Abstract
Background & objectives:
Several studies have suggested an important, but conflicting and controversial role for adipose tissue mass in breast cancer risk. Factors such as insulin-like growth factors, sex steroids, adipokines and obesity-related inflammatory markers have been postulated as potential effectors of the mechanisms by which obesity and associated metabolic disorders influence breast cancer risk. In this study we evaluated the associations between obesity indices, insulin resistance, circulating adipokines, sex steroids and breast cancer.
Methods:
Fasting adiponectin, leptin, insulin resistance (homeostasis model assessment, HOMA-IR), testosterone, estradiol, sex hormone binding globulin (SHBG), LH and FSH were determined in 144 newly-diagnosed histologically confirmed breast cancer patients and 77 controls. Univariate and multivariate regression analyses were used to find the associations of these variables with each other, indices of obesity and with breast cancer.
Results:
BMI, waist circumference, HOMA-IR and leptin were significantly (P<0.001) higher in patients than in controls. Adiponectin level was also significantly (P<0.05) higher in patients compared to controls. Adiponectin and leptin showed significant correlations with insulin and HOMA-IR but only adiponectin was significantly correlated with estradiol and SHBG. Logistic regression analyses showed that factors associated with breast cancer were BMI [OR (95% CI) =2.8 (1.4-5.5), P=0.004]; high levels of adiponectin [5.1 (2.2-11.5), P<0.001); hyperinsulinaemia [1.1 (1.0-1.1), P=0.01], leptin [3.1 (1.7-5.7), P<0.0001], estradiol [2.5 (1.3-4.7), P=0.005] and testosterone [1.3 (1.03-1.7), P=0.03].
Interpretation & conclusions:
Our findings confirm that adipokines, insulin resistance and sex steroids are associated with breast cancer. The paradoxical association of increased adiponectin with breast cancer is a novel finding that deserves further investigation.
Keywords: Adipokines, breast cancer, obesity, sex steriods
Obesity is known to be a risk factor for certain cancers such as colon, prostate, endometrium and breast. As adipose tissue expresses several sex-steroid metabolizing enzymes such as aromatase, it is believed that the increased risk of breast cancer in post-menopausal women is due to the increased synthesis of estrogens in fat deposits1. However, clinical, biological and epidemiological data suggest that the link between excess adiposity and breast cancer risk cannot be explained by obesity-induced changes in serum levels of sex hormones alone2. Other mechanisms that have been proposed include roles for insulin and insulin-like growth factor-1 and local and systemic effects of adipocyte-specific secretory proteins - the so-called adipokines (e.g. leptin and adiponectin)2. The dysregulation in the secretion of the adipokines in a positive or a negative manner may modulate the insulin signaling pathway, and this could be one of the mechanisms by which insulin resistance is linked to obesity as well as obesity-related cancers3. Lower circulating adiponectin levels have been shown to be associated with increased risk of breast cancer independent of age and menopause status4. The association between circulating leptin and cancer or cancer risk is still unclear despite in vitro data that suggest a potential role in cancer development5,6. Insulin and insulin like growth factors are known for their potential roles in cancer development through favouring cell proliferation and inhibition of apoptosis7.
Many of the mechanisms that have been proposed to link obesity with breast cancer could be altered by ethnic factors8. Therefore, we undertook this study to explore the association between obesity and breast cancer in Kuwaiti patients; and to investigate the association between obesity related factors such as hyperinsulinaemia, insulin resistance, adipokines (adiponectin and leptin) as well as sex steroids and breast cancer.
