Abstract
Background & objectives:
In vitro assays are an important tool to assess baseline sensitivity and monitor the drug response of Plasmodium falciparum over time and place and, therefore, can provide background information for the development and evaluation of drug policies. This study was aimed at determining the in vitro sensitivity of P. falciparum isolates to antimalarials.
Methods:
The in vitro activity of 108 P. falciparum isolates obtained from five States of India was evaluated using WHO microtest (Mark III) to chloroquine, monodesethylamodiaquine, dihydroartesunate and mefloquine. Samples were collected from the States of Orissa, Jharkhand, Karnataka, Goa and Chhattisgarh from September 2007 to August 2009. In addition, representative samples from different States of India cryopreserved and culture adapted in the Malaria Parasite Bank of National Institute of Malaria Research, New Delhi, were also evaluated.
Results:
The proportion of isolates resistant to chloroquine and monodesethylamodiaquine was 44.4 and 25 per cent, respectively. Of the 27 isolates resistant to monodesethylamodiaquine, 16 (59.3%) were cross-resistant to chloroquine. No isolate showed resistance to dihydroartesunate and mefloquine. Isolates from Orissa showed the highest degree of resistance to chloroquine and amodiaquine followed by Jharkhand. Forty two isolates were genotyped for pfcrt T76K chloroquine resistant mutation; mutations were seen in 38 (90.47%) isolates.
Interpretation & conclusions:
The Indian P. falciparum isolates showed a high degree of resistance to chloroquine followed by monodesethylamodiaquine. No resistance was recorded to mefloquine and dihydroartesunate.
Keywords: In vitro sensitivity, India, malaria, Plasmodium falciparum
India reports about two million cases and 1000 deaths attributable to malaria annually1. One of the greatest challenges facing malaria control is the spread and intensification of parasite resistance to antimalarial drugs. The availability of limited number of effective drugs has led to increasing difficulties in the development of antimalarial drug policies and adequate disease management2. Surveys have shown rates of treatment failure higher than 50 per cent for chloroquine in most affected regions, as well as poor efficacy of sulphadoxine-pyrimethamine in sub-Saharan Africa and Southeast Asia3.
In India, chloroquine resistance in Plasmodium falciparum was first reported in Assam in 19734 and subsequently reported from several parts of the country5,6. India is a vast country with several eco-epidemiological subtypes of malaria and because of variability of drug pressure, it is necessary to evaluate efficacy of first and second line antimalarial drugs at several sites so as to help adopt alternative strategies for treatment6,7.
The National Vector Borne Disease Control Programme in India has introduced artemisinin based combination therapy (ACT)8 for falciparum malaria; hence there is a need to estimate the baseline sensitivity to the partner drugs. The in vitro assays provide information on the quantitative drug response of P. falciparum and serve as a tool for assessing baseline sensitivity and monitoring drug response of P. falciparum over time. In vitro assays can help to monitor drug sensitivity of even single antimalarial medicines. Sometimes changes in drug sensitivity can precede the clinical resistance, and in vitro assays can provide early warning of drug resistance. In the current study, we evaluated in vitro sensitivity of P. falciparum isolates from five different States to four antimalarials.
Material & Methods
P. falciparum isolates: From September 2007 through August 2009, P. falciparum isolates were collected from the States of Orissa, Jharkhand, Karnataka, Goa and Chhattisgarh. In addition, representative samples from different States of India cryopreserved and culture adapted in the Malaria Parasite Bank of National Institute of Malaria Research (NIMR), New Delhi, were also evaluated.
Active case detection was carried out by NIMR personnel by fever surveys in community to find malaria patients. Thick and thin blood films were prepared from persons suspected of malaria, and stained with Jaswant Singh and Bhattacharji stain. All patients were detected by active surveillance only. Patients having mono infections of P. falciparum and asexual parasitaemia of 1000 to 80000 per μl blood were considered suitable for testing. Persons receiving quinine, artemisinin or its derivatives within the last seven days, 4 - aminoquinolines within the last 14 days, pyrimethamine and/or sulphonamides within the last 28 days, or mefloquine within the last 56 days were excluded from the study. This was assessed by eliciting the history of consumption of the antimalarials.
The study was approved by the institutional ethics committee of National Institute of Malaria Research, New Delhi. Written consent was obtained from each patient. Consent from parents/guardians was obtained in case of children.
Treatment: All the diagnosed cases of malaria were treated as per the national drug policy. Artesunate and sulphadoxine pyrimethamine combination was given to falciparum malaria cases in designated resistant areas. Chloroquine was given to falciparum malaria cases in other areas and vivax malaria cases in all areas after collection of specimens. Antipyretics and antiemetics were administered as per need.
