Abstract
Hypertension is the very common chronic disease in rural, urban and semi-urban areas of today's world, which needs continuous monitoring and treatment through out the life. Lack of education, lifestyle modification, and low level of understanding on disease management in rural people will influence directly on their quality of life (QOL). The objective of this study was to know the impact of clinical pharmacist interventions on medication adherence and QOL. It was a prospective, randomized and interventional study. Fifty-six patients were enrolled; only 52 patients completed the study. Interventional group patients received patient counselling, patient information leaflets (PILS), and frequent telephonic reminding. In the baseline, first and second follow-ups, medication adherence and QOL were assessed by using Morisky Medication Adherence Scale (MMAS) and Medication Adherence Report Scale (MARS) Questionnaires and SF-12v2 Quality of life Questionnaire in both the groups. The results showed that systolic blood pressure P value in the second follow-up was 0.086+ when compared to baseline follow-up P value 0.094. The diastolic blood pressure reading of the intervention group at the second follow-up was 77.73 ± 3.63 in mmHg when compared to the baseline, i.e. 86.62 ± 11.35 in mmHg. The MMAS and MARS scores P values were 0.007**, 1.000, <0.001**; 0.007, 0.014 and 0.000 at the baseline, first and second follow-ups, respectively. The QOL score P values of physical component scale and mental component scale showed highly significant. This study concluded/showed that the impact of clinical pharmacist provided patient counselling had a positive impact on medication adherence and QOL.
Keywords: Blood pressure, hypertension, medication adherence behaviour
INTRODUCTION
Hypertension (HTN) is the one of the cardiovascular diseases estimated to cause 7.1 million deaths annually, accounting for 13% of all deaths globally. Overall 26.4% (972 million) of the adult world population was estimated to have HTN in the year 2000. This is projected to increase by 29.2% (1.56 billion) for the year 2025.
In India, the prevalence of HTN reports was increasing rapidly in the urban, i.e. 25% of adults, and gradually even in rural areas, i.e. 10% of individuals are affected. The same study estimated that there were about 66 million hypertensive patients in India (out of 66 million hypertensive patients—34 million are in urban areas and 32 million in rural areas). This clearly indicates that medication nonadherence is the multifaceted problem, responsible for increasing the important medical and public health issues like worsened therapeutic outcome, higher hospitalization rates, and increased health care costs.[1]
According to WHO Medication Adherence is defined as “the extent to which a person's behaviour [in] taking medication corresponds with agreed recommendations from a health care provider” (World Health Organization, 2003).[2] Therefore, medication adherence is one of the important factors helps in keeping the vital link between the treatment and the therapeutic outcomes in medical care.[3]
The two methods available for measuring adherence are direct and indirect methods. In direct methods measurement of concentrations of a drug or its metabolite in blood or urine, and detection or measurement in blood of a biologic marker added to the drug formulation are used. These approaches are expensive, burdensome to the health care provider, and susceptible to distortion by the patient. The indirect methods include asking the patient about how easy it is for him or her to take prescribed medication, assessing clinical response, performing pill counts, ascertaining rates of refilling prescriptions, collecting patient questionnaires, using electronic medication monitors, measuring physiologic markers, asking the patient to keep a medication diary, and assessing children's adherence by asking the help of a caregiver, school nurse, or teacher. These questioning the patient methods will help the healthcare provider for estimating the medication adherence indirectly without pain.[4]
Quality of life (QOL), a broad multidimensional concept, usually includes subjective evaluations of both positive and negative aspects of life. Health is one of the important domains of overall QOL; health related quality of life (HRQOL) questions about perceived physical and mental health and function have become an important component of health surveillance and are generally considered as valid indicators of service needs and intervention outcomes.[5–11]
This study was carried out in a 750 bedded tertiary care rural hospital of General Medicine Department, for the first time to know the impact of clinical pharmacist interventions on medication adherence and QOL.
MATERIALS AND METHODS
