Abstract
Human biomonitoring has evolved beyond margins to ascertain exposure-response relationship in environmental associated human diseases. As occupational ailments continue to dominate global concerns, biomonitoring strategies have evolved better in terms of evaluating health risks associated with systemic uptake from chronic (long-term) environment exposures. Even though contributions of acute toxic exposures (short-term) towards initiation of disease processes have been gradually recognized, a comprehensive approach delineating mechanistic insights of such an implication remains elusive. Molecular biomonitoring in a strictly selected defined surviving cohort of the infamous Bhopal gas tragedy “as a model”, could provide an unparallel opportunity to discern the long standing implications of acute exposures. Besides comprehending clinical significance of isocyanate toxicity, the results might provide a framework for understanding the molecular repercussions pertaining to a host of other such acute environmental exposures. The investigative strategy might also be helpful in identification of biomarkers with potential for translational research.
Keywords: Bhopal gas tragedy, biomarkers, environmental medicine, genome wide studies, health risk assessment, translational research
Introduction
The developments in the post-genomics era has provided immense opportunities for pragmatic assessment of environmental and occupational exposure to noxious substances. Identification and validation of various biomarkers, toxicogenomic effects and gene-susceptibilities have revolutionized our understanding of human occupational diseases1. Risk assessment is often of great clinical relevance but as occupational diseases continue to dominate global concerns, biomonitoring strategies have been evolved for evaluation of health risks associated with systemic uptake from chronic (long-term) exposures in comparison to acute (short-term and peak) environmental exposures2. Acute exposures to relatively high concentrations or doses are a regular cause of concern. An acute exposure may be encountered through food intoxication (the dioxin-crises in Belgium and The Netherlands) or through accidental releases of contaminants from industries (the Seveso incident, the Bhopal disaster) or from a fire (in waste storage in The Netherlands)3. In such scenarios, questions emerge about whether acute short-term exposure (1 h to 10 days) to highly toxic substance/s could possibly contribute to long-standing repercussions and if so, whether these implications can be quantified employing definitive measures.
Biomonitoring of the 570,000 ailing survivors of the infamous Bhopal gas tragedy as a model, could provide an unparallel opportunity to comprehend the long-term implications of acute environmental toxic exposures. On December 3, 1984, the accident was caused by a runaway reaction in a gigantic storage tank in which 42 tons of methyl isocyanate (MIC) was stored in the Union Carbide India Limited (UCIL) pesticide plant in Bhopal, capital of Madhya Pradesh, a State situated in Central India. As a result, the huge poison cloud of MIC gas and its breakdown products leaked into a densely populated area of the city and drifted across 75-100 km2, killing thousands of people and injuring hundreds of thousands4. Systematic studies conducted in the exposed cohort revealed that a large fraction of the surviving population continues to be chronically ill5. Higher incidence of chronic health problems including pulmonary fibrosis, bronchial asthma, chronic obstructive pulmonary disease (COPD), emphysema, recurrent chest infections, keratopathy and corneal opacities have been reported to still persist in the MIC affected population6,7. Though there has been an international consensus on the nature, severity of damage and sufferings of the survivors being of superlative magnitude, efforts at understanding the molecular repercussions and lapses in strategic planning to institutionalise studies to uncover the carnage has recently been summarised8.
