Abstract
To study the diuretic effects of cleistanthin A and cleistanthin B, phytoconstituents were isolated from the leaves of Cleistanthus collinus in Wistar rats. The in vivo diuretic effects of cleistanthins A and B were determined according to the Lipschitz test. Prior to the experiment, the animals were fasted for 5 h and placed individually in metabolic cages. Cleistanthins A and B (12.5, 25, and 50 mg/kg) and furosemide (5 mg/kg) were suspended in 0.5% w/v carboxymethyl cellulose and administered orally. The urine was collected up to 5 h after administration and subsequently up to 24 h after administration. The acidity and urine volume were measured immediately. The urinary sodium and potassium levels were determined using a flame photometer, and the chloride level was determined by argentometric titration. The diuretic index and diuretic activity were calculated mathematically. While cleistanthins A and B showed a diuretic index of more than one, the diuretic activity of these compounds was less than one, indicating inferior activity compared with furosemide. Both cleistanthin A and B produced a significant increase in the urine volume and alterations in urinary electrolyte levels. However, the effect of the compounds was not dose dependent. Cleistanthin A and cleistanthin B exert diuretic effects in male Wistar rats without affecting the urinary acidity.
Keywords: Cleistanthin A, cleistanthin B, diuretic activity
INTRODUCTION
Cleistanthin A and cleistanthin B are phytoconstituents of Cleistanthus collinus Roxb. (Euphorbiaceae). This plant was commonly used as a source of suicidal and homicidal poison in Southeast Asian countries.[1] Cleistanthins A and B have been reported to be toxic, but scientific evidence for this claim is lacking.[2] Arylnaphthalide lignans are found in significant levels in plants of the genus Cleistanthus. Arylnaphthalide lignans include diphyllin, collinusin, cleistanthin, taiwanin, and other related compounds. The sugars/glycones of the glycosides are O-methyl xylose and, to a lesser extent, d-glucose and its O-methyl derivatives.[3] Diphyllin glycosides such as cleistanthin and cleistanthoside are also present in Cleistanthus patulus, Haplophyllum bucharicum, and Phyllanthus toxodiifolius.[3–5] P. toxodiifolius is commonly used as a diuretic agent in Thailand, and this plant contains various diphyllin glycosides, such as cleistanthin A, cleistanthoside A, and cleistanthin A methyl ether.[4] Nothing has been published on the effect of cleistanthins A and B on urine volume and renal functions. Hence, this study was planned to determine the diuretic effects of cleistanthin A and cleistanthin B in Wistar rats.
MATERIALS AND METHODS
Plant material
Taxonomically identified C. collinus (Roxb.) (Euphorbiaceae) plant parts were collected in Pondicherry and in rural parts of Villupuram and Cuddalore districts of Tamil Nadu, India. They were identified and certified by the Botanical Survey of India (BSI), Coimbatore (BSI/SC/5/23/08-09/Tech.241). Leaves of C. collinus were collected during February–April every year. A voucher specimen of the plant is kept in the Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), for further reference.
Chemicals
An activated neutral alumina (column chromatography grade) was purchased from Spectrum Reagents and Chemicals Pvt. Ltd., Cochin, India, and Sisco Research Laboratory Ltd., Mumbai, India. Silica gel-G for thin layer chromatography (TLC) was purchased from Sisco Research Laboratory Ltd. Furosemide was purchased from Sanofi Aventis, India (Tablet Lasix 40 mg). All other solvents and chemicals were of analytical grade, and were purchased from Sisco Research Laboratory Ltd. and Merck Chemicals, India.
Animals
Healthy adult male Wistar rats (180–200 g) of inbred colony strains were used in the study. The animals were obtained from the Central Animal House, JIPMER, and allowed to adapt to the laboratory conditions for a period of 1 week. All the experimental animals were housed at a temperature of 25 ± 2°C and 40–50% humidity in a 12:12 ± 1 h light–dark cycle. The rats were fed with standard rat pellets (VRK Nutritional Solution, Sangli, Maharastra) and water ad libitum. The study protocol was approved by the Institute Animals Ethics Committee (IAEC), and all the animal experiments were carried out in accordance with the guidelines of the Committee for the Purpose of control and supervision of experiments on animals (CPCSEA), India.
