Abstract
Background & objectives:
Ocimum sanctum (OS) is known to possess various therapeutic properties. We have earlier isolated and characterized three OS compounds; Ocimarin, Ocimumoside A and Ocimumoside B. However, their role in modulating stress-induced central changes is unexplored. Thus, the present study was aimed to investigate the effect of these OS compounds on restraint stress (RS)-induced changes in the monoaminergic and antioxidant systems in the frontal cortex, striatum and hippocampus of rats.
Methods:
RS was produced by immobilizing (restraining) the Sprague Dawley rats for a period of 2.5 h inside cylindrical steel tubes. The monoamine levels and the in vivo antioxidant status in brain regions were evaluated by HPLC-EC and spectrophotometric assays, respectively.
Results:
RS significantly increased the dopamine levels in the frontal cortex and decreased in the striatum and hippocampus, and accompanied with selective increase of dopamine metabolites compared to the NS control group. The serotonin and its metabolite levels were significantly increased, while noradrenaline levels were decreased by RS in the three brain regions studied. The activities of superoxide dismutase and glutathione peroxidase in the frontal cortex and striatum were significantly increased by RS with decreased glutathione levels and increased lipid peroxidation. Pre-treatment with Ocimumoside A and B (40 mg/kg po) for a period of 3 days prevented the RS-induced changes with an efficacy similar to that of standard anti-stress (Panax quinquefolium; 100 mg/kg po) and antioxidant (Melatonin; 20 mg/kg ip) drugs, while, Ocimarin failed to modulate these changes. OS compounds per se had no effect on these parameters.
Interpretation & conclusions:
The present findings showed the anti-stress potential of Ocimumoside A and B in relation to their simultaneous modulatory effects on the central monoaminergic and antioxidant systems implicating their therapeutic importance in stress-related disorders. Further studies are required to understand the mechanism of action of these compounds.
Keywords: Antioxidant system, dopamine, noradrenaline, Ocimum sanctum, restraint stress, serotonin
Stress-induced changes in the monoaminergic system with subsequent enhancement of oxidative load are associated with a wide range of central and peripheral disorders1. Interestingly, most of the workers studied stress-induced changes using chronic stress models, as these simulate human related clinical conditions1–3. However, investigating the effect of single stress exposure on central nervous system is also crucial, specifically in terms of initial brain region specific monoaminergic and oxidative responses, and in understanding the mechanism of various anti-stress agents at the early stage of stress exposure.
Ocimum sanctum Linn (OS), is one of the most widely used medicinal herb in indigenous system of medicine, it belongs to the family Lamiaceae and is commonly known as sacred basil or “Tulsi”. Stress alleviating and immuno-modulatory potential of OS has already been established4,5. OS leaves contain 0.7 per cent volatile oil comprising about 71 per cent eugenol and 20 per cent methyl eugenol along with carvacrol, sesquiterpine hydrocarbon caryophyllene, apigenin, luteolin, apigenin-7-O-glucuronide, orientin, olludistin and ursolic acid6,7. Previously, we have reported the anti-stress activity of ethanol extract of OS leaves and its n-butanol fraction, further Ocimarin, Ocimumoside A and B were isolated and identified for the first time6. However, the underlying anti-stress mechanism of these novel OS compounds, particularly with reference to their modulatory role on the monoaminergic systems and oxidative routes of damage in acute stressful condition remains to be elucidated. Thus, the current study was designed to evaluate the effects of novel Ocimarin, Ocimumoside A and B on acute restraint stress (RS)-induced changes in these central stress systems in rats. The brain regions selected in the study were the frontal cortex, striatum and hippocampus, as these are intricately connected through monoaminergic systems and play an important role in stress responses8. To understand the effect of OS compounds on RS-induced monoaminergic changes, the levels of nor-adrenaline (NA), dopamine (DA), serotonin (5-HT) and their metabolites were evaluated. We also attempted to explore the RS-induced changes in the in vivo antioxidant status and the modulatory role of OS compounds by measuring the activities of Cu-Zn superoxide dismutase (SOD), catalase (CAT), selenium dependent glutathione peroxidase (GSH-Px), the extent of lipid peroxidation (malondialdehyde levels) and glutathione (GSH) levels in the selected brain regions.