Utility and clinical significance of biomonitoring in Bhopal
For a developing economy like India which is fraught with human, environmental and economical perils, dissecting out the long standing effects of the Bhopal disaster will be of immense value and significance in dealing with future chemical hazards, if any9. It is imperative to design a coherent strategy for human biomonitoring (Table). This could be best achieved by initiating a debate involving experts and linking national and international institutions of repute working on environmental health risk assessments. Restructuring of research teams owing responsibility of investigating the long-term ill effects will be essential to develop a committed inter-disciplinary talent pool best available in the biomedical research enterprise. Empirical multi-variant epidemiological exposure analysis may not be able to untangle all complexities of risk-assessment unless mechanistic molecular studies evaluating dose-response relationships from available clinical data and resources are scientifically analyzed. Investigative biomonitoring will require (i) Suitable biological matrices - easily accessible in sufficient volumes under routine conditions. Samples should be collected and stored using standard operating procedures. (ii) Selection of parameters of the study should be able to reflect internal incidental exposure and biochemical effects. (iii) Quality control and assurance of the analytical methods employed should be validated for efficient interpretation of results. Since 28 years have elapsed following the exposure, a careful and deliberate attempt should be made to identify and recruit subjects to ensure that they were indeed exposed to MIC10. Besides recruiting exposed individuals of 27 to 50 yr age from affected zones (within 2.5 km from the plant) for the studies, age and gender matched non-exposed healthy controls should be recruited from places within the geographical region of Bhopal but unaffected zones (more than 25 km from the plant) from places well outside geographical region of Bhopal (more than 200 km from the plant). Confounding factors such as socio-economic conditions, history and duration of stay, tobacco use/alcohol consumption, and any significant medical history impeding the evaluatory framework of exposure-response relationship should be assessed during subject recruitment. Exposure of the subjects to MIC must be ascertained on the basis of the following criteria: (i) Physical presence and address of stay on the night of MIC leak from the factory; (ii) Distance from the factory; (iii) Protective measures taken at the time of gas leak, if any; (iv) Remained outdoor or indoor; (v) If outdoor, what was the activity profile, i.e. running or exertion or walking or driving, etc.; (vi) The overall symptom profile like respiratory, cutaneous and ocular symptoms on exposure on that night to corroborate exposure11.
Table.
List of biological measures, endpoints and indices proposed for inclusion in the investigative strategy for human biomonitoring in the methyl isocyanate exposed surviving population of the Bhopal gas tragedy
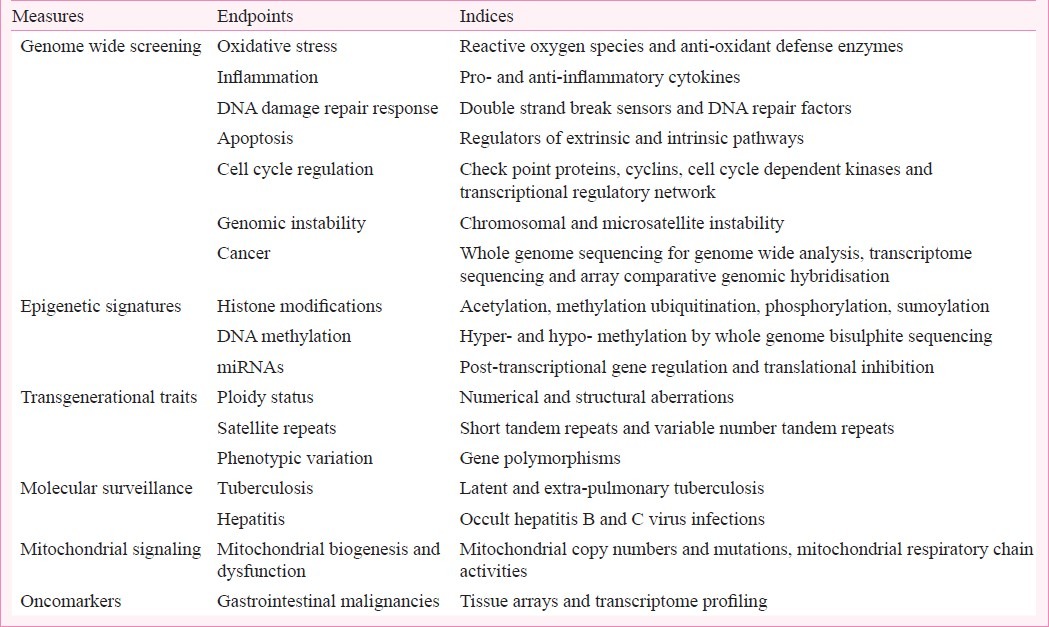
Genome wide screening
The process of human biomonitoring includes the assessment of three basic end points of chemico-biological interaction: mutagenesis, abnormal cell proliferation and alteration of the expression of genes at transcriptional, translational, or post-translational levels. Acute environmental toxic exposure has the potential to induce one or more of these end points. But ultimately to illustrate the causal links of exposure and a human disease, we need to use cutting edge molecular technologies that have provided revolutionary insights to understand the genesis of environment associated diseases12. Theoretically, gene-gene, gene-environment, and individual genetic heterogeneity in signalling mechanisms appear to be important contributors of persisting ailments. However, a population-based large scale genome wide association study (GWAS) of the exposed cohort is warranted to ascertain the often debated weakest link of exposure-response relationship. The sequence of events that leads to oxidative stress/inflammation, DNA damage response/apoptosis, overcome damage repair mechanisms, genomic instability, and oncogenic transformation following in vitro exposure to MIC have been documented systematically13–17. A parallel study conducted in surviving cohorts of the Bhopal gas tragedy not only displayed significant enhanced levels of circulating inflammatory biomarkers presenting a persistent and subtle toxic effect but also corroborated with the results of in vitro findings11. Proposal to refine the existing information and seek to decipher any persistent and subtle genotoxic effect of MIC in well designed genome wide studies might enhance understanding to the extent and gravity of long term effects.