Isolation of cleistanthin A and cleistanthin B
Cleistanthins A and and B were isolated from the leaves of the C. collinus plant. An extract was obtained from defatted powered leaves of this plant using acetone. The acetone extract was used for isolation of cleistanthins A and B through column chromatography. The acetone extract was dissolved in benzene, passed through the neutral alumina column and eluted with benzene, benzene: ethyl acetate (4:1), benzene: ethyl acetate (1:1), and methanol: chloroform (9.5:0.5) to isolate the fatty alcohol, collinusin, cleistanthin A and cleistanthin B fractions, respectively.[6,7] The fractions of cleistanthins A and B were purified using preparative thin layer chromatography and crystallization method, respectively. The functional groups and facial arrangement of atoms in cleistanthin A and B molecules were confirmed by FT-IR spectroscopy (Avatar FT-IR 330) and nuclear magnetic resonance (Bruker 300 MHz) spectroscopy.[8]
Dose calculation for cleistanthin A and cleistanthin B
The dose of cleistanthins A and B was determined according to the OECD guidelines (new OECD Test Guideline 420, 421)[9] using the fixed dose method. Female Swiss albino mice weighing 20–25 g were used for the study. Cleistanthin A and B were administered successively in different groups at fixed dose levels of 5, 50, 300, 1000, and 1200 mg/kg and observed for a specified period. Three mice in a group were used per dose and closely observed for 24 h and monitored for 14 days to record the general behavior and mortality.[10]
Determination of renal effect of cleistanthin A and cleistanthin B
Diuretic activity of cleistanthins A and B were determined according to the Lipschitz test. Healthy male Wistar rats (180–200 g) were used for the study. The animals were divided into eight groups of six animals each and treated as follows:
Group I : Control
Group II : Standard drug treatment (furosemide, 5 mg/kg)
Group III : Cleistanthin A, 12.5 mg/kg
Group IV : Cleistanthin A, 25 mg/kg
Group V : Cleistanthin A, 50 mg/kg
Group VI : Cleistanthin B, 12.5 mg/kg
Group VII : Cleistanthin B, 25 mg/kg
Group VIII : Cleistanthin B, 50 mg/kg
Five hours prior to the experiment, food and water were withdrawn, and the animals were housed individually in metabolic cages. cleistanthins A and B at various concentrations (12.5, 25, and 50 mg/kg) and the reference standard, furosemide (5 mg/kg), were suspended in 0.5% w/v carboxymethyl cellulose and administered orally in a single dose. Additionally, 5 ml of 0.9% NaCl solution per 100 g body weight was given by oral gavage. Urine was collected continuously up to 5 h after dosing and from then up to 24 h after dosing, and the urine volume and acidity (pH) were measured immediately after collection. The urinary electrolyte contents were analyzed within 24 h of collection. The urinary sodium and potassium levels were determined by flame photometry, and the chloride level was determined by argentometric titration.[11–13] The diuretic action/index and diuretic activity were calculated mathematically as follows:[14]

Statistical analysis
The mean±SEM values were calculated for each parameter. Since the variation within a group was large, the values of each parameter were log transformed to carry out statistical analysis. Significant differences between the groups were determined using one-way ANOVA followed by a Bonferroni comparison of the groups. A P value less than 0.05 was considered statistically significant.
RESULTS
There was clear TLC separation of cleistanthin at a Rf value of 0.37–0.42. The purified cleistanthin A and B were identified using UV-visible, FT-IR, and NMR spectroscopy. The percentage yield of isolated cleistanthin A and cleistanthin B were 0.9–1.0% w/w and 0.6–0.8% w/w, receptively. The single oral dose of cleistanthin A and B up to 800 mg/kg did not lead to mortality, but these compounds caused death in 50% of the animals at 1200 and 1000 mg/kg, respectively. Based on the dose determination study results, less than 1/10 of the toxic dose was selected for pharmacological evaluation.