Material & Methods
Animals: Experimental protocols were approved by the Institutional Ethical and Usage Committee of Central Drug Research Institute (CDRI), Lucknow. Adult male Sprague Dawley rats, weighing 180-220 g procured from National Laboratory Animal Centre, CDRI, were used in the study. Rats were housed three to four per cage in a room with temperature regulated at 22 ± 2°C and a 12h/12h light/dark cycle. Standard chow pellet diet and water were given ad libitum, except during the experimental period when food or water deprivation was applied.
Constituents of Ocimum sanctum (OS): The leaves of OS were collected from Lucknow in the month of November and identified by Botany division of CDRI; voucher specimen (no. 38) is kept in the herbarium of the Institute. The dried and powdered leaves of OS were extracted with ethanol and concentrated at 45°C. The resulting dried ethanol extract was suspended in water and sequentially partitioned with chloroform and n-butanol. The n-butanol-soluble fraction was separated by a combination of chromatographic procedures, which afforded three new compounds designated as Ocimarin (coumarin), Ocimumoside A and Ocimumoside B (glycoglycerolipids)6.
Chemicals: Dopamine (DA), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), nor-adrenaline (NA), 5-hydroxy tryptamine (5-HT), 5-hydroxyindole acetic acid (5-HIAA), dihydroxybenzylamine (DHBA), epinephrine, t-butyl hydroperoxide, reduced glutathione (GSH), glutathione reductase, thiobarbituric acid, 1,3,3 -tetraethoxypropane and 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB), standardized Panax quinquefolium (PQ) crude root powder [Rg: 0.13%, Re: 1.32%, Rb1< 0.10%, Rc: 1.49, Rb2: 0.14%, Rd: 0.54%, others: 1.17%] and melatonin (MEL) were purchased from Sigma Aldrich (St. Louis, MO, USA). All the other chemicals used in the study were of analytical or HPLC grade, and were purchased locally.
Restraint stress (RS) model: After 18 h fasting (food deprivation) of rats, one stress session consisting of a 2.5 h immobilization period inside the cylindrical steel tube (7cm diameter, 17.5 cm long, with holes for ventilation) at room temperature was performed during the early phase of the light cycle (07:00 to 09:30 h) and after 1 h the animals were sacrificed9.
Dose selection and drug preparation: In a pilot investigation, the dose dependent effect of OS ethanolic extract (100-400 mg/kg po), its fractions (chloroform, n-butanol and aqueous; 200 mg/kg po) and pure compounds (10-40 mg/kg po) was examined on peripheral stress biomarkers6. The OS compounds prevented RS-induced peripheral changes at an oral dose of 40 mg/kg6. Thus, the same dose was selected for evaluating the effect on central stress pathways. For comparative analysis, Panax quinquefolium (PQ; 100 mg/kg po) and melatonin (MEL; 20 mg/kg ip) were used as standard anti-stress and antioxidant drugs, respectively, and their respective doses were selected on the basis of pilot studies and available literature10,11. Suspensions of OS compounds, PQ and MEL were freshly prepared in 1 per cent sodium carboxy methyl cellulose (CMC) just before administration.
Experimental protocol: The rats were acclimatized under laboratory conditions and handled daily for a week prior to commencement of experiment. The rats (n= 80) were randomly divided into 10 groups of 8 rats each.
Group I: The rats were kept undisturbed and served as non-stress control group (NS).
Groups II: The rats were subjected to immobilization stress for 2.5 h (RS).
Two types of NS and RS groups were run in the present study, one was untreated and another was vehicle-treated (1% CMC). As there were no significant differences between the two groups in all the parameters assayed (data not given), for further comparison purposes untreated NS and RS rats were used as respective controls.
Groups III, IV and V: Normal rats were pretreated with OS compounds (40 mg/kg po) daily for 3 consecutive days to obtain baseline data of drug per se effect (Ocimarin, Ocimumoside A and Ocimumoside B).
Groups VI, VII, and VIII: The rats were pre-treated with OS compounds (40 mg/kg po) daily for 3 days. On the second day after feeding drug or vehicle, rats were fasted overnight with free access to water. On the third day, 45 min after feeding the compounds rats were subjected to RS (Ocimarin + RS, Ocimumoside A + RS and Ocimumoside B + RS).
Groups IX and X: The rats were pretreated with PQ (100 mg/kg po) and MEL (20 mg/kg ip) for 3 days as described above and served as anti-stress and antioxidant standard groups respectively (PQ + RS, MEL + RS).