In vitro test: About two ml blood was collected by venepuncture and diluted to 1:10 with RPMI medium. Pre-culture thick and thin films were prepared. WHO microtest predosed plates of chloroquine (1-64 pmol/well), monodesethylamodiaquine (0.25-16 pmol/well), mefloquine (2-128 pmol/well) and dihydroartesunate (0.15-150 pmol/well) procured from the University Sains, Malaysia, were charged with 50 μl of the blood medium mixture. Plates were incubated at 37°C for 24-30 h, depending on the developmental stage of the trophozoites in the preculture slide.
After incubation, a series of thick films was prepared using deposits. The thick films were stained with Giemsa stain. Post culture blood slides were examined and the counts were read as the number of schizonts with three or more nuclei out of a total of 200 asexual parasites.
pfcrt K76T mutation analysis: Genomic DNA of parasite was isolated from filter paper using QIAamp DNA mini kit (Qiagen, Valencia, California, USA) according to the manufacturer's instructions in isolates. The pfcrt genotyping was done only in isolates from Karnataka. Mutations were detected using a set of allele-specific primers PfcF 5’- CCG TTAATAATAAATACAGGCAG-3’, PfcR 5’-CTTTTAAAAATGGAAGGGTGTATAC -3’ , K76 T Forward 5’ GGC TCA CGT TTA GGT GGA-3’ and K76T Reverse 5’ TGA ATT TCC CTT TTT ATT TCC AAA-3’ and restriction fragment length polymorphism (RFLP)9 using enzyme ApoI. RFLP products were electrophoresed on 2 per cent agarose gels and visualized under Gel documentation system (Alpha Innotech, USA) after staining with ethidium bromide.
Data management: The data obtained were analysed by nonlinear regression analysis. The IC50 of individual samples for each drug was determined as the concentration that inhibited 50 per cent parasites. HNnonLin version V1.1 software (www.malaria.farch.net) was used for this purpose10. The cut-off values for determining sensitivity to antimalarials were based on the WHO microtest protocol11. The critical minimum inhibitory concentrations for chloroquine, monodesethylamodiaquine and mefloquine were 160, 80 and 640 nmol/l, respectively. The protocol does not recommend cut-off for dihydroartesunate, hence the cut-off IC50 value of 10.5 nmol/l for artesunate as described by Pradines et al12 was used. The values are based on the logarithmic values of drug concentrations along the X axis. Statistical analyses were done using SPSS software (Version II, SPSS Inc., Chicago).
Results
A total of 218 P. falciparum isolates were obtained from five States, the average parasite count in the patients subjected to in vitro testing was 17540/μl blood. In addition, 19 isolates were also studied from the Malaria Parasite Bank of NIMR. These isolates were collected from Rajasthan, Orissa, Chhattisgarh, Assam and Gujarat. The proportion of successful assays was 58.8 per cent (n=117) and 36.8 per cent (n=7) in fresh and cultured isolates, respectively. Of these 124 isolates tested, 108 yielded sensitivity results for all the four antimalarials.
The geometric means of IC50 values were 33.7, 9.67, 3.4 and 46.9 nmol/l for chloroquine, monodesethylamodiaquine, dihydroartesunate and mefloquine, respectively (Table I). Fig. 1 shows a representative graph illustrating the typical response of a P. falciparum isolate to chloroquine.
Table I.
In vitro susceptibility of P. falciparum to four drugs
Fig. 1.
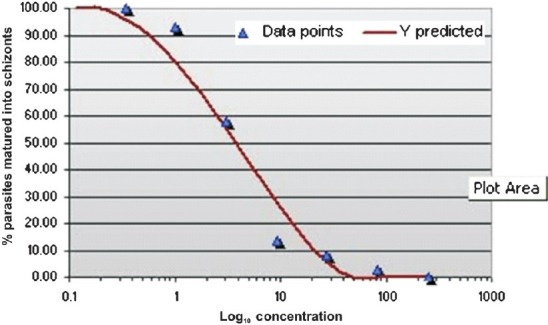
Response of a P. falciparum representative isolate to chloroquine.
Chloroquine resistance was observed in 44.4 per cent (48/108)isolates. A higher proportion of resistance was seen in Orissa followed by Jharkhand. Monodesethylmodiaquine resistance was also recorded (27 isolates, 25%) in all the studied States. Of these 27 isolates, 16 (59.3%) were also resistant to chloroquine (Fig. 2). No isolate showed resistance to dihydroartesunate or mefloquine (Table II).
Fig. 2.
