Abstract
Background & objectives:
Nigella sativa Linn. is extensively used in the Indian diasporas as spice, which may interact with co-administered drugs and affect their intestinal availability. The purpose of this study was to investigate the effect of Nigella on bioavailability of amoxicillin in animal model.
Methods:
Everted rat intestinal sacs were used for in vitro experiment to study the transfer of amoxicillin across the gut. Amoxicillin (6 mg/ml) was co-infused with 3 and 6 mg of methanol and hexane extract of Nigella seeds separately. The amount of amoxicillin that traversed the gut was followed spectrophotometrically at 273 nm. For in vivo studies Wistar albino rats were used. Amoxicillin (25 mg/kg, po) was co-administered with hexane extract of Nigella seeds (25 mg/kg, po). The amount of amoxicillin in rat plasma was determined by UPLC-MS/MS method.
Results:
The in vitro studies both with methanol and hexane extracts of Nigella increased the permeation of amoxicillin significantly (P<0.001) as compared to control. Permeation was also found to be significantly higher for the hexane extract (P<0.001) in comparison to methanol extract at the same dose levels. In vivo experiments revealed that Cmax of amoxicillin in rat plasma when administered orally alone and in combination with hexane extract increased correspondingly from 4138.251 ± 156.93 to 5995.045 ± 196.28 ng/ml while as AUC0→t increased from 8890.40 ± 143.33 to 13483.46 ± 152.45 ng/ml.h.
Interpretation & conclusions:
Nigella enhanced amoxicillin availability in both in vivo and in vitro studies. As the increase in bioavailability is attributed, in part, to enhanced diffusivity across intestine, our study indicated that Nigella increased intestinal absorption of amoxicillin.
Keywords: Absorption enhancement, amoxicillin, bioavailability, everted rat sac, Nigella sativa
Nigella, belonging to Ranunculaceae, is a small genus of annual herb found in Southern Europe and Western Asia, especially in the mediterranean region. Three species are recorded in India. N. sativa Linn. is one of these species, which is found as a weed in Punjab, Himachal Pradesh, Bihar and Assam1. Its seeds are angular, dark gray in colour and are locally known as ‘Kalajira’ or ‘Kalongi’. Its oil is generally regarded as safe (GRAS) by Food and Drug Administration2. The seeds are considered carminative, stimulant, diuretic, emmenagogue, galactagogue, whereas its oil is applied externally for skin eruptions. It is extensively used in the Indian diasporas as spice, which may interact with co-administered drugs and affect their availability. The purpose of this study was to investigate the effect of Nigella on bioavailability of amoxicillin in rats.
Amoxicillin is a beta-lactam antibiotic classified as a class III drug with low permeability according to Biopharmaceutics Classification System3. The drug is conventionally administered as capsule or as oral suspension. The drug shows absorption through two mechanisms- energy dependent active mechanism and electro-chemical gradient dependent passive mechanism but has poor oral absorption due to complete ionization under gastrointestinal pH conditions and exhibits very low lipid solubility4. In rat plasma profile, the drug has revealed low bioavailability5. It was also confirmed by the reports obtained from a regional perfusion technique in vivo in humans6. Among the reasons for poor oral bioavailability of drugs, the permeability of intestinal epithelium plays an important role in passive transport. Several factors seem to influence the permeability of intestine and thereby affect the absorption of substances by the gut. These include herbs, chemicals, stress, inflammation, toxins and probiotics7.
In the present study the effect of concomitantly infused Nigella extracts on the absorption of amoxicillin was studied using in vitro models. As a model for the in vitro absorption enhancement studies excised rat intestinal segments were used. Amoxicillin permeation with hexane extract was significantly higher than methanol extract in in vitro studies and was thus evaluated in in vivo studies.
Material & Methods
Plant materials: Nigella seeds were collected from Green Earth Products Pvt. Ltd, Delhi, India. Samples were identified by Dr H.B. Singh, Head, Raw Materials Herbarium and Museum, National Institute of Science Communication and Information Resources (NISCAIR), Delhi. A voucher specimen with reference number NISCAIR/RHMD/1147/179/01 has been retained in the herbarium for future reference.
Chemicals: Amoxicillin was obtained as gift from Ranbaxy Pvt. Ltd., Gurgaon, India. Potassium dihydrogen orthophosphate, sodium hydroxide, acetonitrile (LC-MS grade, Assigned purity 99.9%; Lot No: 90525) and ammonium acetate were purchased from Sigma-Aldrich, Germany. Dichloromethane was purchased from Merck Specialties Pvt. Ltd. (E. Merck, India). Water used in the entire analysis was prepared in-house with Milli-Q water purification system procured from Millipore (Millipore Corporation, USA). Other chemicals used were of analytical grade from commercial sources.
Preparation of extracts: Seeds were cleaned, milled and then passed through a 35 mm (42 mesh) sieve. The grounded seeds (25 g) were separately extracted with hexane and methanol for 6 h using Soxhlet apparatus. The solvent was evaporated under reduced pressure. The extracts were stored at 4°C in the dark till further used.
