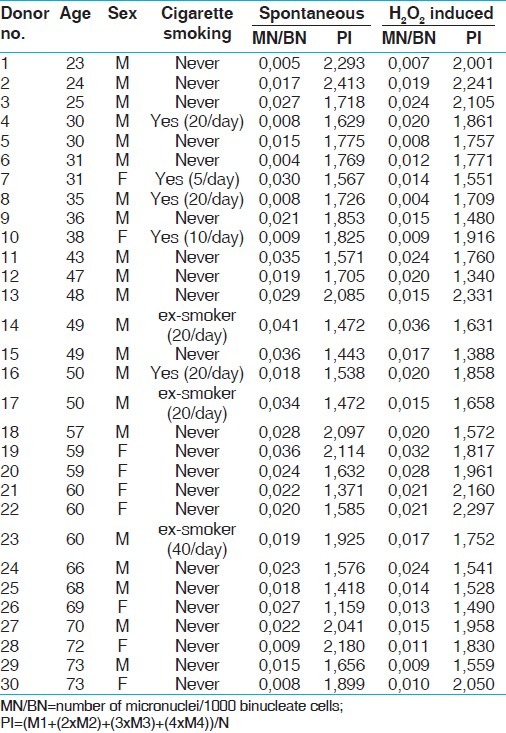
One thousand BN were scored for each volunteers. Their spontaneous MN frequencies were between 0.004 and 0.041 with the mean (SD) of 0.021 (0.01) and proliferative indexes were between 1.159 and 2.413 with the mean (SD) of 1.750 (0.29). H2O2-induced MN frequencies were between 0.004 and 0.036 with the mean (SD) 0.017 (0.007) and proliferative indexes were between 1.340 and 2.331 with the mean (SD) of 1.796 (0.27). When the comparisons were made between male and female, and between smokers and non-smokers there were no differences in the scores of either MN frequencies or proliferative indexes before and after H2O2 treatment. Therefore, the scores under the same heading are pooled together.
MN frequencies before H2O2 treatment were significantly higher than MN frequencies after H2O2 treatment (P = 0.010). The relationships between spontaneous and H2O2-induced MN frequencies and differing ages are given in Figure 1.
Figure 1.
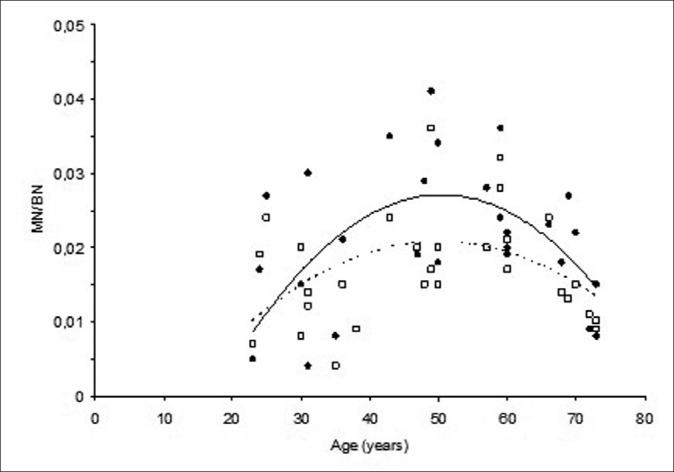
Changes in spontaneous (●; straight line) and H2O2-induced (□; dotted line) MN frequencies with increasing age
Spontaneous and H2O2-induced MN frequencies of donors showed an increase first starting from 20 years of age up to around 50 years of age and then decreased. The best fit line obtained by applying quadratic (2nd degree equation) regression analysis to the data (in order to see the biphasic character of the relationship) had a tendency to decrease after 50 years.
Nuclear buds (blebs and extrusions) and nucleoplasmic bridges were also scored in all volunteers’ binucleates, untreated or treated with H2O2 in 1000 BN cells [Table 2]. But nuclear bridges and nuclear extrusions were occasionally scored. Number of extrusions was added into the scores of nuclear blebs summing up to nuclear buds. Therefore, comparison was made in terms of nuclear buds.
