Abstract
Background & objectives:
The interleukin (IL)-17 producing T-helper cells have been linked to pathogenesis of autoimmunity and mostly investigated in rheumatoid arthritis (RA). In this study we tested the IL-17 levels, as well as the levels of nitric oxide (NO) as possible IL-17-induced product, in patients with primary Sjögren's syndrome (pSS), an intricate and complex chronic autoimmune disorder of exocrine glands.
Methods:
Serum IL-17 levels and nitrite concentrations determined in patients with pSS (n=30) were compared with the values obtained in patients with RA (n=10) and healthy controls (n=15). The values obtained for IL-17 in pSS patients were also associated with the patients’ clinical characteristics, particularly the rheumatoid factor (RF) and total antinuclear antibodies (tANA) levels.
Results:
Serum concentrations of IL-17 were significantly (P<0.01) higher in patients with pSS (12.9 ± 28.0 pg/ml) as compared to those obtained in healthy individuals (0.2 ± 0.6 pg/ml), but not as high as the values obtained for the patients with RA (34.5 ± 56.2 pg/ml). The mean IL-17 levels were significantly (P<0.05) higher in the pSS patients positive for rheumatoid factor (20.3 ± 33.3 pg/ml) than in RF-negatives (0.3 ± 0.6 pg/ml). Mean serum concentrations of IL-17 were also higher in antinuclear antibody (ANA)-positive samples (19.8 ± 33.5 pg/ml) in comparison to ANA-negative sera (1.1 ± 3.1 pg/ml) (P<0.05). The NO levels also showed elevated values in both pSS and RA patients, as compared to the healthy controls, since mean nitrite levels in patients with pSS and RA were 38.2 ± 29.2 μM and 41.7 ± 21.1 μM, respectively, while those in healthy controls were significantly lower, at 19.2 ± 10.5 μM.
Interpretation & conclusions:
The findings of this study showed that there was increased IL-17 and NO production in patients with primary SS, especially if they had associated elevated rheumatoid factor and antinuclear antibody values.
Keywords: Interleukin-17, nitric oxide, primary Sjögren's syndrome, rheumatoid arthritis
Interleukin-17 (IL-17) and its receptor are members of emerging cytokine family involved in pathogenesis of inflammation and host defense1, as IL-17 exhibits proinflammatory activity and appears to have a role in regulation of granulopoiesis and erythropoiesis2,3. Also, newly recognized subset of IL-17-secreting T (Th17) cells has been identified and linked to pathogenesis of autoimmunity4. The significance of IL-17 was mostly investigated in the pathology of rheumatoid arthritis and other T cell dependent tissue damages5. A hallmark feature of IL-17 is its ability to induce the production of a number of inflammatory mediators and in particular nitric oxide (NO) by stimulated chondrocytes, osteoblasts, fibroblast, endothelial cells and astrocytes6. NO itself is well known free radical implicated in cytotoxic tissue injury in a variety of diseases and its increased production has been linked to pathogenesis of autoimmune and inflammatory diseases7,8.
Sjögren's syndrome (SS) is a chronic autoimmune disorder of exocrine glands characterized as an autoimmune exocrinopathy, and there are limited reports concerning the levels, as well as the role, of both IL-17 and NO in the in pathogenesis of primary Sjögren's syndrome. Increased NO production has been previously reported in patients with SS, especially if they had associated arthritis9. Recently, the possible role of systemic and local IL-17 was associated with primary Sjögren's syndrome (pSS)10. Cytokines as important component in pathogenesis are considered as potential targets in pSS11,12. Due to complex pathogenesis of pSS, heterogeneity of disease and for determination of new potential therapeutic targets the role of IL-17 should be further elucidated13. In order to gain more data on the presence and the influence of IL-17 in the pathology of pSS, we investigated the levels of IL-17 and NO in patients with pSS and compared the values with those obtained in patients with rheumatoid arthritis (RA) and in healthy controls. Additionally, the IL-17 levels in the pSS patients were associated with their clinical characteristics, particularly the rheumatoid factors (RF) and total antinuclear antibodies (tANA) levels.