In vitro experimental design and surgical procedure: The study was performed in Phytochemistry Research Laboratory, Hamdard University, New Delhi, and the protocol was duly approved by the Institutional Animal Ethics Committee (No. 639/09). Adult male Wistar albino rats weighing 120-150 g were procured from central animal facility and housed in cages under standard laboratory conditions (10 h dark/ 14 h light, temperature 20-25 °C, relative humidity 65%) for seven days. Animals were fasted for 18 h before the experiment. After decapitation, the abdomen of rats was opened by a midline incision. The entire small intestine was removed quickly by cutting across the upper end of duodenum and the lower end of ileum, and by stripping the mesentery manually8. The small intestine was washed out with normal saline solution (0.9% w/v NaCl) using a syringe equipped with blunt end. Intestinal segments (8 ± 2 cm) were everted. After being blotted with a piece of filter paper, a glass weight (1 g) was fixed and tied to the end of everted gut segment to make an empty gut sac. This was important to prevent peristaltic muscular contractions, which may otherwise alter the shape and internal volume of the sac.
In vitro everted intestinal sac permeation study: The methanol and hexane extracts were tested with amoxicillin using everted rat sac model. Wilson and Wiseman method with slight modifications was followed9. The empty sac was filled with 1 ml of amoxicillin (6 mg/ml) in PBS (pH 7.4) using a blunt-end syringe. The needle was then slipped off carefully, and the loose ligature on the proximal end was tightened. The serosal compartment contained buffer in the sac. The distended sac was placed inside organ tube of organ bath containing 50 ml PBS. This gut sac bath was surrounded by a water jacket maintained at 37 ± 0.5 °C. The mucosal fluid compartment content was continuously mixed with air bubbles using an aerator. At predetermined intervals, 5 ml of sample was withdrawn from the organ tube and same volume was replaced with fresh buffer. The concentration of drug that traversed intestinal surface was monitored at 273 nm spectrophotometrically. Experiment was repeated with amoxicillin along with 3 mg and 6 mg of methanol and hexane extracts separately. All experiments were conducted in triplicate.
A solution of amoxicillin (6 mg/ml) was prepared in PBS (pH 7.4). PBS was prepared by mixing 250 ml 0.2 M potassium dihydrogen orthophosphate and 393.4 ml of 0.1 M sodium hydroxide, both dissolved in distilled water and final volume made up to one litre10. Amoxicillin (10 mg) was dissolved in 100 ml of PBS to prepare a stock solution 100 μg/ml. Different dilutions ranging from 10 to 100 μg/ml amoxicillin were prepared by serial dilution method. Absorbance was recorded at 273 nm11. PBS was used as blank and absorbance was plotted against concentration. Permeated concentration of amoxicillin was calculated by standard plot of amoxicillin in PBS.
In vivo experimental design: Adult male Wistar albino rats (200-250 g) were used for the experiments. Animals were fed on standard pellet diet (Ashirwad Industries, Chandigarh) and water was provided ad libitum. The rats were fasted overnight before the day of the experiment. Animals were randomly distributed in two groups (n = 6), one group received amoxicillin (25 mg/kg body weight, po) while the other group received amoxicillin (25 mg/kg b.w., po) and Nigella hexane extract (25 mg/kg b.w., po). Blood samples were collected from tail vein in pre-heparinized glass tubes at different time intervals post-dosing (0, 0.250, 0.500, 0.750, 1, 1.5, 2, 4, 6 and 8 h). Blood samples were centrifuged (2500×g; 10 min; 20 °C) to separate plasma fractions. The collected plasma samples were preserved in deep freezer until analysis.
In vivo pharmacokinetic investigation: UPLC-MS/MS method for estimation of amoxicillin in rat plasma was used12 with suitable modification. The chromatographic separation was achieved on a Waters Acquity UPLC™ system (Miliford, MA, USA) equipped with Waters Acquity BEH C18 column (100.0 × 2.1 mm; 1.7 μm) using isocratic mobile phase acetonitrile-ammonium acetate (85:15 v/v). For isocratic elution, the flow rate of degased mobile phase was maintained at 0.2 ml/min and 10 μl of sample solution was injected in each run. The Waters Q-TOF premier detector was operated in multiple reactions monitoring mode via positive ionization interface using the transitions m/z 366.1→349.1 for amoxicillin and m/z 350.1→192.1 for ampicillin used as internal standard. The recovery of the analytes from rat plasma was optimized using liquid-liquid extraction (LLE) technique in dichloromethane13. The total run time was 3.0 min and the elution of amoxicillin occurred at 0.88 ± 0.05 min. Concentration-time curves were established for each analyte and used for the determination of pharmacokinetic parameters such as peak plasma concentration (Cmax), peak time (Tmax) and extent of absorption (AUC) by a non-compartmental analysis using PK Solutions Version 2.0; Summit Research Services, USA.
Data analysis: Three-group comparisons were analyzed by paired Student's two-tailed t-test. P value was calculated by ANOVA.