Table 2.
Spontaneous and H2O2-induced nuclear buds* and nucleoplasmic bridges
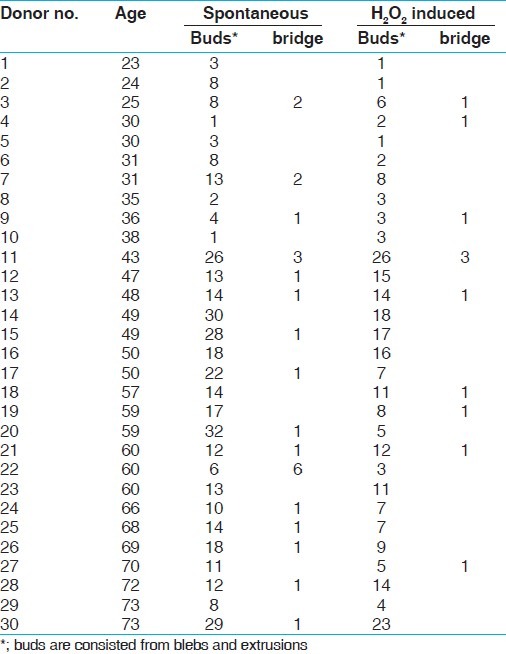
Nuclear buds before H2O2 treatment were significantly higher than nuclear buds after H2O2 treatment (P = 0.0003). This relationships between spontaneous and H2O2-induced nuclear bud frequencies and differing ages is given in Figure 2. Clearly, the pattern of change in the nuclear bud frequencies with age was quite similar to that of change in MN frequencies, showing the similar biphasic character.
Figure 2.
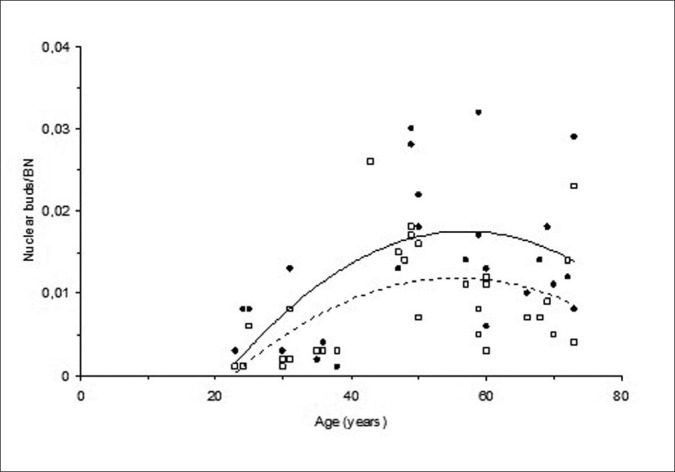
Changes in spontaneous (●; straight line) and H2O2-induced (□; dotted line) nuclear bud frequencies with an increasing age
When proliferative indexes before and after treatment were compared, there was no any difference (P = 0.42). The similarity in relationships between proliferative indexes and aging are shown in Figure 3.
Figure 3.
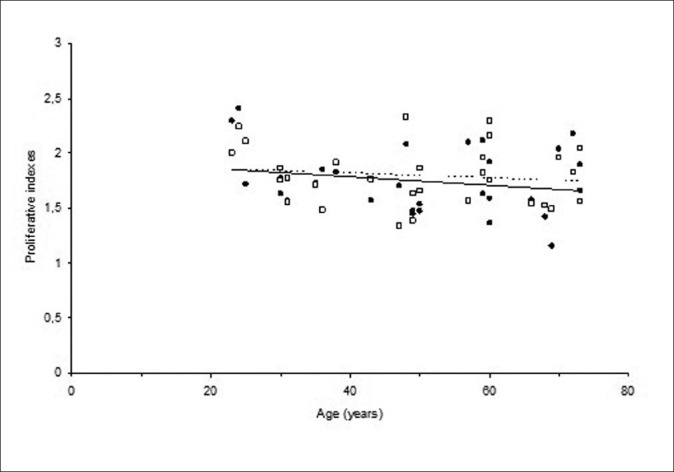
Changes in spontaneous (●; straight line) and H2O2-induced (□; dotted line) proliferative indexes with an increasing age
Although spontaneous and H2O2-induced proliferative indexes had a tendency to decrease with increasing aging, correlation coefficients (R2) of regression analysis for spontaneous PI line was 0.045 and for H2O2-induced PI line was 0.018. There were no variations in proliferative indexes with increases in donor's ages.