This was a prospective randomized and interventional study conducted in the Medicine department of Adichunchanagiri Hospital and Research Center, B G Nagara, for a period of 7 months. Ethical committee clearance was obtained prior to the study from Adichunchanagiri Hospital and Research Centre.
Study criteria
Inclusive criteria
Inpatients and outpatients of General Medicine Department who were diagnosed and on medication for hypertension over a period of 6 months.
18 years and above patients of either sex.
Patients who are willing to participate and give the consent form.
Exclusion criteria
Patients with comorbidities (more than four diseases).
Pregnant/lactating women.
Sources of data
Inpatients
Patient case records, medication charts and lab reports.
Outpatients
Prescriptions.
Materials used
Informed consent form, patient data collection form, patient information leaflets (PILS) regarding disease and drugs, diary card, questionnaires [Morisky Medication Adherence Scale (MMAS), Medication Adherence Report Scale (MARS), SF-12v2 Quality of Life (QOL) Scale, and patient satisfaction questionnaire (PSQ)].
Study procedure
After obtaining the patient consent, the patients were randomized into the intervention and control group by a simple randomization technique [i.e. odd (in the control group) and even numbers (in an interventional group)] in order to minimise/prevent the bias. The required details/data were obtained from outpatient cards (OP card), case records of inpatients, and by direct interviews. The patients were also informed to come for the first and second follow-ups after 1 month from the base line or from the date of enrolment.
The control and intervention group patients were interviewed and their sociodemographic details were recorded in the patient data collection forms along with baseline blood pressure levels and body mass index (BMI). In order to know the medication adherence behaviour (MAB) of both control and intervention groups, they were provided with the dairy card and which was collected at the end of the study. The control group did not provide with any counselling and PILS (patient information leaf lets) at the baseline and in the first follow-up. However, they were provided with oral instruction and PIL at the end of the second follow-up. The intervention group patients were counselled on various aspects such as, drugs, lifestyle changes, and their disease management, and told them to inform if any unwanted and unintended effects of drugs occurs at any follow-ups. These patients mediation adherence and QOL were assessed by using standard questionnaires, i.e. morisky medication adherence scale (4 items), MARS (5 items), and SF-12v2 QOL to know their medication adherence and QOL, respectively, in the baseline and follow-ups. The answers given by them were recorded. In each follow-ups and baseline the patient's blood pressure values were noted/measured. At the end of the second follow-up, diary cards were collected back.
The patient satisfaction questionnaire (PSQ) was prepared by selecting the suitable questions from the validated osteoporosis patient satisfaction questionnaire (OPSQ) and the expert's opinion was taken to administer for the intervention group, to know the impact of clinical pharmacy services and types of counselling services provided by the clinical pharmacist. The obtained data were subjected for statistical analysis.
Statistical methods
Descriptive statistical analysis has been carried out in this study. Results on continuous and categorical measurements are presented on mean±SD (min–max) and in number (%). Significance is assessed at the 5% level of significance.[12–15]
RESULTS
A total of 118 patients were approached and explained about the study and study procedure briefly, out of which only 56 patients agreed to participate in the study. Four patients were dropped out because of negligence, left the place, illiteracy, and age factor. The total number of patients who had completed the study was 52 (26C+26I).
The basic demographic variables of patients showed, at the age group of 51–60 (34.6%), 61–70 (38.5%) years was found to be more in the control and in the intervention group. In gender wise, males were more, 16 (61.5%) and 21 (80.8%), than the females, 10 (38.5%) and 5(19.2%), in both the interventional and control group. The BMI results showed normal range patients were more in both the groups. Education, occupation, and annual income details showed more illiterates, farmers, Rs. <25,000 in both the groups, respectively. The clinical variable of patients showed that only 14 (53.8%) had alcohol habit in the control group and non-alcoholics were found to be 14 (53.8%) in the intervention group. Nonsmokers were found more in both the groups. The diabetes mellitus was the one of the more common comorbid conditions and there is no suggestive of family history of disease in both the groups [Tables 1 and 2].
Table 1.
Patients demographic details