Epigenetic signatures
Epigenetic studies have abruptly changed our vision to the determinants of exogenous influence on human ailments18. Mechanistic laboratory investigations and clinico-epidemiological data have provided insights of heritability of epigenetic traits suggesting molecular influence of the environment to extend well beyond the interaction with the DNA sequence19,20. A persistent hyper-responsive cellular and humoral immune state observed two decades later in individuals, in utero exposed to MIC during the Bhopal gas tragedy offers a potential insight into epigenetic modulation of the immune system10. Downstream investigations could prove to be fundamental in determining the individual risks and help us in assortment of heritable epigenetic traits likely to be transmitted to future generations.
Transgenerational traits
Whether genetic and epigenetic changes in somatic cells resulting from in utero, early life and adulthood exposure can be correlated to deleterious transgenerational consequences is uncertain. In addition to the increasing incidence of genetic abnormalities in the resulting offspring, risks related toxic exposures have been associated with accelerated ageing, Alzheimer's disease, congenital heart defects, non-familial schizophrenia, diabetes, gonadal dysgenesis and many other life threatening diseases including cancer21. Experimental evidence from studies conducted on spermatogonial stem cells and ovarian epithelial cells treated with MIC manifested chromosomal aberrations, telomere anomaly, aneuploidy and variable amplification of microsatellite repeats. This was accompanied by evidence of a deregulation of cell cycle progression, such as substantial fold changes in the expression of proliferating cell nuclear antigen, cyclin D1, Bcl-2 and Bax genes, aberrant expression of p53, cyclin A, cyclin E, cyclin dependent kinase 2 protein (CDK-2) and aurora kinase-B proteins. Immuno-FISH analysis illustrated early loss of telomeric repeat-binding factor2 protein (TRF2) protein suggestive of telomeric dysfunction due to premature senescence and plausible association with chromosomal and microsatellite instability22,23. Apart from direct consequence of the initial lesions produced in cellular DNA, non-targeted effects of toxic exposure such as bystander responses, genomic instability, gene induction, adaptive responses and low hypersensitivity may also induce transgenerational health effects. These effects may be modulated by the specific germ-line stage exposed and cellular repair proficiency associated with the transmission of heritable genomic alterations. Screening of MIC-induced transgenerational alterations in germline genome in F0, F1 and F2 generations of Bhopal gas victims may address the less understood environmental influence of heritable or familial component of susceptibility to infectious and chronic non-communicable diseases.
Molecular surveillance of infectious disease trends
Patterns of antimicrobial resistance, spread of infectious diseases and molecular disease surveillance studied in recent decades have been comprehensively attributed to global rise in accidental or occupational exposure to environmental contaminants24. Pesticides, air pollutants, industrial wastes and heavy metals through a broad array of genetic and epigenetic mechanisms have significantly altered immune cell function thereby imposing a greater threat to human lives from infections25. A retrospective analysis of molecular diagnostics records, as a data source for monitoring possible health effects for the surveillance of hepatitis and Mycobacterium tuberculosis infections amongst the first and second generations of survivors of Bhopal gas tragedy revealed HBV infections to be most common among the MIC exposed cohort, followed by extra-pulmonary and pulmonary TB and HCV infections. Genotype 3 was the most prevalent HCV genotype among the survivors. Failure to detect HBsAg, anti-HBc and anti-HCV through ELISA and tuberculosis by culture and Ziehl-Neelson stain in significant number of samples indicated higher prevalence of occult hepatitis and latent tuberculosis in the affected population26. As the risk of progress of infection is often influenced by conditions and periods of environmental chemical exposure, insights of interconnected molecular pathways might illuminate gene environment association and offer valuable information for shaping better strategies of prevention and management of these infectious trends in the surviving population.