Discussion
The aim of this research was to investigate the effect of aging on chromosome instability and cellular reproduction in human peripheral blood lymphocytes. Studies that were investigating the relationship between aging and chromosome instabilities showed an increase in MN frequencies with an increasing age.[14,16,22,23] This increase in MN frequencies in these studies was followed by a decrease in proliferating efficiencies of cells. Milosevic-Djordjevic et al.,[23] showed a decrease of MN frequencies in older aged groups and explained this might be the result of declining in proliferation capacity of cells with an increasing age.
An increased frequency of chromosomal damage was associated with increasing chromosome instability in aged people. In this study, the increase in the frequency of micronuclei was first observed up to 50 years of age and this increase was followed by a decrease with a further increasing age. This biphasic character of the curve was also observed in the relationship between nuclear buds, which are the result of disorientation of nucleus integrity and age. But the decrease of buds occurred later fifties.
Non-toxic levels of an oxidant, H2O2, were also used in this study in order to investigate if there is a differential effect on differing ages. The alterations in MN frequencies after H2O2 treatment according to donor ages had a similar characteristic pattern with spontaneous frequencies, but the line representing this relationship was lower showing decreased frequency levels. When the paired t-test applied, there was an evidence of difference (P < 0.05) between the two groups. However, there was a low correlation (r2 = 0.018) between H2O2-induced proliferative efficiency and age, and this was not different (P = 0.42) than the relationship between spontaneous PI values and age. This similarity between H2O2-induced and spontaneous PI lines defines that the H2O2 treatment was not toxic.
Increases in the chromosome damage measured by either chromosome aberration test or MN test with increasing age were shown by many authors and this relationship was also shown in this study. But we also observed a trend in the decrease of chromosome instability measured by two different measures at late ages. Regarding this decrease in the chromosomal instability in the most elderly donors, we investigated the proliferative capacity of peripheral blood lymphocytes. Proliferation capacity of cells was worked on the same amount of cells, 1000 binucleate cells. Change in proliferative capacity with an increasing age did not follow the same relationship with chromosome instability with the slope not different from zero (P = 0.26). Increasing age did not have any influence on the proliferation indexes. Therefore, proliferative capacity of cells can not be responsible from the change pattern (rising and falling or fluctuation) of chromosome instability with age.
As a result, MN frequencies change with an increasing age and this change have a biphasic character; first, there is a positive correlation up to 50 years of age and second, there is a negative correlation after 50 years. although the pattern of changing of spontaneous MN frequencies depending on age is similar compared to the literature but when the regression analysis applied on the effect of age on the proliferative efficiency of cells, there was no correlation (R2 = 0.045) between the PI and age. May be the age range we used were unable to detect the decreasing proliferative capacity as we forced cells to go division. The decrease in MN frequencies at old ages may not be explained by the effect of proliferative indexes. The reason of decrease in H2O2-induced MN frequencies compared to spontaneous MN frequencies may be inducing an apoptosis by H2O2 treatment leading to underscore cells because of damaged cells, which requires further investigation.
Acknowledgments
This work was supported by Istanbul University, Research Fund (Project no: 248/23082004).
Footnotes
Source of Support: Istanbul University, Research Fund (Project no: 248/23082004).
Conflict of Interest: None declared.