Material & Methods
Cases and controls: This cross-sectional study was performed at Kuwait Cancer Control Center (KCCC), Shuwaikh, Kuwait and included 144 consecutive female patients with breast cancer during June 2007 - June 2009. The diagnosis of cancer was made on histological basis. Only newly diagnosed patients with breast cancer were recruited immediately before treatment commencement. Cases who were not histologically proven, male patients, patients with previous history of breast or other cancers, patients with history of diabetes mellitus on treatment were excluded. Also subjects who reported a recent (within the previous 1-6 months) weight gain or loss of 5 per cent or more of their current weight were excluded. Control subjects (n=77) comprising apparently healthy females were enrolled from the Blood Bank, Safat, Kuwait. In the control group exclusion of breast cancer was made on the basis of questionnaire and clinical examination. At the time of the study, 57 of 144 patients (39.58%) and 27 of 77 (35.06%) women of the control group were post-menopausal. None of the subjects was on hormone replacement therapy. All study subjects signed an informed voluntary consent form. The study protocol was approved by the local ethics committee. Women having menstrual cycles at the time of study were asked to report after fasting for 12-14 h on the next 2nd to 4th day of the cycle.
Blood pressure of each patient and control subject was measured in the sitting position after resting for 5 to 10 min using a mercury sphygmomanometer. Body weight (kg) was measured in light clothing without shoes. Height (cm) was measured as the distance from the top of the head to the bottom of the feet using a fixed stadiometer. Body mass index (BMI, kg/m2) was calculated. Waist circumference (cm) at the level of the umbilicus was also taken.
Blood (10 ml) was collected from patients and controls after fasting for 12-14 h and plasma was separated for analysis.
Adiponectin assay: Fasting plasma adiponectin was measured using a commercially available enzyme-linked immunoassay (ELISA) kit (Linco Research, Missouri, USA) with a sensitivity of 0.39 μg/ml. The inter- and intra-assay coefficients of variation on pooled plasma specimen with adiponectin concentration of 8.2 μg/ml were 4.7 and 6.8 per cent, respectively.
Leptin assay: Plasma leptin concentration was determined with the DSL-10-23100 ACTIIVE ELISA kit (Diagnostics Systems Laboratories, Texas, USA) with an assay sensitivity of 0.5 ng/ml. The inter- and intra-assay coefficients of variation on pooled plasma specimen with leptin concentration of 23.6 ng/ml were 4.1 and 5.3 per cent, respectively.
Other laboratory methods: All routine biochemistry tests were performed immediately after sample collection. Fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG) and high density lipoprotein cholesterol (HDL-cholesterol) were analyzed on an automated analyzer (Beckman DXC 800, Beckman Corporation, USA). The low density lipoprotein cholesterol (LDL-cholesterol) was calculated using the Friedewald formula9.
Fasting serum insulin was determined by an ELISA (DSL-10-1600 ACTIVE, Diagnostics Systems Laboratories, Texas, USA). Insulin resistance was calculated using the homeostasis model assessment (HOMA-IR) using a calculator downloaded from http://www.dtu.ox.ac.uk/index.html?maindoc=/publications/ (Diabetes Trials Unit, Oxford, 2004). We used HOMA-IR > 2 as the cut-off point for determination of insulin resistance10.
Quantitative determinations of luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2), total testosterone and sex hormone-binding globulin (SHBG) were performed on an automated analyzer (Immulite 1000, Siemens Healthcare Diagnostics, Deerfield, USA).
Statistical analysis: Statistical Package for the Social Sciences (SPSS) version 16.0 for windows software (SPSS Inc., Chicago, IL) was used for statistical analysis. Several variables (BMI, adiponectin, leptin, insulin, and HOMA-IR) were not normally distributed. These variables were log-transformed when parametric tests were used. Spearman correlation coefficients (r) were used to describe the association between variables and comparison between two groups was performed with the unpaired Student's t test. Binary logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association between the variables and breast cancer. In the regression models age and estradiol (except in the association with estradiol when only age was used) were included as potentially confounding variables.
Results
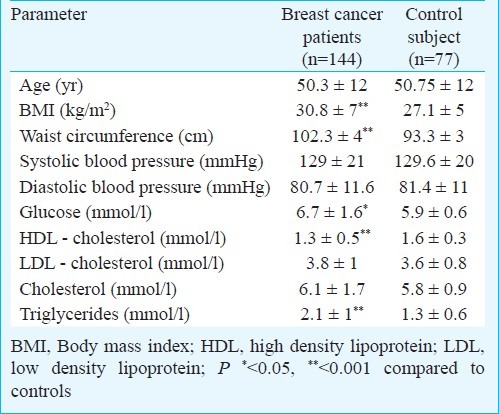
Table I summarizes the demographic, anthropometric and biochemical characteristics of the study population. The mean age of patients (50.3±12 yr) was not significantly different from that of controls. Cancer patients had significantly (P<0.001) higher BMI than control subjects. They also had significantly higher (P<0.0001) waist circumference. There were no significant differences in systolic and diastolic blood pressure between cases and controls. Mean glucose and triglycerides were significantly higher in patients than controls whereas HDL was significantly lower (Table I).