Material & Methods
Patients: The study was conducted at the Institute of Rheumatology, Belgrade, Serbia, and 40 patients [30 with primary Sjogren's syndrome (pSS) and 10 with rheumatoid arthritis (RA)] were included with a written consent, during one year period (January to December 2004). The diagnosis of pSS was made according to the European classification criteria14 since the average duration of disease of patients was 10 years. The clinical samples were formed in a such manner that each patient who met the entry criteria of the study during the research period was included in the corresponding group. Randomization of the samples was a result of indeterminable patients reporting to the clinic. All patients were women, mean age 55.2 ± 10.1 yr with the mean duration of the disease 10.6 ± 5.6 yr. The patients with pSS were on substitution therapy for dry eyes and mouth, four of them only on substitution, 25 were treated with glycocorticoids, in addition, 11 of them with chloroquine, five with azathioprine, one with cyclophosphamide. Seven patients with RA were treated with nonsteroid anti inflammatory drugs and monotherapy with disease modifing anti-rheumatic drug (Methotrexate, Salazopyrine, Hloroquine, Azathioprine), and three were on low doses of prednisolone (5-7.5 mg/day). The blood samples (10 ml) were drawn from patients and serum was separated and investigated by routine clinical analyses for the presence of rheumatoid factor (RF) by the nephelometric test (The Binding site, Birmingham, UK), total antinuclear antibodies (tANA) by an indirect immunofluorescent test15 and antibodies against SS-A and SS-B by ELISA test (Varelisa, Pharmaciadiagnostics, Freiburg, Germany). Extraglandular manifestations occurred in all pSS patients studied.
RA patients fulfilling the revised criteria of the American College of Rheumatology were included16. The mean age of RA patients (all women) was 60 ± 13.8 yr and the mean duration of the disease 8.4 ± 7.7 yr; 80 per cent of RA patients had extra-articular manifestations.
Fifteen healthy volunteers (13 female and 2 male), after written consent obtained, were studied as controls and their mean age was 49 ± 11.1 yr. Blood samples were drawn at the Institute of Rheumatology, Belgrade. Serum was separated immediately and stored at -20°C until analysis. The study was approved by the Committee of Ethics, Institute of Rheumatology, Belgrade.
Enzyme linked immunosorbent assay (ELISA) for human IL-17: Serum IL-17 levels were determined in duplicate by ELISA kit for human IL-17 (sensitivity-2 pg/ml) purchased from BioSource Europe S.A. (BioSource IL-17 cytoscreen kit, Belgium), following manufacturer's instruction.
Nitric oxide determination: Production of NO was determined by measuring accumulation of nitrite in serum using the Griess reaction with sodium nitrate as the standard. Briefly, 50 μL samples of serum were mixed with equal volumes of 1 per cent sulphanil-amide and 0, 1 per cent N-1-naphthylethylene diamine-dihydrochloride in 0.5 per cent H3PO4. After 10 min at room temperature, the absorbance at 540 nm was measured in a microplate reader.
Statistical analyses: Descriptive statistical methods were used. Data were not normally distributed, as tested by one-sample Kolmogorov-Smirnov test (KSZ). Therefore, non-parametric tests were used, Kruskal-Wallis chi-squared (KW χ2) for differences between three groups, and Mann-Whitney test (U) for inter-group differences. P<0.05 was considered significant.
Results
The clinical and laboratory characteristics of the patients with pSS and RA are shown in the Table. The most common extraglandular manifestations in patients with pSS were dermal changes (70%), Raynaud's phenomenon (63.3%), sensitive neuropathy (53.3%), arthralgia and arthritis (50%), and haematological manifestations (40%). In these patients RF and ANA were present in 19 (around 63%) patients, while anti-SS-A and anti-SS-B in 18 (60%) and 12 (40%) patients, respectively. In two patients with RA, rheumatic nodules as extra-articular manifestations were found.
Table.
Clinical and laboratory characteristics of patients with primary Sjögren's syndrome (pSS) and rheumatoid arthritis (RA)
Serum IL-17 levels were significantly elevated in patients with pSS as compared to the healthy individuals (12.9 ± 28.0 vs. 0.2 ± 0.6 pg/ml, P<0.01). Patients with RA also had significantly elevated serum levels of IL-17 when compared to the controls (34.5 ± 56.2 vs. 0.2 ± 0.6 pg/ml, P<0.001) (Fig. 1A). Though the mean serum IL-17 levels were higher in patients with RA than in the patients with pSS, the difference was not statistically significant.