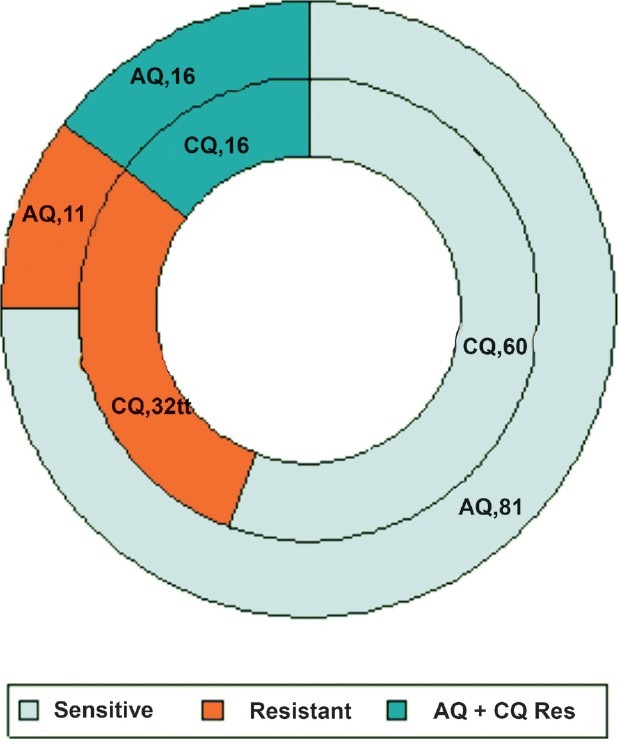
Cross-resistance between chloroquine and amodiaquine. CQ, chloroquine ; AQ, amodiaquine; figures succeeding drug name indicate number of isolates.
Table II.
Drug sensitivity pattern
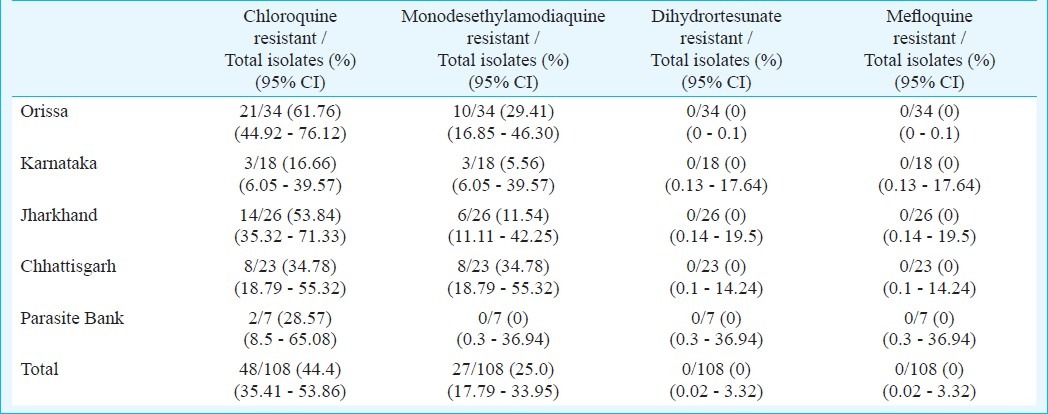
Forty two isolates were genotyped for pfcrt K76T chloroquine resistant mutation; mutations were seen in 38 (90.47%) isolates. A 264 bp region surrounding the Pfcrt K76T mutation was amplified by nested PCR, and the mutation was detected using the ApoI restriction enzyme. ApoI digestion produced two fragments in wild-type alleles, whereas the mutant allele remained undigested (Fig. 3). RFLP analysis revealed the presence of the mutant T76 allele in all 38 (100%) isolates. None of isolates carried the wild-type K76 alleles.
Fig. 3.
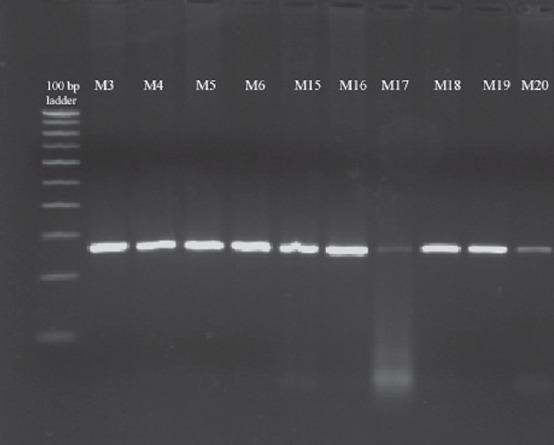
Restriction fragment length polymorphism analysis of pfcrt. Representative agarose gel electrophoresis of the pfcrt K76T polymorphism. Presence of the K76T mutation was detected using the ApoI restriction enzyme. ApoI does not cut the mutant allele (lanes 2-17 up & 2-7 down). Lane 1, 100 bp ladder.