References
- 1.Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer. 2001;1:203–13. doi: 10.1038/35106045. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair DA, Oberdoerffer P. The ageing epigenome: Damaged beyond repair? Ageing Res Rev. 2009;8:189–98. doi: 10.1016/j.arr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziêtkiewicz E, Wojda A, Witt M. Cytogenetic perspective of ageing and longevity in men and women. J Appl Genet. 2009;50:261–73. doi: 10.1007/BF03195682. [DOI] [PubMed] [Google Scholar]
- 4.Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 5.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 6.Tucker JD. Low-dose ionizing radiation and chromosome translocations: A review of the major considerations for human biological dosimetry. Mutat Res. 2008;659:211–20. doi: 10.1016/j.mrrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Fenech M, Bonassi S, Turner J, Lando C, Ceppi M, Chang WP, et al. Intra-and inter-laboratory variation in the scoring of micronuclei and nucleoplasmic bridges in binucleated human lymphocytes: Results of an international slide-scoring exercise by the HUMN project. Mutat Res. 2003;534:45–64. doi: 10.1016/s1383-5718(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res. 2006;600:58–66. doi: 10.1016/j.mrfmmm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Norppa H, Falck GC. What do human micronuclei contain? Mutagenesis. 2003;18:221–33. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 10.Norppa H, Bonassi S, Hansteen IL, Hagmar L, Strömberg U, Rössner P, et al. Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat Res. 2006;600:37–45. doi: 10.1016/j.mrfmmm.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Iarmarcovai G, Ceppi M, Botta A, Orsiere T, Bonassi S. Micronuclei frequency in peripheral blood lymphocytes of cancer patients: A meta-analysis. Mutat Res. 2008;659:274–83. doi: 10.1016/j.mrrev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey MJ, Moore DH, 2nd, Briner JF, Lee DA, Olsen L, Senft JR, et al. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat Res. 1995;338:95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- 13.Bolognesi C, Abbondandolo A, Barale R, Casalone R, Dalprà L, De Ferrari M, et al. Age-related increase of baseline frequencies of sister chromatid exchanges, chromosome aberrations, and micronuclei in human lymphocytes. Cancer Epidemiol Biomarkers Prev. 1997;6:249–56. [PubMed] [Google Scholar]
- 14.Ganguly BB. Cell division, chromosomal damage and micronucleus formation in peripheral lymphocytes of healthy donors: Related to donor's age. Mutat Res. 1993;295:135–48. doi: 10.1016/0921-8734(93)90015-u. [DOI] [PubMed] [Google Scholar]
- 15.Fenech M. Chromosomal damage rate, aging, and diet. Ann N Y Acad Sci. 1998;20:23–36. doi: 10.1111/j.1749-6632.1998.tb09889.x. [DOI] [PubMed] [Google Scholar]
- 16.Bolognesi C, Lando C, Forni A, Landini E, Scarpato R, Migliore L, et al. Chromosomal damage and ageing: Effect on micronuclei frequency in peripheral blood lymphocytes. Age Ageing. 1999;28:393–7. doi: 10.1093/ageing/28.4.393. [DOI] [PubMed] [Google Scholar]
- 17.Ames BN. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 18.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103:17589–94. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorhead PS, Nowell PC, Mellmann WJ, Battıps DM, Hungerford DA. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;206:613–6. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 20.Hungerford DA. Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCL. Stain Technol. 1965;40:333–8. doi: 10.3109/10520296509116440. [DOI] [PubMed] [Google Scholar]
- 21.Eastmond DA, Tucker JD. Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environ Mol Mutagen. 1989;13:34–43. doi: 10.1002/em.2850130104. [DOI] [PubMed] [Google Scholar]
- 22.Wojda A, Zietkiewicz E, Witt M. Effects of age and gender on micronucleus and chromosome nondisjunction frequencies in centenarians and younger subjects. Mutagenesis. 2007;22:195–200. doi: 10.1093/mutage/gem002. [DOI] [PubMed] [Google Scholar]
- 23.Milosevic-Djordjevic O, Grujicic D, Novakovic T, Arsenijevic S, Marinkovic D. Micronuclei and ageing in a sample of Yugoslavian population. Genetika. 2002;38:264–7. [PubMed] [Google Scholar]