Table I.
Anthropometric and biochemical characteristics of study population
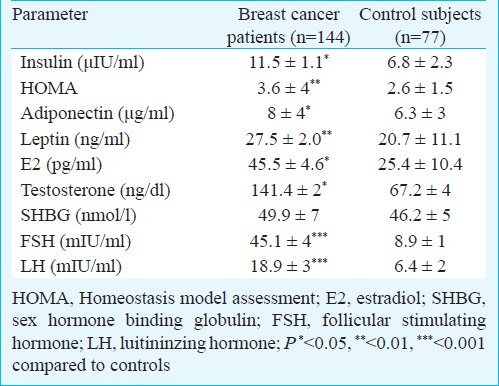
Patients had significantly higher insulin (P<0.05) levels and HOMA-IR (P<0.01) compared to controls (Table II). Leptin and adiponectin concentrations were also higher in cancer patients than in control subjects (Table II). Patients also had significantly higher estradiol and testosterone levels compared to controls.
Table II.
Obesity related parameters and cancer biomarkers of the study population
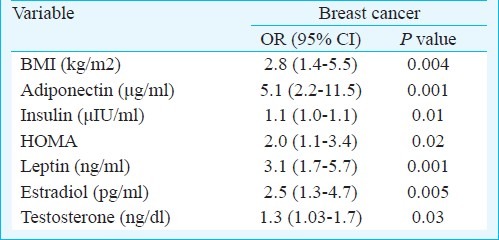
Leptin, insulin and HOMA-IR correlated positively with BMI; while adiponectin showed direct correlations with estradiol and SHBG and inverse correlation with insulin levels (Table III). Using binary logistic regression analysis, BMI, insulin, leptin, HOMA-IR, estradiol and testosterone were found to be significantly associated with breast cancer. High levels of adiponectin were also significantly associated with breast cancer [OR=5.1, 95% CI (2.2-11.5) P< 0.0001] (Table IV).
Table III.
Spearman's rank correlations of variables of interest among all subjects
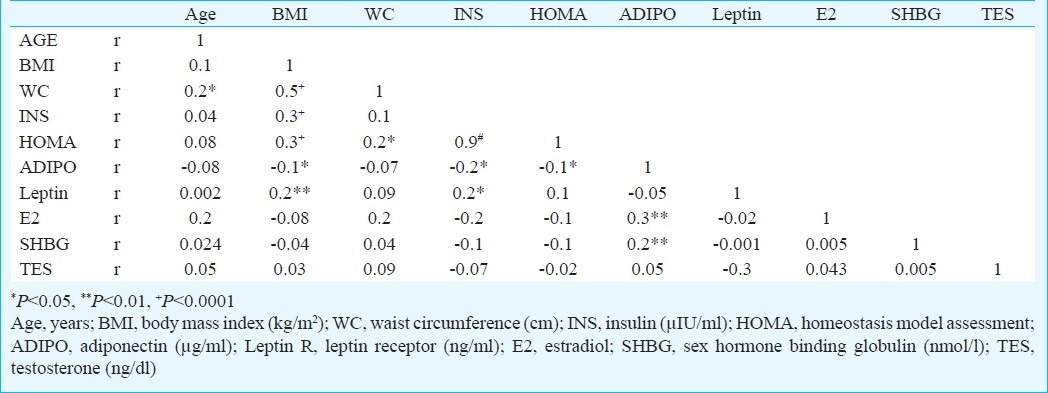
Table IV.