Mitochondrial signalling
Given the central role of mitochondria in energy production and utilization, the premise that mitochondrial dysfunction might play an impending role in environmental toxin induced pathophysiology of various human diseases has been realized27. Mitochondria contain their own extranuclear DNA (mtDNA), which is a potentially susceptible target of various environmental mutagens/carcinogens. Lack of protective histones, chromatin structure, introns, proof reading apparatus of DNA repair, and close proximity to free radicals makes mtDNA more vulnerable to environmental insult. The mutation rate of mtDNA is greatly increased compared with nuclear DNA, and an increase in the reactive oxygen species (ROS) burden induced by environmental toxic exposure could further lead to a vicious cycle of oxidative damage and organellar dysregulation28. In addition to deletions, a reduction in the copy number of mtDNA could alter mitochondrial function, which might also lead to increased cellular ROS. mtDNA alterations may result in changes in mitochondrial oxidative phosphorylation (OXPHOS), and the emergence of the glycolytic phenotype over OXPHOS, promoted by mitochondrial dysfunction29. Experimental evidence implicate that in vitro exposure to MIC enhances mutations and oxidative damage to mtDNA thereby eliciting oxidative stress, oxidative DNA damage, failure to maintain regulatory gene mechanisms controlling the process of OXPHOS and an imbalance of anti-oxidant defence system30. Oxidative stress measurement techniques have neither been fully employed nor exploited to improve the monitoring of populations exposed to environmental toxins, particularly the relationship between oxidative stress and disease outcomes. Dispelling out the status of mitochondrial genome, mutation in genes and factors affecting its biogenesis and dysfunction could offer prospective significance for investigating relationship between mitochondrial function and acute environmental toxic exposure of MIC.
Oncomarkers
The establishment of a tumour biorepository from malignant tissues of Bhopal gas victims with linkage to clinical and epidemiologic data will provide an invaluable resource for cancer research. This translational objective might address issues pertaining to aetiology, progression, and prognosis of different cancer patterns in relation to MIC exposure but might, as well help in validating biomarkers for environmental carcinogenesis. Observing high frequency of gastrointestinal carcinomas in the MIC exposed cohort31, a retrospective analysis of gene signatures in gall bladder carcinomas was conducted. In the pilot study, tissues from 92 cases of gall bladder carcinoma were examined for K-ras, p-53, cyclin-E and Rad-50 expression. Microsatellite instability was determined from PCR amplifications of six-microsatellite marker loci (D16S539, D13S317, D7S820, F13A01, FES/FPS, vWA). Preliminary evidence suggests Rad-50 and cyclin-E as potential biomarkers for early diagnosis of environmental stress induced gall bladder carcinoma32,33. However, a larger study on archived tumour tissues is warranted to establish the cause-effect relationship of acute MIC exposure with cancer risk.
Issues and challenges
Scientific and technological advances in genomics, proteomics, and cytomics have unravelled several processes involved in the genesis of environment associated ailments. Mechanistic investigations coupled with epidemiological studies (both case-control and cohort) have demonstrated that individual risk of developing an environment associated disease phenotype involves complex interplay of molecular reprogramming. Establishment of an effective dose-response relationship is often impeded by stochastic determinants such as magnitude and duration of exposure, genetic make-up, immune susceptibility, activity patterns, nutrition, socio-economic status, and lifestyle. In case of a lag between acute toxic exposure and manifestation of the illness due to latent sub-cellular injury, comprehension of such a relationship is an arduous task to achieve. Such difficulties are only compounded by deficiency in knowledge of a specific biomarker directly related to the class of exposure, like in the case of Bhopal disaster. Moreover, for biological monitoring of the population that survived the Bhopal gas tragedy, identification of an exposed cohort would be a major challenge. Heterogeneous composition resulting from population migration during the past 28 years would require painstaking efforts to identify a defined exposed cohort for the suggested investigations. Wide array of biomarkers proposed to be studied would also necessitate substantial investment in a constrained research setting. Therefore, for meaningful implementation of such a monumental task, institutional support and organizational impetus are highly imperative. Besides documenting the morbidity and increasing the understanding of MIC toxicity, the results might provide a framework to understand the molecular repercussions pertaining to a host of other such acute environmental exposures. The investigative strategy might also aid identification of novel biomarkers thereby stimulating efforts to design newer and more effective diagnostic and therapeutic strategies with potential of research translation.