The effects of cleistanthins A and B on urine volume and urinary electrolytes are presented in Tables 1 and 2. Cleistanthin A, cleistanthin B and furosemide produced a significant increase in the urine volume from 5 to 24 h though the test compounds did not cause a significant increase in the urine volume during the first 5 h. Cleistanthin A, cleistanthin B and furosemide caused an increase in the urinary sodium and potassium levels in the first 5 h (P<0.01), but no changes in the levels were observed with urinary sodium from 5 to 24 h. The potassium levels in the urine were comparable in the control and furosemide groups from 5 to 24 h, but cleistanthin A reduced the potassium level while cleistanthin B increased excretion of potassium in urine. Neither of the test compounds altered the pH value of the urine and the chloride excretion in comparison with the control group.
Table 1.
Effects of cleistanthin A and cleistanthin B on urine volume and electrolyte concentration (0–5 h)
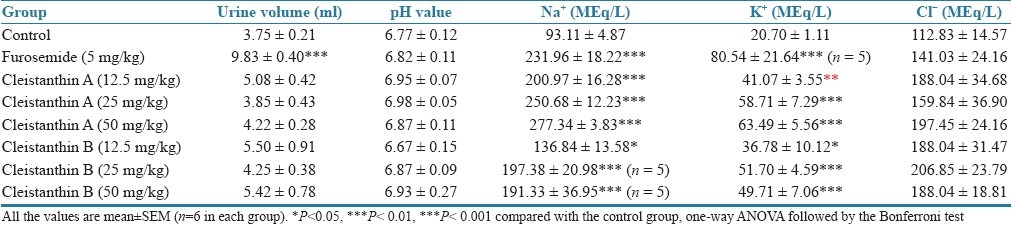
Table 2.
Effects of cleistanthin A and cleistanthin B on urine volume and electrolyte concentration (5–24 h)
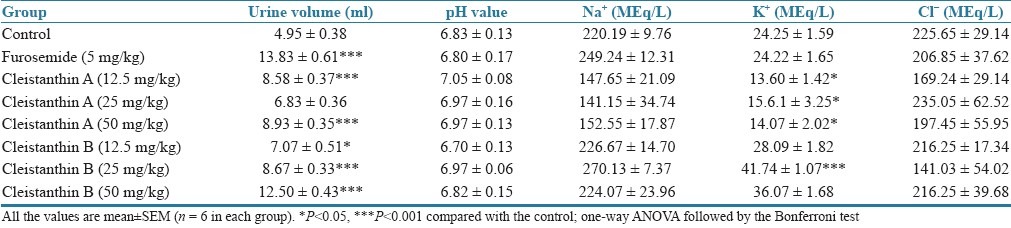
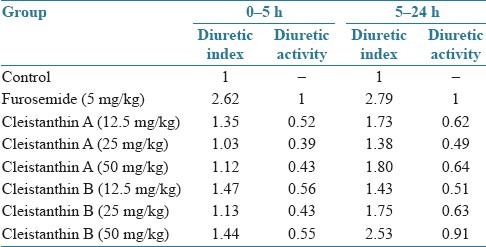
The calculated diuretic index and diuretic activity values are presented in Table 3. The diuretic index and diuretic activity of both cleistanthin A and cleistanthin B are less than those of furosemide.
Table 3.
Effects of cleistanthin A and cleistanthin B on diuretic action and diuretic activity
DISCUSSION
The fixed dose method of safety dose determination shows that cleistanthin A and B are safe up to 800 mg/kg. Cleistanthin A and B caused 50% mortality at 1200 and 1000 mg/kg, respectively. Cleistanthins A and B belong to class 4 (slightly toxic) of Hodge and Sterner toxicity Scale.[15]
The results of this study show that cleistanthins A and B have diuretic effects. Both the compounds significantly increase the urine volume between 5 and 24 h after administration but not up to 5 h after administration. This suggests a delayed action of the compounds, in contrast to furosemide, which acts within a relatively short time. In spite of a significant increase in the excretion of urinary electrolytes such as sodium and potassium during the period from 0 to 5 h after administration, there is no corresponding increase in the urine volume in comparison with control animals. Almost all diuretics increase the excretion of sodium and potassium, the exception being potassium-sparing diuretics, which increase the excretion of sodium but conserve potassium. It is difficult to offer an explanation as to why the urine volume in animals treated with cleistanthins A and B did not show an increase corresponding with the increased excretion of electrolytes. It could be inferred that the action of the test compounds starts late in the first 5 h after administration and continues 5–24 h after administration. The urine volume starts increasing, but it does not reach a statistically significant level before 5 h. During the 5–24 h period, the increase in urine excretion continues to become significant, but the urinary electrolyte levels are not different in the urine of the control animals and in that of those treated with the compounds. This could be due to compensatory mechanisms that come into action in the later part of the period from 5 to 24 h, after the action of the compounds is over. Hence there is no difference in the electrolyte levels of the treated and control groups. The increase in the urinary flow during the period from 5 to 24 h could have mainly occurred in the initial part of that period. The electrolyte values in the control group differ during 0–5 and 5–24 h. This could be due to salt loading leading to dilution of electrolytes in 0–5 h and hence the electrolyte levels, especially sodium and chloride, appear to be excreted more during 5–24 h.