Table 2.
Clinical variables of hypertensive patients

The distribution of systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the patients, at the base line and second follow-ups, i.e., SBP (in mmHg) of control and intervention groups was 138.85 ± 16.03, 147.54 ± 20.45 and 131.08 ± 5.16, 128.27 ± 6.35, respectively. The DBP (in mmHg) of the control and intervention groups at the base line and second follow-ups was 81.12 ± 7.16, 86.62 ± 11.35 and 78.46 ± 4.12, 77.73 ± 3.63, respectively [Table 3].
Table 3.
Comparative distribution of blood pressure of hypertensive patients

The distribution of medication adherence scores of MMAS and MARS statistically showed a strongly significant P value, in both the baseline and second follow-up, i.e. 0.007** and <0.001** for MMAS and for MARS the P values are 0.000, 0.082, 0.003, 0.164, and 0.000 from questions 1–5 at the second follow-up. The overall total of MARS score P value at the second follow-up was 0.000 [Tables 4 and 5].
Table 4.
Comparison of Morisky medication adherence scale scores

Table 5.
Comparison of medication adherence reporting scale scores

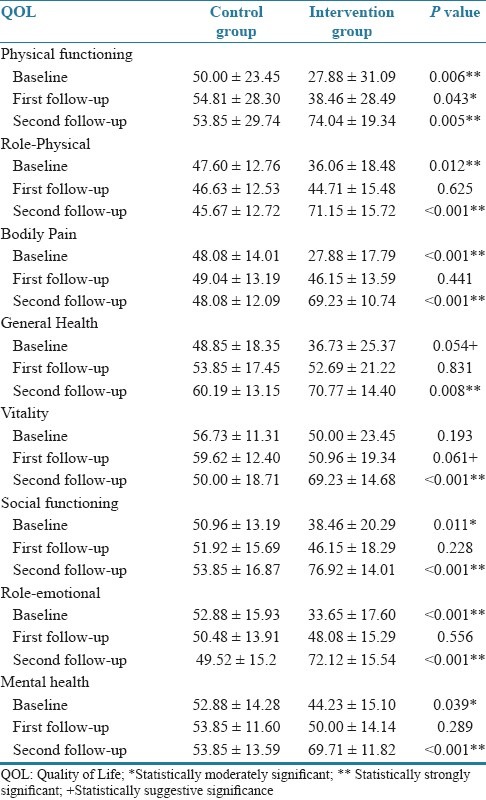
The comparative distribution of individual QOL domain scores (i.e. PF, RP, BP, GH, VT, SF, RE, and MH) of patients at the base line and second follow-up results was 0.006**, 0.012**, <0.001**, 0.054+, 0.193, 0.11*, <0.001**, 0.039FNx01 and 0.005**, <0.001**, <0.001**, 0.008**, <0.001**, <0.001**, <0.001**, <0.001**, showing highly significant values [Table 6].
Table 6.
Comparative distribution of quality of life (SF-12v2 QOL) domain scores in HTN patients
The distribution of over all QOL, i.e. physical component scale (PCS) and mental component scale (MCS) scores in two groups of patients showed at the baseline, first and second follow-ups were (0.003**, 0.153, <0.001**) and (0.006**, 0.394, <0.001**), respectively, this shows a good improvement in both the component/over all [Table 7].
Table 7.
Comparative distribution of quality of life (SF-12v2 QOL) PCS and MCS Scores of patients

The patient satisfaction about the pharmacist involvement in the disease management showed the average score of 33.73 ± 4.3 (max score 45) for the clinical pharmacy services and 18.38 ± 1.88 (max score 25) for types of counselling [Table 8]. The dairy cards were returned by only 29 patients (11 control and 18 intervention) at the end of the follow-up.
Table 8.
Patient satisfaction questionnaire about the pharmacist provided clinical pharmacy services and types of counselling