Variables associated with cancer using Binary logistic regression analysis
Discussion
In this study significant associations were noted between obesity-associated indices and breast cancer. Therefore, our study confirms reports from other populations that showed associations between obesity and breast cancer11. Our results also suggest role for insulin resistance, sex steroids and adiponectin.
Insulin resistance is one of the most pronounced metabolic changes associated with obesity. Although insulin is most widely known for its metabolic effects, studies showing that insulin has mitogenic effects on both normal and malignant breast tissue provide the biologic basis for an association of hyperinsulinaemia with breast cancer but results have been inconsistent. Some studies found no association between hyperinsulinaemia and breast cancer development12 whereas others are in agreement with our study and showed significant associations between hyperinsulinaemia, insulin resistance and breast cancer13,14. The inconsistencies in the associations of insulin with breast cancer risk may be attributable to the size and design of the studies, methodological differences as well as differences in the menopausal status of the study populations. Less controversial is the extensive experimental evidence that sex steroids and insulin interact in their actions on tissues.
Insulin stimulates the synthesis of sex steroids and inhibits the synthesis of SHBG. One of the postulated mechanisms linking obesity and breast cancer risk is an increase in circulating bioavailable estradiol, which results in increased production of estrogens by aromatase in the adipose tissue and a decrease in SHBG. This mechanism is supported in our study by the finding of higher estradiol in the more obese breast cancer patients compared to controls. Estrogens not only act as cancer promoters but also as cancer initiators that are involved in breast cancer carcinogenesis by providing mitotic stimulation as well as tumour growth promotion15,16. Testosterone, on the other hand, could increase breast cancer risk either directly, by increasing cellular growth and proliferation, or indirectly, by conversion to estrogen17. In agreement with previous studies18 we have found an association between testosterone levels and breast cancer that was independent of bioavailable estradiol levels. However, our findings are in contrast to another study19 which reported that the association between levels of total testosterone and breast cancer was not independent of levels of bioavailable estradiol. The mechanism for the association of testosterone with breast cancer may not be entirely due to conversion of testosterone to estradiol because some studies have not only confirmed the strong association of elevated serum concentrations of both estradiol and testosterone with breast cancer, but further showed that the association of testosterone was independent of bioavailable estradiol levels20.
Although their physiological and pathological associations are largely in opposition to each other, both leptin and adiponectin have been reported to play roles in the development of breast cancer. In our study, adiponectin was negatively correlated with indices of obesity such as BMI and waist circumference and showed the expected inverse correlations with insulin and HOMA-IR, confirming previous observations on its insulin sensitizing properties10. Breast cancer patients have been shown to here lower adiponectin levels compared to control subjects4. Although the association of adiponectin with obesity and obesity related factors such as insulin resistance are in agreement with what is expected from published studies, our finding of significantly higher adiponectin in breast cancer patients was contrary to earlier reports. It is important to note that the published evidence in support of the anti-cancerous effects of adiponectin has been hypothetical as the anti-cancerous properties have been studied in vitro using certain cancerous cell lines such as breast cancer cell lines (MCF-7)21. These in vitro studies were conducted in serum free environment which may be sufficient to decrease cell proliferation or inhibit cell growth and hence not reflecting the true biological effect of adiponectin in carcinogenesis.
A number of factors may explain our discrepant finding. The first factor relates to the finding of increased levels of tissue adiponectin in breast cancer patients compared to controls suggesting that breast tissue may be producing adiponectin21. This study also reported that, women with high levels of tissue adiponectin are at increased risk of developing breast cancer than women with low tissue adiponectin levels21. However, there are no studies that have evaluated adiponectin production from tumour cells as a tumour marker. It is unlikely that cancer-related weight loss could be a factor in our patients since none of the patients reported significant weight loss. Adiponectin is inversely correlated with body weight and higher levels have been reported in weight-losing patients with advanced lung cancer22.
There is conflicting evidence on the role of leptin in breast cancer. As it has been demonstrated that leptin is produced by breast cancer cells, it is possible that higher levels of leptin in breast cancer patients are caused in part by increased production of leptin independent of the degree of obesity23. Therefore, although the higher leptin in patients with breast cancer in this study could be explained by the higher degree of obesity, our findings support studies that showed association of high serum leptin with increased risk of breast cancer23. However, negative results have also been reported24. Therefore, leptin though has been proposed to be a mitogen that inhibits apoptosis and promotes cell growth25, its role in obesity-related breast cancer remains uncertain.