Sample preparation: 1 h after the stressor the rats were killed by conscious cervical dislocation followed by decapitation, and the brains were immediately removed. The discrete brain regions (frontal cortex, striatum and hippocampus) were dissected on an ice-cold glass plate12. For the estimation of neurotransmitter levels the dissected brain regions were weighed and an internal standard DHBA (25 ng/ml) was added to each sample. Homogenization was performed in 0.17 M perchloric acid with an Ultra-Turrax homogenizer (Model T25, IKA-Laborthechnik, Staufen, Germany). The homogenates were then centrifuged at 35,000 g (Model 3K30, Sigma Centrifuge, Osterode, Germany) at 4°C, supernatants were passed through a 0.22 μm membrane filter and were used for analysis. For the estimations of antioxidant parameters and lipid peroxidation, the dissected brain regions were homogenized in ice cold 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA and centrifuged at 1000 g for 15 min at 4°C, the supernatants thus obtained were used for the analysis.
Estimation of monoamines and their metabolites: The endogenous levels of NA, DA, 5-HT and their non-conjugated metabolites DOPAC, HVA, and 5-HIAA were determined by reverse phase HPLC with electrochemical detection13. The sample (20 μl) was injected via a HPLC pump (Model 1525, Binary Gradient Pump, Waters, Milford, MA, USA) into a column (Spherisorb, RP C18, 5 μm particle size, 4.6 mm id x 250 mm at 30°C) connected to an electrochemical detector (Model 2465, Waters, Milford, MA, USA). Oxidation potential was fixed at 0.80 V using a glass carbon working electrode versus an Ag/AgCl reference electrode. The mobile phase consisted of 32 mM citric acid, 12.5 mM disodium hydrogen orthophosphate, 1.4 mM sodium octanyl sulphonate, 0.05 mM EDTA and 16 per cent (v/v) methanol. The pH of the mobile phase was adjusted to 4.05. Separation was carried out at a flow rate of 1.2 ml/min. The neurotransmitters were quantified using Breeze version 3.2. The levels were expressed in ng/g wet wt. of the brain tissue. Quantification was made by comparing peak heights of the samples to the corresponding standard curve.
Cu-Zn, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) activity: SOD activity was measured based on its ability to inhibit the autoxidation of epinephrine to adrenochrome at alkaline pH12. The absorbance of reaction mixture was followed for 4 min at 480 nm in a spectrophotometer (Model 1201, Shimadzu). Enzymatic activity was expressed as U/mg protein at 30°C. The amount of enzyme that caused 50 per cent inhibition of epinephrine autoxidation was defined as one unit (U).
CAT activity was measured using H2O2 as substrate15. A molar absorption of 43.6 M/cm was used to determine CAT activity. Enzymatic activity was expressed as U/mg protein at 25°C, one unit (U) of which was equal to 1 mole of H2O2 degraded/min/mg of protein. GSH-Px activity was measured using t-butyl hydroperoxide as substrate16. The absorbance of reaction mixture was measured at 340 nm for 180 sec in a spectrophotometer. A molar absorption of 6.22×103M/cm was used to determine enzyme activity, expressed as U/mg protein at 37°C. One unit (U) of activity was equal to mM of NADPH oxidized/min/ mg protein.
Reduced glutathione (GSH) levels: GSH was determined by its reaction with DTNB (Ellman's reagent) yielding a yellow chromophore, the absorbance of which was measured at 412 nm within 15 min spectrophotometrically17. GSH levels were determined from a standard curve and expressed as μM/mg protein.
Lipid peroxidation assay: MDA (a thiobarbituric acid reactive species: TBARS) an indicator of lipid peroxidation was measured using 1,1,3,3 -tetraethoxypropane as standard18. The absorbance of the reaction mixture was measured at 532 nm and the values were expressed as nM MDA/mg protein.
The protein content of the samples was determined by Lowry's method19 using bovine serum albumin as standard .
Statistical analysis: Data were evaluated by One-Way analysis of variance (ANOVA) with post-hoc test by Tukey–Kramer multiple comparison tests. Significant difference of values of RS or OS compounds per se group was compared with the NS control group, while values of OS treatment groups were compared with RS group.