Fig. 1.
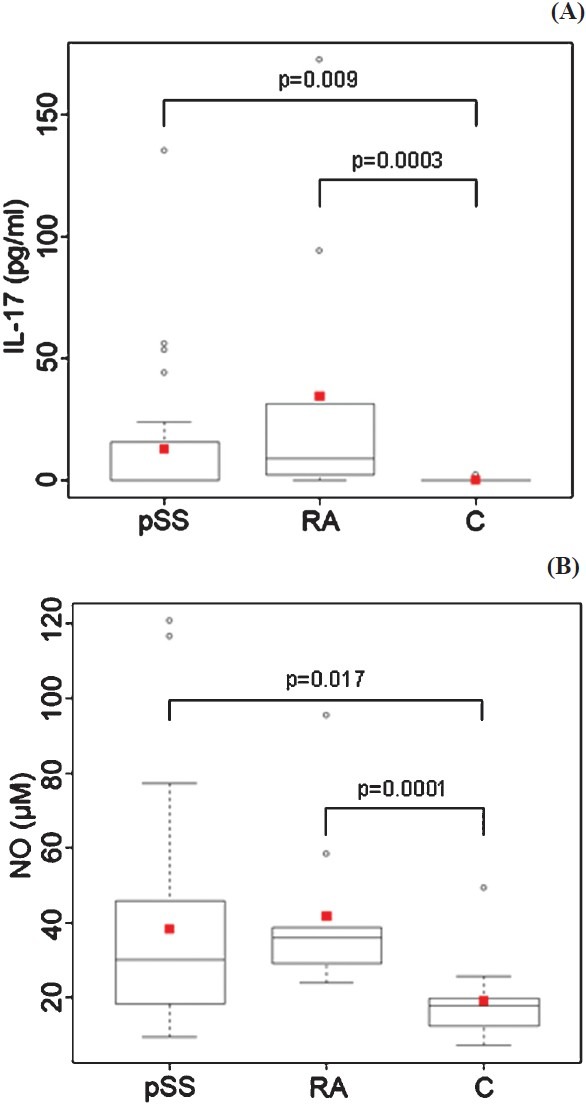
IL-17 (A) and nitrite (B) serum concentrations in patients with pSS (n=30), patients with RA (n=10) and healthy controls - C (n=15). Squares (red) represent mean values, the median is shown as horizontal line within the box. The lower and upper margins of the box represent 25th and 75th percentiles, with the extended arms representing the highest and lowest values. Differences in serum levels between patients’ groups and the control group were analyzed by Mann-Whitney U test.
The NO levels levels in patients with pSS and RA were significantly higher than those in healthy individuals (Fig. 1B). Mean nitrite concentrations in patients with pSS were 38.2 ± 29.2 μM, while those in healthy controls were significantly lower, at 19.2 ± 10.5 μM (P <0.05). In patients with RA, mean NO levels were 41.7 ± 21.1 μM, which was also significantly higher in comparison to the control group (P<0.001).
The possible associated between the elevated IL-17 levels in pSS patients was studied with their clinical characteristics, especially the duration of the disease and the presence of rheumatoid factors (RF) and total antinuclear antibodies (tANA) levels. The mean serum IL-17 concentrations were higher in patients with pSS disease duration lasting longer than 10 years when compared to those with disease duration < 10 yr. The difference was not statistically significant. The results also demonstrated significantly higher IL-17 concentrations in RF-positive than in RF-negative patients (P<0.05) (Fig. 2B), as well as in ANA-positive in comparison to ANA-negative patients (P<0.05) (Fig. 2C).
Fig. 2.