In conclusion, our results showing associations of the obesity-related factors, insulin resistance, adiponectin, leptin, and sex steroids suggest potential roles in the development of breast cancer. The novel finding in this study was the association of high levels of adiponectin with breast cancer and the possibility that adiponectin could be a tumour marker which is shed by cancer cells into the circulation. Studying the expression of adiponectin in cancer tissue sections using immunohistochemistry will be of importance in confirming such a possibility.
Acknowledgment
Authors acknowledge KFAS for financial support of this project (2006-1302-05), and thank Mrs. Maha Alash for secretarial assistance, and all nurses and doctors in KCCC for their co-operation.
References
- 1.Key T, Allen N, Verkasalo P, Banks E. Energy balance and cancer: the role of sex hormones. Proc Nutr Soc. 2001;60:81–9. doi: 10.1079/pns200068. [DOI] [PubMed] [Google Scholar]
- 2.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–65. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajala M, Scherer P. The adipocyte - At the crossroads of homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 4.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 5.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin PJ, Ennis M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, et al. Is leptin a mediator adverse prognostic effects of obesity in breast cancer? J Clin Oncol. 2005;23:6037–42. doi: 10.1200/JCO.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 8.Karliner LS, Kerlikowske K. Ethnic disparities in breast cancer. Women Health. 2007;3:679–88. doi: 10.2217/17455057.3.6.679. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Mojiminiyi O, Abdella N, Al-Arouj M, Ben Nakhi A. Adiponectin, insulin resistance and clinical expression of the metabolic syndrome in patients with type 2 diabetes. Int J Obesity. 2007;31:213–20. doi: 10.1038/sj.ijo.0803355. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7:605–25. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 13.Papa V, Belfiore A. Insulin receptors in breast cancer: Biological and clinical role. J Endocrinol Invest. 1996;19:324–33. doi: 10.1007/BF03347871. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson SE, Schernhammer ES. Insulin-like growth factor and breast cancer risk: evidence from observational studies. Breast Dis. 2003;17:27–40. doi: 10.3233/bd-2003-17104. [DOI] [PubMed] [Google Scholar]
- 15.Kasid D, Lipman L. Regulation of growth factors by estrogen through the estrogen ereceptor. J Steroid Biochem. 1987;27:465–70. doi: 10.1016/0022-4731(87)90341-4. [DOI] [PubMed] [Google Scholar]
- 16.Russo J, Russo IH. Genotoxicity of steroid estrogens. Trends Endocrinol Metab. 2004;15:211–4. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Dorgan J, Longcope C, Stephenson HE, Falk RT, Miller R, Franz C, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:533–9. [PubMed] [Google Scholar]
- 18.Berrino F, Pasanisi P, Bellati C, Venturelli E, Krogh V, Mastroianni A, et al. Serum testosterone levels and breast cancer recurrence. Int J Cancer. 2005;113:499–502. doi: 10.1002/ijc.20582. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Splegelman D, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–9. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR Study of osteoporotic fractures research group. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Ann Intern Med. 1999;130:270–7. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 21.Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol. 2007;24:361–6. doi: 10.1007/s12032-007-0021-0. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson NB, Brown DJ, Michael WA, McMillan DC. Adiponectin and the systemic inflammatory response in weight-losing patients with non small cell lung cancer. Cytokine. 2004;27:90–2. doi: 10.1016/j.cyto.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 24.Stattin P, Soderberg S, Biessy C, Lenner P, Hallmans G, Kaaks R, et al. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat. 2004;86:191–6. doi: 10.1023/B:BREA.0000036782.11945.d7. [DOI] [PubMed] [Google Scholar]
- 25.Artwohl M, Roden M, Hölzenbein T, Freudenthaler A, Waldhäusl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577–80. doi: 10.1038/sj.ijo.0801947. [DOI] [PubMed] [Google Scholar]