Results
Effect of OS compounds and PQ on the brain monoamine changes induced by RS: RS significantly increased the levels of frontal cortical DA (P<0.05), HVA, 5-HT (P<0.001) and 5-HIAA (P<0.05), and decreased the NA (P<0.01) levels in the frontal cortex as compared to the NS control group. Pretreatment of Ocimumoside A, Ocimumoside B and PQ significantly prevented these changes as compared to the respective RS group, however, no significant effect of Ocimarin was observed. OS compounds failed to prevent the RS-induced decrease in the NA levels. On the other hand, Ocimarin, Ocimumoside A and B per se did not cause any significant change in the monoamine and metabolite levels in all the three brain regions (Fig. 1 A, B).
Fig. 1.
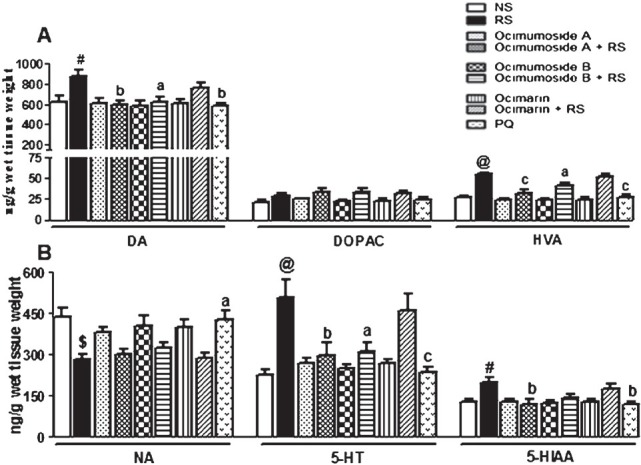
Histogram representing the levels of DA, DOPAC, HVA (A) NA, 5-HT and 5-HIAA (B) in the frontal cortex of the control (NS), stressed (RS) and OS treated groups. Results are represented as mean ± SEM; (n=8) (ANOVA, Tukey-Kramer post hoc test). #P<0.05, $P<0.01, @P<0.001 compared to NS group, aP<0.05, bP<0.01, cP<0.001 compared to RS group.
RS exposure significantly increased the striatal DOPAC (P<0.05), HVA (P<0.001), 5-HT (P<0.01) and 5-HIAA (P<0.05) levels, while significantly (P<0.001) decreased the DA levels in striatum in comparison to the NS control group (Fig. 2 A and B). Pretreatment with Ocimumoside A, Ocimumoside B and PQ significantly prevented these RS-induced changes, while, Ocimarin insignificantly altered these changes. The DA and NA levels were significantly (P<0.05, P<0.01, respectively) decreased by RS, while, the levels of 5-HT and 5-HIAA significantly (P<0.05, P<0.01, respectively) increased in hippocampus as compared to the NS control group (Fig. 3 A, B). Pretreatment with Ocimumoside A, Ocimumoside B and PQ significantly prevented the increased levels of 5-HT and 5-HIAA as compared to the respective RS group, while, Ocimarin insignificantly altered these changes.
Fig. 2.
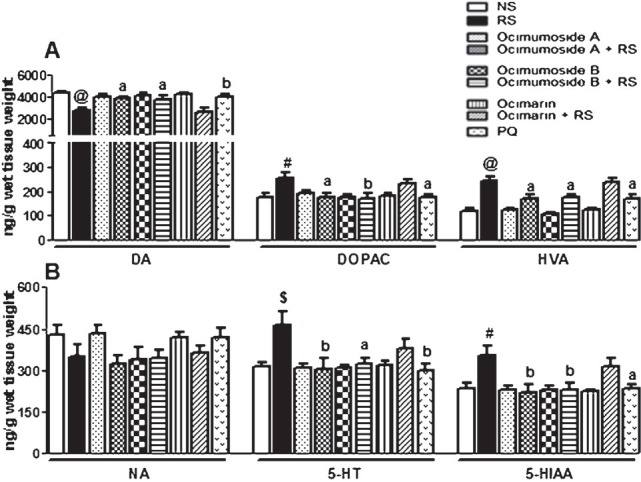
Histogram representing the levels of DA, DOPAC, HVA (A) NA, 5-HT and 5-HIAA (B) in the striatum of the control (NS), stressed (RS) and OS treated groups. Results are represented as mean ± SEM; (n=8) (ANOVA, Tukey-Kramer post hoc test). #P<0.05, $P<0.01, @P<0.001 compared to NS group, aP<0.05, bP<0.01 compared to RS group.
Fig. 3.