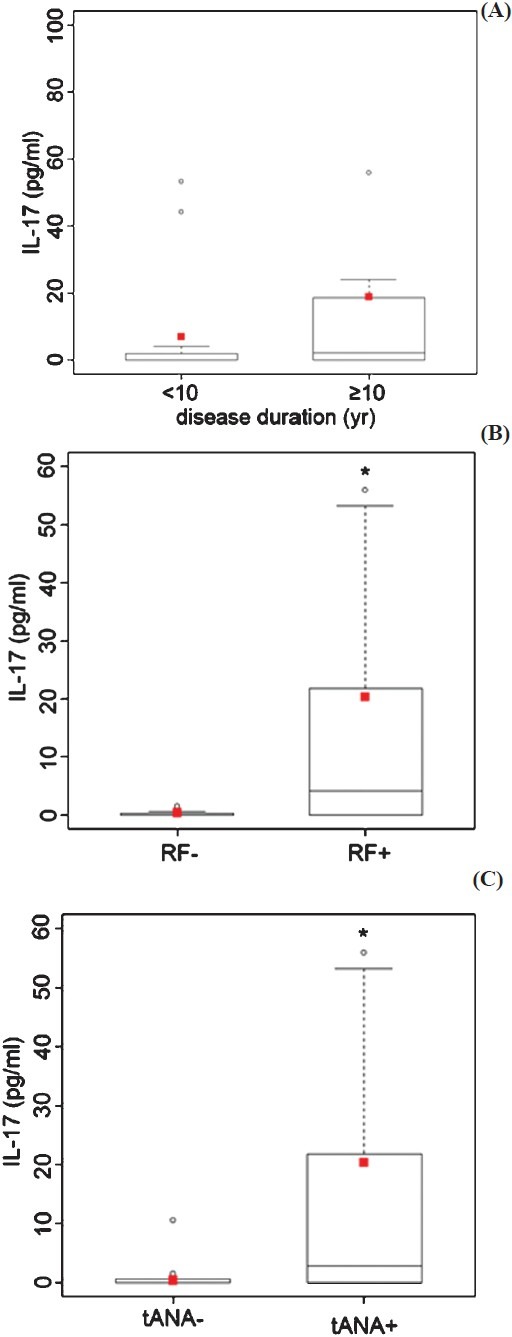
IL-17 concentration in serum of the patients with pSS (n=30). The IL-17 levels were associated with (A) the disease duration, (B) between the RF-negative and RF-positive patients, and (C) between the tANA-negative and tANA-positives. Squares represent mean values, the median is shown as horizontal line within the box. The lower and upper margins of the box represent 25th and 75th percentiles, with the extended arms representing the highest and lowest values. Differences in IL-17 levels between the tested groups were analyzed by Mann-Whitney U test (*P<0.05).
No differences in the mean concentrations of IL-17 were found between the anti-SS-A positive and anti-SS-A negative patients (13.6 ± 18.7 vs. 15.4 ± 44.9 pg/ml), nor between the anti-SS-B positive and anti-SS-B negatives (13.6 ± 18.5 vs. 12.5 ± 33.4 pg/ml). Although no relation between presence of anti-SS-A, anti-SS-B antibodies and elevated IL-17 concentrations was found, the values of anti-SS-A antibodies in patients (n=11) with elevated IL-17 concentration were considerably higher than in those with normal (n=19) IL-17 values (72.3 ± 54.7 vs. 37.7 ± 37.5 U/ml, P<0.05).
Discussion
This study demonstrated elevated IL-17 and NO levels in the circulation of the patients with pSS, which is in agreement with reported data9,10,17,18. The elevated IL-17 levels in pSS patients are most probably a reflection of the systemic response to the inflammation, like often seen in autoimmune diseases. Proinflammatory effects of IL-17 were clearly demonstrated in various autoimmune diseases. Stimulation of its production and the release of inflammatory mediators from synovial fluid monocytes, synoviocytes and peripheral blood mononuclear cells5,19, as well as the additive and synergistic effects with interleukin-1 (IL-1) and tumour necrosis factor (TNF) in inducing joint pathology have been described in rheumatoid arthritis20. Similarly, the role of IL-17 as a crucial proinflammatory mediator was demonstrated in the pathogenesis of other autoimmune diseases, including multiple sclerosis21, systemic lupus erythematosus22 and autoimmune encephalomyelitis23. IL-17 was, therefore, shown to be incorporated in cytokine network acting in tissue damage24. In patients with pSS, systemic levels of Th17-associated cytokines, including IL-17, significantly varied between different subgroups of patients as related to the histopathological features25. Other studies showed the presence of IL-17 and other factors fostering Th17 lineage polarization, such as IL-23, TGF-β, IL-6, in the local salivary gland milieu10,17, that correlated with the degree of inflammation and objective clinical evidence. These data pointed towards the important role of IL-17 in the immunopathogenesis of pSS and indentified this cytokine as a potential therapeutic target. In a mouse model of pSS, blood levels of IL-17 were detected at early time points of the disease and were decreasing further, indicating that early induction of a CD4+ Th1/Th17 pathway leads to systemic release of IL-1717. However, based on the current knowledge, the question of when Th17 cells become involved in the autoimmune response and whether these act directly through secretion of inflammatory IL-17 family cytokines or by activating autoimmune T and B cells remains still to be defined. In our study, IL-17 was detected in the one third of our patients with pSS, and showed tendency to be higher in those patients with long disease duration (more than 10 yr), implying that the blood levels of this cytokine might be associated with natural progression of the SS disease. Doreau et al26 have shown that IL-17 can influence the