DISCUSSION
In this study, only 56 patients were accepted and involved/participated because maximum people had an afraidness to give the consent, location and difficulty in follow-ups and what the pharmacist can do. The consented people were participated and only four were dropped out, due to inability to come for regular follow-up, due to negligence, age factor, illiteracy, and left the place.
The results of the blood pressure (both SBP and DBP values) show a very good improvement from the base line to the second follow-up. This strongly showed that there is a clinical pharmacist influence/positive impact on patient counselling (i.e., interventions made and provided PILs).
The assessment of medication adherence scores by MMAS clearly showed that there was a good improvement in MAB of the patients both in control and intervention groups. The control group, showed little improvement because of repeated follow-ups that made them to think about life maintenance. However there was a very good improvement in intervention when compared to the control group because the intervention group patients were provided with counselling, PILS, and frequent telephone reminding makes them to strongly adapt to think about disease management.
Medication adherence report scale
The comparative results of the baseline to the second follow-up shows that there is a good improvement in medication adherence and the various factors influencing for the nonadherence rate was reduced from the baseline to the second follow-up. This point strongly suggests that the pharmacist influence had a very important role in the MAB. The MAB directly influences positively on QOL of a chronic patient
The various QOL domains scores showed a good improvement when compared the baseline to first follow-up and from first follow-up to second follow-up, and the baseline to second follow-up. The final individual domains suggest that the overall QOL was improved. This strongly suggests a positive influence on their QOL. However, still there is a need of continuous monitoring to manage their disease/QOL in a constant manner.
The stress is the one of the factor which will influence on the QOL (i.e. physical and mental health). Comparison of QOL PCS and MCS scores of patients after intervention demonstrated a larger improvement in PCS and MCS than control. The mental strength/stamina is the one which is directly proportional to the physical activity so more improvement in the MCS clearly states that there was an improvement in the management of their disease. Even the control group patients stated/felt that there was a need of more information on other drugs. The comparison of overall QOL domains shows that there are strongly significant values.
The dairy cards were provided to control and interventional group patients as a reminder to their medications returned back was less. This may be due to forgetfulness, lack of education, and negligence. This showed more observation on returning of this dairy card will also enhance the disease management.
Seventy six percent of the patient satisfied about the pharmacist provided clinical pharmacy services and types of counselling was observed. This strongly suggest that the pharmacist provided clinical pharmacy services and the patient counselling had a very good impact and need to be find out reasons and strategies to improvise it to 100%.
CONCLUSION
This study showed that clinical pharmacist intervention among rural population has a very strong positive impact in creating awareness about the disease, and its maintenance by increasing their medication adherence and QOL.
This study also concluded that pharmacist involvement/need is very important in other chronic disease managements of rural population for increasing the QOL by preventing recurrence of disease, its progression, and minimizing of hospital admissions.
ACKNOWLEDGMENTS
Our sincere pranamas to Sri Dr. Balagangadharanatha Maha Swamiji, Chairman of Sri Adichunchanagiri Shikshana Trust. We would like to thank Dr. B. Ramesh, the Principal, SACCP, staff and PGs of Department of Medicine AH and RC, Dana Kopec and Donald E Morisky, ScD, ScM, MSPH, for providing permission to use the SF12v2 (QOL) and Morisky Medication Adherence Scale (©MMAS-4 Item), respectively. Their timely support and suggestions at all stages of this work are greatly appreciated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Palanisamy S, Sumathy A. Intervention to improve patient adherence with antihypertensive medications at a tertiary care teaching hospital. Int J Pharm Tech Res. 2009;1:369–74. [Google Scholar]
- 2.Adult Meducation. Improving Medication Adherence in Older Adults. American Society on Aging and American Society of consultant Pharmacist foundation. c2006. [Last accessed on 2011 Aug 25]. Available from: http://www.adultmeducation.com/index.html .
- 3.Parthasarathi G, Mahesh PA. Medication adherence. In: Parthasarathi G, Nyfort-Hansen K, Nahata MC, editors. A text book of clinical pharmacy practice. Chennai: Orient Longman Private Limited; 2004. p. 54. [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Health related quality of Life (HRQOL) 2010. [Last accessed on 2011 Mar 17]. Available from: http://www.cdc.gov/hrqol/concept.htm .
- 6.Albercht S. The pharmacist's role in medication adherence. U S Pharm. 2011;36:45–8. [Google Scholar]
- 7.Krousel-Wood MA, Islam T, Webber LS, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in hypertensive seniors. Am J Manag Care. 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 8.McKenney JM, Slining JM, Henderson HR, Devins D, Barr M. The effect of clinical pharmacy services on patients with essential hypertension. Circulation. 1973;48:1104–11. doi: 10.1161/01.cir.48.5.1104. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcante MA, Bombig MT, Filho BL, Carvelho AC, Paola AA, Povoa R. Quality of life of hypertensive patients treated at an outpatient clinic. Arq Bras Cardiol. 2007;89:245–50. doi: 10.1590/s0066-782x2007001600006. [DOI] [PubMed] [Google Scholar]
- 10.Isabelle C, Jean-Pierre G, Jocelyne M, Isabelle C. Quality of life in hypertension: The Sf-12 compared to the Sf-36. Can J Clin Pharmacol. 2004;11:e232–8. [PubMed] [Google Scholar]
- 11.Adepu R, Ari SM. Influence of structured patient education on therapeutic outcomes in diabetes and hypertensive patients. Asian J Pharm Clin Res. 2010;3:174–8. [Google Scholar]
- 12.Rosner B. Duxbury. 5th ed. 2000. Fundamentals of biostatistics; pp. 80–240. [Google Scholar]
- 13.Riffenburg RH. 2nd ed. Academic Press; 2005. Statistics in medicine; pp. 85–125. [Google Scholar]
- 14.Sunder Rao PS, Richard J. New Delhi: Prentice Hall of India; 2006. An introduction to biostatistics, a manual for student in health sciences; pp. 86–160. [Google Scholar]
- 15.Eng J. Sample size estimation: How many individuals should be studied? Radiology. 2003;227:309–13. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]