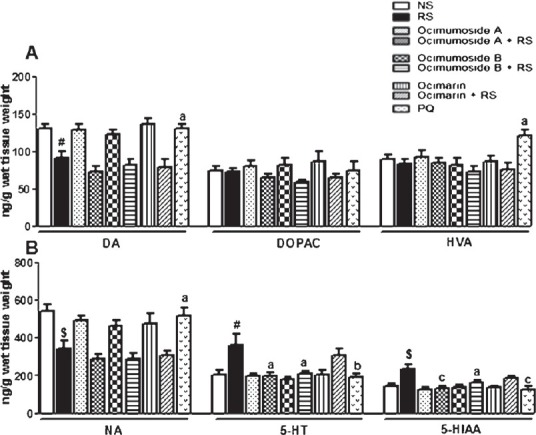
Histogram representing the levels of DA, DOPAC, HVA (A) NA, 5-HT and 5-HIAA (B) in the hippocampus of the control (NS), stressed (RS) and OS treated groups. Results are represented as mean ± SEM; (n=8) (ANOVA, Tukey-Kramer post hoc test). #P<0.05, $P<0.01 compared to NS group, aP<0.05, bP<0.01, cP<0.001 compared to RS group.
Effect of OS compounds and MEL on the changes in the brain antioxidant systems induced by RS: The activities of SOD and GSH-Px were significantly (P<0.01, P<0.001) increased in the frontal cortex and striatum by RS, while the CAT activity remained unaltered in the three brain regions as compared to the NS control group. The RS-induced changes in the antioxidant enzymes were restored to the control values by Ocimumoside A, Ocimumoside B and MEL pretreatment, while, Ocimarin showed no significant changes. In the hippocampus, the SOD, CAT and GSH-Px activities remained unaltered by RS and following the treatment of OS compounds (Fig. 4A, B).
Fig. 4.
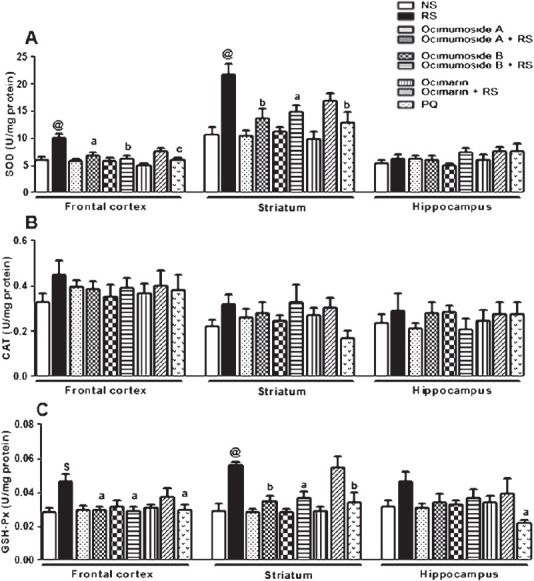
Histogram representing the activities of SOD (A) , CAT (B) and GSH-Px (C) in the frontal cortex, striatum and hippocampus of the control (NS), stressed (RS) and OS treated groups. Results are represented as mean ± SEM; (n=8). $P<0.01, @P<0.001 compared to NS group (ANOVA, Tukey-Kramer post hoc test). aP<0.05, bP<0.01, cP<0.001 compared to RS group.
Effect of OS compounds and MEL on RS-induced changes in the brain GSH and MDA levels: The GSH content was significantly (P<0.05) reduced in all the three selected brain regions by RS when compared to the NS control group. Administration of Ocimumoside A, Ocimumoside B and MEL restored the GSH levels while, Ocimarin showed no changes (Fig. 5A).
Fig. 5.
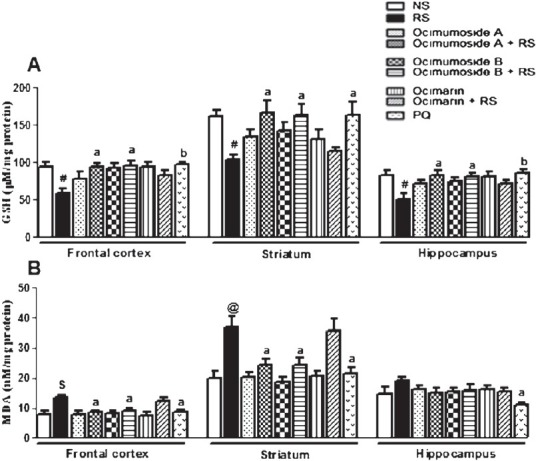
Histogram representing the levels of GSH (A) and MDA (B) in the frontal cortex, striatum and hippocampus of the control (NS), stressed (RS) and OS treated groups. Results are represented as mean ± SEM; (n=8) #P<0.05, $P<0.01, @P<0.001 compared to NS group (ANOVA, Tukey-Kramer post hoc test). aP<0.05, bP<0.01, cP<0.001 compared to RS group.