References
- 1.Manno M, Viau C, Cocker J, Colosio C, Lowry L, Mutti A, et al. Biomonitoring for occupational health risk assessment (BOHRA) Toxicol Lett. 2010;192:3–16. doi: 10.1016/j.toxlet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Neumann HG. Risk assessment of chemical carcinogens and thresholds. Crit Rev Toxicol. 2009;39:449–61. doi: 10.1080/10408440902810329. [DOI] [PubMed] [Google Scholar]
- 3.Bos PM, Baars BJ, van Raaij MT. Risk assessment of peak exposure to genotoxic carcinogens: a pragmatic approach. Toxicol Lett. 2004;151:43–50. doi: 10.1016/j.toxlet.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Sriramachari S, Chandra H. The lessons of Bhopal [toxic] MIC gas disaster scope for expanding global biomonitoring and environmental specimen banking. Chemosphere. 1997;34:2237–50. doi: 10.1016/s0045-6535(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 5.Report on health effects of the toxic gas leak from the methyl isocyanate plant in Bhopal. New Delhi: ICMR; 2004. Indian Council of Medical Research (ICMR) [Google Scholar]
- 6.Mishra PK, Samartha RS, Pathak N, Jain SK, Banerjee S, Maudar KK. Bhopal gas tragedy: review of clinical and experimental findings after 25 years. Int J Occup Med Environ Health. 2009;22:193–202. doi: 10.2478/v10001-009-0028-1. [DOI] [PubMed] [Google Scholar]
- 7.Vijayan VK. Methyl isocyanate (MIC) exposure and its consequences on human health at Bhopal. Int J Env Stud. 2010;67:637–53. [Google Scholar]
- 8.Balaram P. Bhopal: The tragedy of collective amnesia. Curr Sci. 2010;98:1547–8. [Google Scholar]
- 9.Broughton E. Bhopal disaster and its aftermath: a review. Environ Health. 2005;4:6. doi: 10.1186/1476-069X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra PK, Dabadghao S, Modi G, Desikan P, Jain A, Mittra I, et al. In-utero exposure to methyl isocyanate in the Bhopal gas disaster: evidence of persisting hyper-activation of immune system two decades later. Occup Environ Med. 2009;66:279. doi: 10.1136/oem.2008.041517. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava A, Punde RP, Pathak N, Dabadghao S, Desikan P, Mishra PK, et al. Status of inflammatory biomarkers in the population that survived the Bhopal gas tragedy: a study after two decades. Ind Health. 2010;48:204–8. doi: 10.2486/indhealth.48.204. [DOI] [PubMed] [Google Scholar]
- 12.Angerer J, Ewers U, Wilhelm M. Human biomonitoring: state of the art. Int J Hyg Environ Health. 2007;210:201–28. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Mishra PK, Panwar H, Bhargava A, Gorantla VR, Jain SK, Banerjee S, et al. Isocyanates induces DNA damage, apoptosis, oxidative stress, and inflammation in cultured human lymphocytes. J Biochem Mol Toxicol. 2008;22:429–40. doi: 10.1002/jbt.20260. [DOI] [PubMed] [Google Scholar]
- 14.Mishra PK, Gorantla VR, Akhtar N, Tamrakar P, Jain SK, Maudar KK. Analysis of cellular response to isocyanate exposure in cultured mammalian cells. Environ Mol Mutagen. 2009;50:328–36. doi: 10.1002/em.20469. [DOI] [PubMed] [Google Scholar]
- 15.Mishra PK, Bhargava A, Raghuram GV, Jatawa SK, Akhtar N, Khan S, et al. Induction of genomic instability in cultured human colon epithelial cells following exposure to isocyanates. Cell Biol Int. 2009;33:675–83. doi: 10.1016/j.cellbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Mishra PK, Bhargava A, Raghuram GV, Gupta S, Tiwari S, Upadhyaya R, et al. Inflammatory response to isocyanates and onset of genomic instability in cultured human lung fibroblasts. Genet Mol Res. 2009;8:129–43. doi: 10.4238/vol8-1gmr544. [DOI] [PubMed] [Google Scholar]