survival and proliferation of B cells, and their differentiation into immunoglobulin-secreting cells. It has been assumed that the activation of B cells is an important event in the pathogenesis of the disease27. The presence of autoantibodies in the circulation is one of the evidence supporting B-cell activation in pSS, and presence of RF and ANA in the serum of more than 60 per cent of our patients is in line with this assumption. As in these pSS patients, either RF-positive or ANA-positive, the average level of IL-17 was increased, a role of IL-17 in maintenance of disease activity could be supposed. Therefore, the consideration of the disease heterogeneity might be important for finding the most adequate therapeutic strategy.
In addition to IL-17, the results of this study revealed increased NO levels in the circulation of pSS patients, thus confirming that NO may contribute to inflammatory damage in pSS9. Several studies have established that the proinflammatory action of IL-17 depends considerably on its ability to induce inducible NOS (iNOS) and stimulates significant NO production in various cell types, including chondrocytes, astrocytes, fibroblasts and endothelial cells6. The possible role of NO in the pathogenesis of different systemic autoimmune disorders has also been indicated, since elevated NO levels have been shown in pSS, SLE and RA patients8,28,29.
The functional outcome of the increased NO production in patients with pSS, as well as the mechanisms by which NO contributes to pathophysiology, are still not resolved. Recent data presented a role of the NO-mediated nitrosylation in downregulation of salivary function in a mouse model for SS30. However, other possible mechanisms of NO action are not excluded; such is its effect in triggering apoptosis, since in low concentrations NO may protect cells from apoptosis31, but at high concentrations, resulting from the activation of iNOS, NO can induce apoptosis32. As concerning SS apoptosis of the acinar and ductal epithelial cells of the salivary and lacrimal glands has been suggested as a possible mechanism responsible for the impairment of secretory function33. In addition, in SS patients increased apoptosis and increased iNOS expression were detected in salivary epithelial cells , after the exposure to inflammatory cytokines, such as INF-gamma34.
In our study, there was no relation between the NO production and the other clinical characteristics, such as the disease duration, rheumatoid factors or antinuclear antibody positivity, as well as with the elevated IL-17 concentrations (data not shown). However, one cannot assert the objectivity of such findings, bearing in mind that the majority of patients were under appropriate therapy and that steroids are known to suppress NO production35. As the NO is also known to be implicated in the enhancement of Th1 cell proliferation and function36, it will be of great interest for future work to investigate the impact of NO on the development and function of Th17 effector's T cells.
In summary, the present data demonstrated increased IL-17 and NO production in patients with primary SS. The elevated IL-17 levels were associated with elevated rheumatoid factors and antinuclear antibody values, confirming that IL-17 is an important cytokine linked to the immunopathogenesis in patients with pSS. But, since SS is a very intricate and complex chronic autoimmune disease, further studies are needed to determine the exact role of both IL-17 and NO, as well as their mutual link, in pSS disease pathogenesis.
Acknowledgment
This work was supported by a grant (#175062) from the Ministry of Science and Technological Development, Republic of Serbia.