Exposure to RS significantly increased the MDA levels in the frontal cortex (P<0.01) and striatum (P<0.001) as compared to the NS control group. Pre-treatment with Ocimumoside A, Ocimumoside B and MEL significantly (P<0.05) prevented the RS-induced increase of MDA levels, while, Ocimarin showed no changes (Fig. 5B).
Discussion
The stress-induced effects are an outcome of altered activity of different systems such as central neurotransmitters, neurohormonal factors particularly those linked with the pituitary-adrenal axis, and free radical generation3,8. These responses are believed to be developed during the first exposure of stress and/or novel environment. In the present study the initial response of monoaminergic and oxidative systems in important brain regions was evaluated under RS condition. Acute immobilization (restraint) procedure was need to investigate the changes induced by a short acting severe stressor. Among the various stress models, immobilization has been used extensively and accepted widely for studying stress-induced alterations20.
RS significantly increased the DA levels in the frontal cortex and decreased in the striatum and hippocampus with selective increase of their metabolites. The variable response of DA in the frontal cortex and striatum by RS can be explained on the basis that the terminals of DA neurons on the frontal cortex may differ from those in the striatum21. In the striatum, the decreased DA levels were accompanied by subsequent increase in the DOPAC and HVA levels suggesting increased conversion of DA to its metabolite. However, the decreased DA levels in the hippocampus could be due to a possible decrease in the cholinesterase activity22. Pre-treatment with Ocimumoside A and B prevented RS-altered DA changes in the frontal cortex and striatum, while Ocimarin failed to prevent any of these changes towards normal values. The NA system is known to be activated by stress; although only for a short duration23, which could explain the decreased levels of NA by RS, as the stressor applied was of relatively longer duration (2.5 h). Administration of Ocimumoside A and B prevented the RS-induced decrease of NA levels. Impaired monoamine function is one of the major contributors to stress-mediated disorders like anxiety and depression24. The exact mode of action of these two compounds cannot be explained, however, a corticosterone like effect can be hypothesized as such effects are inhibited either by alpha-helical CRF, a CRF antagonist or adrenalectomy, suggesting the possible mediation through corticosterone25.
Our results demonstrated that RS led to an increase of 5-HT and 5-HIAA levels similar to the reports where physical and psychological stressors increased the serotonergic transmission26,27. A report suggested that an acute stress exposure activates tryptophan hydroxylase (TH) in the cortex and midbrain causing an increased level of 5-HT25. Hence, it can be assumed that the prevention of these serotonergic changes by Ocimumoside A and B may operate by influencing either the activity of TH or the reuptake of 5-HT. However, the differential monoaminergic responses under RS condition suggested regional variations in their levels of distribution, synthesis and degradation. PQ was found to be effective in preventing these RS-induced monoaminergic changes in the three brain regions which could be associated to the presence of its major constituent ginsenosides, known to possess corticosteroid like actions11. The ginsenosides of PQ also modulated the nerve transmissions by altering the availability of neurotransmitters28.
It was observed that RS significantly enhanced the activities of SOD and GSH-Px in the frontal cortex and striatum, while, CAT activity was increased but did not reach statistical significance. In hippocampus, there was no significant change in the activity of these enzymes. The increased activity of SOD during RS is an indicator of a relative increase in the superoxide radical production, which could stimulate the second line of defence including GSH-Px and CAT29. However, a lesser change in the CAT activity as compared to GSH-Px indicated that in the brain CAT activity has been found to be low and confined to peroxisomes30. The increased GSH-Px activity may also indicate the increase in cellular peroxide levels, whereas, the decreased GSH concentrations could be due to its increased rate of utilization during oxidative stress2. Pre-treatment with Ocimumoside A and B prevented the RS-induced perturbations in the antioxidant enzyme activities, GSH content and the extent of lipid peroxidation. However, Ocimarin failed to alter such oxidative processes. These differential alterations in the antioxidant systems and variable effect of OS compounds in selected brain regions may arise from the differences in antioxidant buffering capacities or differential susceptibilities to oxidative stress. The prevention of altered redox state in RS group by MEL is in agreement with reports demonstrating its potent antioxidant capacity10.