Lanes 2-17 = Samples M3, M4, M5, M6, M15, M16, M17, M18, M19, M20, M21, M22, M23, M24, M25 Lanes
2-7 = Samples M26, M27, M28, M29 M30, M39.
Discussion
The study reports the drug sensitivity pattern of four antimalarials in 108 isolates of P. falciparum from different parts of India. Isolates collected from Orissa showed the higher proportion of resistance to chloroquine and amodiaquine as compared to other States. This was followed by Jharkhand. ACT was introduced both in Orissa and Jharkhand on the basis of chloroquine resistance for treatment of falciparum malaria; while there is no change in the policy in the other States studied13. There was wide variation in the proportion of chloroquine and amodiaquine resistant isolates in Orissa, Jharkhand and the other States. Similar results were seen in a study carried out in four States of the country in 199614. Therapeutic efficacy studies conducted in Orissa and Jharkhand also showed an efficacy of 57 and 54 per cent, respectively in chloroquine5. The wide variations may be attributed to various factors related to the host, parasite and the environment, antimalarials recommended in the area, immunity of the population and the parasite polymorphism. Further, transmission settings are also different in these regions.
It is important to monitor the in vitro drug susceptibility of P. falciparum regularly to guide the national drug policy. Artesunate + sulphadoxine-pyrimethamine was introduced in India in 2004 and later was extended to the whole country in 2010 for treatment of malaria14,15. One of the important criteria for deciding the partner drug is that it should have at least 80 per cent efficacy. The clinical efficacy of individual antimalarials is difficult to assess, since artemisinin monotherapy has been banned by the World Health Organization16. Thus, molecular markers and in vitro drug sensitivity assays are optimal to assess resistance to individual drugs. Molecular markers are not available for detecting resistance to all antimalarials. Thus, in vitro assays can be a useful tool in determining resistance and also its extent.
The fact that there was more success in carrying out the assay in field isolates rather than cultures isolates explains the difficulty in carrying out the test in isolates in running culture. This finding indicated that the assay was more suited for field isolates rather than cultures isolates. Similar success rates have been observed in other studies17.
All the isolates were sensitive to dihydroartesunate and mefloquine in vitro. Mefloquine has been sparsely used in the country only for short term chemoprophylaxis. The IC50 value for mefloquine obtained in this study was lesser than that obtained in Thailand five years back18. It would be important to monitor the levels after the drug consumption increases in the country. Artesunate showed IC50 level of 3.45 nmol/l (95% C.I. 3.29-3.62), similar to the one observed in isolates from Thailand, Bangladesh and Cambodia. The Cambodian isolates showed an IC50 value of 3.34 nmol/l in 2006-200719. But since predosed plates supplied by the WHO were used in the study, which had minimum drug concentration of 3 nmol/l; values below 3 nmol/well could not be detected. Considering the fact that there was no growth in the first well having 3 nmol/l dihydroartesunate in as much as 71(65.7%) isolates, it is highly probable that the actual IC50 values might have been much less than 3 nmol/l in these samples.
The National Drug Policy in India uses artesunate + sulphadoxine–pyrimethamine combination. Resistance to sulphadoxine-pyrimethamine is well known in India20. The results of in vitro sensitivity can guide on the selection of the partner drug of ACT apart from monitoring the minimum inhibitory concentrations of artemisinin derivatives as well as partner drug. Thus, this study suggests that mefloquine could be a better partner than amodiaquine. Further, the study confirmed the widespread resistance to chloroquine. Chloroquine was being used in many areas during the study period. Earlier also, chloroquine resistance was shown by therapeutic efficacy studies, and India switched over to ACT for falciparum malaria all over the country21.
To conclude, the study showed high levels of resistance to chloroquine; and also to monodesethylamodiaquine. No resistance was recorded in mefloquine and dihydroartesunate. Regular monitoring will help the policy makers in choosing the alternative ACT in future.
Acknowledgment
The authors acknowledge the Drugs for Neglected Diseases initiative (DNDi), Geneva, for financial support and thank Dr Harald Noedl, Associate Professor, Medical University of Vienna for kindly permitting the HN Non Lin software. Authors also acknowledge the help provided by the scientists and staff of the National Institute of Malaria Research, the Malaria Parasite Bank in particular and its field units at Rourkela, Ranchi, Goa, Bangalore and Raipur, and Shrimati Bina Srivastava, Technical Assistant, for standardizing the techniques, Dr Naman Shah, University of North Carolina for reviewing the manuscript.