References
- 1.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–47. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–9. [PubMed] [Google Scholar]
- 3.Jovčić G, Bugarski D, Petakov M, Stanković J, Stojanović N, Milenković P. Effects of IL-17 on in vitro hematopoietic progenitor cells growth and cytokine release in normal and post-irradiated murine bone marrow. Growth Factors. 2001;19:61–71. doi: 10.3109/08977190109001076. [DOI] [PubMed] [Google Scholar]
- 4.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Miljković D, Trajković V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004;15:21–32. doi: 10.1016/j.cytogfr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, et al. Role of arginine metabolism in immunity and immunopathology. Immunobiology. 2007;212:795–812. doi: 10.1016/j.imbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Nagy G, Clark JM, Buzás EI, Gorman CL, Cope AP. Nitric oxide, chronic inflammation and autoimmunity. Immunol Lett. 2007;111:1–5. doi: 10.1016/j.imlet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Wanchu A, Khullar M, Sud A, Bambery P. Elevated nitric oxide production in patients with primary Sjögren's syndrome. Clin Rheumatol. 2000;19:360–4. doi: 10.1007/s100670070028. [DOI] [PubMed] [Google Scholar]
- 10.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roescher N, Tak PP, Illei GG. Cytokines in Sjögren's syndrome: potential therapeutic targets. Ann Rheum Dis. 2010;69:945–8. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker H, Pavenstaedt H, Willeke P. Emerging treatment strategies and potential therapeutic targets in primary Sjögren's syndrome. Inflamm Allergy Drug Targets. 2010;9:10–9. doi: 10.2174/187152810791292935. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjögren's syndrome using adenovirus-mediated gene transfer. Lab Invest. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Moutsopoulos HM, Coll J, Gerli R, Hatron PY, et al. Assessment of the Europen classification criteria for Sjögren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The Europen Study Group on Diagnostic Criteria for Sjögren's Syndrome. Ann Rheum Dis. 1996;55:116–21. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabiedes J, Núñnex-Álvarez CA. Antinuclear antibodies. Reumatol Clin. 2010;6:224–30. doi: 10.1016/j.reuma.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariette X, Gottenberg JE. Pathogenesis of Sjögren's syndrome and therapeutic consequences. Curr Opin Rheumatol. 2010;22:471–7. doi: 10.1097/BOR.0b013e32833c36c5. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol. 2008;35:515–9. [PubMed] [Google Scholar]
- 20.Zwerina J, Redlich K, Polzer K, Joosten L, Krönke G, Distler J, et al. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci USA. 2007;104:11742–7. doi: 10.1073/pnas.0610812104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 24.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 25.Reksten TR, Jonsson MV, Szyszko EA, Brun JG, Jonsson R, Brokstad KA. Cytokine and autoantibody profiling related to histopathological features in primary Sjögren's syndrome. Rheumatology (Oxford) 2009;48:1102–6. doi: 10.1093/rheumatology/kep149. [DOI] [PubMed] [Google Scholar]
- 26.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 27.Hansen A, Lipsky PE, Dorner T. Immunopathogenesis of primary Sjögren's syndrome: implications for disease management and therapy. Curr Opin Rheumatol. 2005;17:558–65. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 28.Wanchu A, Khullar M, Sud A, Deodhar SD, Bambery P. Elevated urinary nitrite and citrulline levels in patients with rheumatoid arthritis. Inflammopharmacology. 1999;7:155–61. doi: 10.1007/BF02918387. [DOI] [PubMed] [Google Scholar]
- 29.Wanchu A, Khullar M, Deodhar SD, Bambery P, Sud A. Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol Int. 1998;18:41–3. doi: 10.1007/s002960050055. [DOI] [PubMed] [Google Scholar]
- 30.Caulfield VL, Balmer C, Dawson LJ, Smith PM. A role for nitric oxide-mediated glandular hypofunction in a non-apoptotic model for Sjögren's syndrome. Rheumatology (Oxford) 2009;48:727–33. doi: 10.1093/rheumatology/kep100. [DOI] [PubMed] [Google Scholar]
- 31.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–7. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehsel K, Krancke KD, Meyer KL, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–65. [PubMed] [Google Scholar]
- 33.Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. Semin Arthritits Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 34.Ping L, Ogawa N, Sugai S. Novel role of CD40 in Fas-dependent apoptosis of cultured salivary epithelial cells from patients with Sjögren's syndrome. Arthritis Rheum. 2005;52:573–81. doi: 10.1002/art.20789. [DOI] [PubMed] [Google Scholar]
- 35.Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA. 1990;87:10043–7. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]