In conclusion, this study identified the anti-stress potential of Ocimumoside A and B in relation to their simultaneous modulatory effects on the central monoaminergic and antioxidant systems during acute stressful condition. Further studies need to be done to understand the mechanism of such pharmacological interventions with OS constituents in the prevention of stress-induced neurological and related disorders.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, India, for providing financial support.
References
- 1.Ahmad A, Rasheed N, Banu N, Palit G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress. 2010;13:355–64. doi: 10.3109/10253891003667862. [DOI] [PubMed] [Google Scholar]
- 2.Sahin E, Gumuslu S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav Brain Res. 2004;155:241–8. doi: 10.1016/j.bbr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12:167–77. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]
- 4.Archana R, Namasivayam A. Effect of Ocimum sanctum on noise induced changes in neutrophil functions. J Ethnopharmacol. 2000;73:81–5. doi: 10.1016/s0378-8741(00)00281-6. [DOI] [PubMed] [Google Scholar]
- 5.Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol. 2002;80:15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 6.Gupta P, Yadav DK, Siripurapu KB, Palit G, Maurya R. Constituents of Ocimum sanctum with antistress activity. J Nat Prod. 2007;70:1410–6. doi: 10.1021/np0700164. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 8.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai D, Bhatia G, Sen T, Palit G. Comparative study of perturbations of peripheral markers in different stressors in rats. Can J Physiol Pharmacol. 2003;81:1139–46. doi: 10.1139/y03-117. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri S, Singh P, Singh S, Rasheed N, Patit G, Pant KK. Melatonin protects against experimental reflux esophagitis. J Pineal Res. 2009;46:207–13. doi: 10.1111/j.1600-079X.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 11.Rasheed N, Tyagi E, Ahamd A, Siripurapu KB, Lahiri S, Shukla R, et al. Involvement of monoamines and proinflammatory cytokines in mediating the anti-stress effects of Panax quinquefolium. J Ethnopharmacol. 2008;117:257–62. doi: 10.1016/j.jep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Speisky MB, Kharouba SN. Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high-performance liquid chromatography. Differential effect of probenecid, haloperidol and yohimbine on the concentrations of biogenic amines and metabolites in various regions of rat brain. J Chromatogr. 1987;386:25–35. doi: 10.1016/s0021-9673(01)94581-9. [DOI] [PubMed] [Google Scholar]
- 14.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 15.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 16.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 17.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 20.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–48. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 21.Bannon MJ, Michaud RL, Roth RH. Mesocortical dopamine neurons.Lack of autoreceptors modulating dopamine synthesis. Mol Pharmacol. 1981;19:270–5. [PubMed] [Google Scholar]
- 22.Das A, Rai D, Dikshit M, Palit G, Nath C. Nature of stress: differential effects on brain acetylcholinesterase activity and memory in rats. Life Sci. 2005;77:2299–311. doi: 10.1016/j.lfs.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Hellriegel ET, D’Mello AP. The effect of acute, chronic and chronic intermittent stress on the central noradrenergic system. Pharmacol Biochem Behav. 1997;57:207–14. doi: 10.1016/s0091-3057(96)00341-3. [DOI] [PubMed] [Google Scholar]
- 24.Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54(Suppl 1):24–7. doi: 10.1016/j.metabol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Singh VB, Onaivi ES, Phan TH, Boadle-Biber MC. The increases in rat cortical and midbrain tryptophan hydroxylase activity in response to acute or repeated sound stress are blocked by bilateral lesions to the central nucleus of the amygdala. Brain Res. 1990;530:49–53. doi: 10.1016/0006-8993(90)90656-v. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav. 1994;49:911–20. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 27.Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002;926:10–7. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- 28.Tsang D, Yeung HW, Tso WW, Peck H. Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med. 1985;3:221–4. doi: 10.1055/s-2007-969463. [DOI] [PubMed] [Google Scholar]
- 29.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 30.Gaunt GL, de Duve C. Subcellular distribution of D-amino acid oxidase and catalase in rat brain. J Neurochem. 1976;26:749–59. doi: 10.1111/j.1471-4159.1976.tb04448.x. [DOI] [PubMed] [Google Scholar]