References
- 1.Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: Challenges and opportunities. J Biosci. 2008;33:583–92. doi: 10.1007/s12038-008-0076-x. [DOI] [PubMed] [Google Scholar]
- 2.WHO expert committee on malaria. World Health Organ Tech Rep Ser, No. 892. 2000 [PubMed] [Google Scholar]
- 3.Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565–77. doi: 10.1056/NEJMra043207. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal PN, Sharma MID, Sharma SL, Gogai Resistance to chloroquine in falciparum malaria in Assam state. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 5.Valecha N, Joshi H, Mallick PK, Sharma SK, Kumar A, Tyagi PK, et al. Low efficacy of chloroquine: Time to switch over to artemisinin-based combination therapy for falciparum malaria in India. Acta Trop. 2009;111:21–8. doi: 10.1016/j.actatropica.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Dev V, Phookan S, Barman K. Therapeutic efficacies of antimalarial drugs in the treatment of uncomplicated, Plasmodium falciparum malaria in Assam, north-eastern India. Ann Trop Med Parasitol. 2003;97:783–91. doi: 10.1179/000349803225002660. [DOI] [PubMed] [Google Scholar]
- 7.Mohapatra PK, Namchoom NS, Prakash A, Bhattacharya DR, Goswami BK, Mahanta J. Therapeutic efficacy of anti-malarials in Plasmodium falciparum malaria in Indo-Myanmar border area of Arunachal Pradesh. Indian J Med Res. 2003;118:71–6. [PubMed] [Google Scholar]
- 8.National drug policy on malaria. New Delhi: National Anti Malaria Programme, Directorate of Health Services, Government of India; 2005. National Anti Malaria Programme. [Google Scholar]
- 9.Vathsala PG, Pramanik A, Dhanasekaran S, Devi CU, Pillai CR, Subbarao SK, et al. Widespread occurrence of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) gene haplotype SVMNT in P. falciparum malaria in India. Am J Trop Med Hyg. 2004;7:256–9. [PubMed] [Google Scholar]
- 10.Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob Agents Chemother. 2002;46:1658–64. doi: 10.1128/AAC.46.6.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.In vitro micro-test (Mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine / pyrimethamine and artemisinin. Geneva: WHO; 2001. World Health Organization. [Google Scholar]
- 12.Pradines B, Hovette P, Fusai T, Atanda HL, Baret E, Cheval P, et al. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J Clin Microbiol. 2006;44:2404–8. doi: 10.1128/JCM.00623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Vector Borne Disease Control Programme, Directorate of Health Services, Government of India. 2010. [assessed on July 31, 2010]. Available from: http://nvbdcp.gov.in/TAC.html .
- 14.Usha Devi C, Pillai CR, Adak T, Sharma VP, Dwivedi SC. In vitro sensitivity of Indian isolates of Plasmodium falciparum to antimalarials. J Parasitic Dis. 1996;20:177–80. [Google Scholar]
- 15.National Vector Borne Disease Control Programme. National Drug Policy on malaria. National Vector Borne Disease Control Programme, Directorate of Health Services, Government of India. 2010. [accessed on July 31, 2010]. Available from: http://www.nvbdcp.gov.in/
- 16.Rehwagen C. WHO ultimatum on artemisinin monotherapy is showing results. BMJ. 2006;332:1176. doi: 10.1136/bmj.332.7551.1176-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wongsrichanalai C, Lin K, Pang LW, Faiz MA, Noedl H, Wimonwattrawatee T, et al. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am J Trop Med Hyg. 2001;65:450–5. doi: 10.4269/ajtmh.2001.65.450. [DOI] [PubMed] [Google Scholar]
- 18.Noedl H, Attlmayr B, Wernsdorfer WH, Kollaritsch H, Miller RS. Histidine rich protein 2 based malaria drug sensitivity assay for field use. Am J Trop Med Hyg. 2004;71:711–4. [PubMed] [Google Scholar]
- 19.Noedl H, Socheat D, Satimai W. Artemisinin- resistant malaria in Asia. N Engl J Med. 2009;361:540–1. doi: 10.1056/NEJMc0900231. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev V, et al. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob Agents Chemother. 2004;48:879–89. doi: 10.1128/AAC.48.3.879-889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Malaria Research. Guidelines for diagnosis and treatment of malaria in India. National Institute of Malaria Research, New Delhi, India. 2010. [accessed on March 22, 2012]. Available from: http://mrcindia.org/Guidelines_for_Diagnosis2010.pdf .