In the period from 5 to 24 h, there is no change in the urinary sodium level with administration of cleistanthin A and B. However, cleistanthin A reduces the potassium level in the urine while cleistanthin B increases the same. In spite of these conflicting effects of the compounds on the electrolytes, the urine volume of the period from 5 to 24 h is significantly increased. Both the compounds raise the potassium levels in the urine in the first 5 h, but in the period from 5 to 24 h, while cleistanthin A conserves potassium, cleistanthin B continues to increase the potassium excretion. Probably the initial increase in potassium excretion triggers a compensatory mechanism later in the case of cleistanthin A. However, why such a compensation does not take place with cleistanthin B is unexplainable. We are not able to explain the turnaround of urinary potassium that occurs with cleistanthin A.
The chloride level and pH value are not influenced by the test compounds. The use of a C. collinus leaf extract is reported to have reduced the urinary pH value.[16] Since the leaves of C. collinus contain more than 30 compounds, it is possible that the reduction in pH was due to one or more compounds other than cleistanthins A and B. Although the compounds exert a diuretic activity as indicated by the diuretic index being greater than one, the extent of the effect is not comparable with that of furosemide.[11]
Metabolic acidosis, hypokalemia, and hyponatremia are reported to be induced by C. collinus poisoning in humans.[17] In one study, C. collinus poisoning caused metabolic acidosis in all patients, hypokalemia in 60% of the patients, and renal failure in 15.6% of them.[18] The leaf extract of C. collinus produced type I distal renal tubular acidosis and type II respiratory failure in preclinical evaluations, and it was suggested that these changes are due to a decrease in the pH value of the urine, a failure of the acid-secreting mechanism and a decrease in the blood potassium levels.[16] In this study, no changes in urinary pH value (acidity) were observed with either cleistanthin A or cleistanthin B. As discussed earlier, one or more compounds other than cleistanthin A or cleistanthin B present in the leaf extract may be responsible for the acidosis and other effects on the kidneys.
Several plants belonging to the Euphorbiaceae, including P. toxodiifolius, have been used in Thai folk medicine to induce diuresis. P. toxodiifolius is one of the sources of cleistanthin A, cleistanthoside A, and cleistanthin A methyl ether. This offers support to our finding that cleistanthin A is a diuretic.
In this study, no attempt was made to elucidate the mechanism of the diuresis induced by cleistanthins A and B. Useful information would have been obtained if the urine output was measured hourly rather than at 0-5 h and at 5-24 h. This would have revealed the time at which the action of the test compounds starts and the time at which it ends. Since the hourly output of urine in the rats was too small to carry out the analysis with the existing facilities, it was not possible to perform such a study.
In summary, this study explains the diuretic effects of cleistanthin A and cleistanthin B, phytoconstituents isolated from the leaves of C. collinus. Although the compounds have diuretic activity, the effects are not comparable with that of furosemide.