- 17.Mishra PK, Khan S, Bhargava A, Panwar H, Banerjee S, Jain SK, et al. Regulation of isocyanate-induced apoptosis, oxidative stress, and inflammation in cultured human neutrophils: Isocyanate-induced neutrophils apoptosis. Cell Biol Toxicol. 2010;26:279–91. doi: 10.1007/s10565-009-9127-9. [DOI] [PubMed] [Google Scholar]
- 18.Ziech D, Franco R, Pappa A, Malamou-Mitsi V, Georgakila S, Georgakilas AG, et al. The role of epigenetics in environmental and occupational carcinogenesis. Chem Biol Interact. 2010;188:340–9. doi: 10.1016/j.cbi.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–51. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawan C, Vaissière T, Murr R, Herceg Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat Res. 2008;642:1–13. doi: 10.1016/j.mrfmmm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Trosko JE, Upham BL. A paradigm shift is required for the risk assessment of potential human health after exposure to low level chemical exposures: a response to the toxicity testing in the 21st century report. Int J Toxicol. 2010;29:344–57. doi: 10.1177/1091581810371384. [DOI] [PubMed] [Google Scholar]
- 22.Raghuram GV, Pathak N, Jain D, Panwar H, Pandey H, Mishra PK, et al. Molecular mechanisms of isocyanate induced oncogenic transformation in ovarian epithelial cells. Rep Toxicol. 2010;30:377–86. doi: 10.1016/j.reprotox.2010.05.087. [DOI] [PubMed] [Google Scholar]
- 23.Raghuram GV, Pathak N, Jain D, Pandey H, Panwar H, Jain SK, et al. Molecular characterization of isocyanate-induced male germ-line genomic instability. J Environ Pathol Toxicol Oncol. 2010;29:213–34. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i3.50. [DOI] [PubMed] [Google Scholar]
- 24.Crinnion WJ. Environmental medicine, part one: the human burden of environmental toxins and their common health effects. Altern Med Rev. 2000;5:52–63. [PubMed] [Google Scholar]
- 25.Inadera H. The immune system as a target for environmental chemicals: Xenoestrogens and other compounds. Toxicol Lett. 2006;164:191–206. doi: 10.1016/j.toxlet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Mishra PK, Bhargava A, Pathak N, Desikan P, Maudar KK, Varshney S, et al. Molecular surveillance of hepatitis and tuberculosis infections in a cohort exposed to methyl isocyanate. Int J Occup Med Environ Health. 2011;24:94–101. doi: 10.2478/s13382-011-0006-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–34. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5:145–52. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Lim S, Cho YM, Park KS, Lee HK. Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann NY Acad Sci. 2010;1201:166–76. doi: 10.1111/j.1749-6632.2010.05622.x. [DOI] [PubMed] [Google Scholar]
- 30.Mishra PK, Raghuram GV, Panwar H, Jain D, Pandey H, Maudar KK. Mitochondrial oxidative stress elicits chromosomal instability after exposure to isocyanates in human kidney epithelial cells. Free Radic Res. 2009;43:718–28. doi: 10.1080/10715760903037699. [DOI] [PubMed] [Google Scholar]
- 31.Sriramachari S. Health effects of the toxic gas leak from the union carbide methyl isocyanate plant in Bhopal. New Delhi: Indian Council for Medical Research; 2004. Cancer patterns in MIC/toxic gas affected and unaffected areas of Bhopal (1988-2003) pp. 171–7. [Google Scholar]
- 32.Mishra PK, Jatawa SK, Raghuram GV, Pathak N, Jain A, Tiwari A, et al. Correlation of aberrant expression of p53, Rad50, and cyclin-E proteins with microsatellite instability in gallbladder adenocarcinomas. Genet Mol Res. 2009;8:1202–10. doi: 10.4238/vol8-4gmr653. [DOI] [PubMed] [Google Scholar]
- 33.Mishra PK, Raghuram GV, Jatawa SK, Bhargava A, Varshney S. Frequency of genetic alterations observed in cell cycle regulatory proteins and microsatellite instability in gallbladder adenocarcinoma: a translational perspective. Asian Pac J Cancer Prev. 2011;12:573–4. [PubMed] [Google Scholar]