ACKNOWLEDGMENTS
The authors are grateful to Dr. V. Gopal, Professor of Pharmacognosy and Mr. C. N. Arulananda Raj, Lecturer of Pharmaceutical Analysis, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, Pondicherry, for providing laboratory facilities to measure urinary electrolytes and Dr. Sreejith Parameswaran, Assistant Professor of Nephrology, JIPMER, Pondicherry for his suggestions to improve the discussion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Eswarappa S, Chakraborty AR, Palatty BU, Vasnik M. Cleistanthus collinus poisoning: Case reports and review of the literature. Clin Toxicol (Phila) 2000;41:369–72. doi: 10.1081/clt-120022005. [DOI] [PubMed] [Google Scholar]
- 2.Annapoorani KS, Damodaran C, Chabdrasekharan P. High pressure liquid chromatographic separation of aryl-naphthalide lignan lactones. J Liq Chromatogr. 1985;8:1173–94. [Google Scholar]
- 3.Pinho PM, Kijjoa A. Chemical constituents of the plants of the genus Cleistanthus and their biological activity. Phytochem Rev. 2007;6:175–82. [Google Scholar]
- 4.Tuchinda P, Kumkao A, Pohmakotr M, Sophasan S, Santisuk T, Reutrakul V. Cytotoxic arylnapthalide ligan glycoside from the aerial part of Phyllanthus toxodiifolius. Planta Med. 2006;72:60–2. doi: 10.1055/s-2005-873141. [DOI] [PubMed] [Google Scholar]
- 5.Ulubelen A, Ozturk M. Alkaloids, coumarins and lignans from Haplophyllum species. Rec Nat Prod. 2008;2:54–69. [Google Scholar]
- 6.Govindachari TR, Sathe SS, Viswanathan N, Pai BR, Srinivasan M. Chemical constituents of Cleistanthus collinus (Roxb.) Tetrahedron. 1969;25:2815–21. [Google Scholar]
- 7.Parasuraman S, Raveendran R. Effect of cleistanthin A and B on adrenergic and cholinergic receptors. Pharmacogn Mag. 2011;7:243–7. doi: 10.4103/0973-1296.84239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshmi TG, Srimannarayan G, Subbarao NV. A new glucoside from Cleistanthus collinus. Curr Sci. 1970;39:395–6. [Google Scholar]
- 9.Acute Oral Toxicity - OECD Fixed Dose Method. [Last accessed on 2011 May 20]. Available from http://www.hc-sc.gc.ca/ewh-semt/occup-travail/whmis-simdut/acute_oral_toxicity-toxicite_aigue_orale-eng.php .
- 10.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–9. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel HG, editor. 2nd ed. England: Springer; 2002. Drug discovery and evaluation: Pharmacological assays. [Google Scholar]
- 12.El-sayed NH, Awaad AS, Mabry TJ. Phytochemical studies and effect on urine volume of Glossostemon bruguieri Desf. constituents. Indian J Exp Biol. 2004;42:186–9. [PubMed] [Google Scholar]
- 13.Parasuraman S, Kumar EP, Kumar A, Emerson SF. Free radical scavenging property and diuretic effect of triglize, a polyherbal formulation in experimental models. J Pharmacol Pharmacother. 2010;1:38–41. doi: 10.4103/0976-500X.64535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95:57–61. doi: 10.1016/j.jep.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 15.What is an LD50 and LC50. [Last accessed on 2011 Apr 29]. Available from http://www.ccohs.ca/oshanswers/chemicals/ld50.html .
- 16.Maneksh D, Sidharthan A, Kettimuthu K, Kanthakumar P, Lourthuraj AA, Ramachandran A, et al. Cleistanthus collinus induces type I distal renal tubular acidosis and type II respiratory failure in rats. Indian J Pharmacol. 2010;42:178–84. doi: 10.4103/0253-7613.66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subrahmanyam DK, Mooney T, Raveendran R, Zachariah B. A clinical and laboratory profi le of Cleistanthus collinus poisoning. J Assoc Physicians India. 2003;51:1052–4. [PubMed] [Google Scholar]
- 18.Nampoothiri K, Chrispal A, Begum A, Jasmine S, Gopinath KG, Zachariah A. A clinical study of renal tubular dysfunction in Cleistanthus collinus (oduvanthalai) poisoning. Clin Toxicol (Phila) 2010;48:193–7. doi: 10.3109/15563651003641786. [DOI] [PMC free article] [PubMed] [Google Scholar]