Results & Discussion
The solvent extraction of Nigella seeds with hexane yielded a golden yellow colour with a strong aromatic odour while the methanol extract was dark brown in colour with a disagreeable odour. The percentage yield was 30.2 per cent with hexane and 39.32 per cent with methanol. The effect of these extracts on in vitro permeation of amoxicillin was investigated using everted rat sac model. The amounts of amoxicillin permeated alone and in combination with Nigella extracts were calculated. It was observed that the permeation of amoxicillin with 6 mg methanol extract increased significantly (P<0.001) as compared to control. Permeation enhancement with hexane extract showed dose-dependent increase and was significantly higher (P<0.001) than the methanol extract at comparable concentrations (Table I). Having its higher effect on permeation of amoxicillin, the hexane extract was selected for in vivo studies. There was a significant increase (P<0.001) in amoxicillin concentration in rat plasma in presence of hexane extract (25 mg/kg po). The maximum concentrations of amoxicillin achieved in rat plasma when administered orally, alone and in combination with hexane extract, were 4138.251 ± 156.93 to 5995.045 ± 196.28 ng/ml (Table II) while AUC0→tincreased from 8890.40 ± 143.33 to 13483.46 ± 152.45 ng/ml.h, respectively. It indicated that Nigella increased the rate and extent of absorption of amoxicillin.
Table I.
Cumulative concentration of amoxicillin permeated in presence of Nigella extracts in in vitro studies
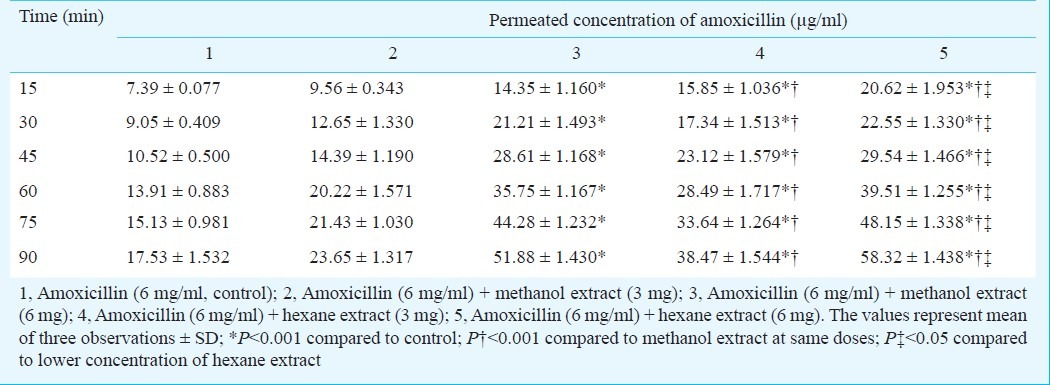
Table II.
Concentration of amoxicillin in rat plasma in presence of Nigella hexane extract after oral co-administration
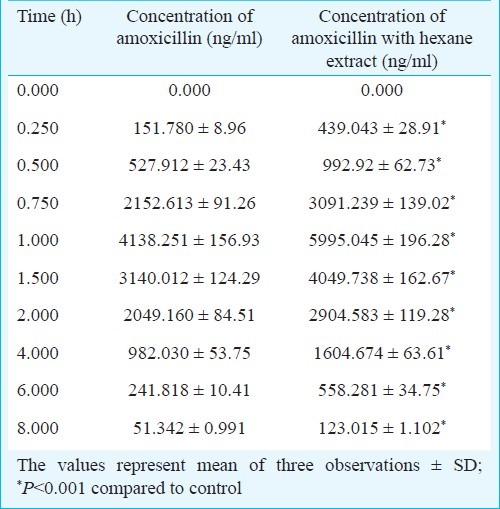
These results suggested that hexane extract of Nigella affected intestinal absorption that might be attributed to the presence of fatty acids in it. Long chain fatty acids (oleic, linoleic acid) and medium chain fatty acids (caprylic, capric acid) have been reported to increase oral bioavailability of peptides, antibiotics and other important therapeutic agents14. The oral bioavailability of cinnarizine was greatly enhanced by oleic acid15. A concentration dependent increase in the oral bioavailability of polar high molecular weight drugs like glycyrrhizin in rats has been found with fatty acids16. Fatty acids have also been reported to produce a dose dependent increase in Cmax of norfloxacin in rabbits17. Fatty acids act as absorption enhancers by increasing the fluidity of the apical and basolateral membranes18,19. Earlier we have studied the effect of hexane extract of Nigella on permeation of carvedilol and documented its fatty acid profile. Nigella was found to exhibit significant enhancement in permeation of carvedilol20. Linoleic acid, oleic acid, margaric acid, cis-11, 14-eicosadienoic acid and stearic acid were identified as main fatty acids21. It was thus postulated that since the hexane extract contains both long and medium chain fatty acids, a synergistic effect cannot be ruled out.
In conclusion, our results indicated that Nigella when co-administered with amoxicillin increased its absorption across gut wall. Thus, Nigella was found to be efficient absorption enhancer for amoxicillin and can be tested as absorption (bioavailability) enhancing system for various low permeable drugs.
Acknowledgment
Authors acknowledge the financial support provided by Council for Scientific and Industrial Research (CSIR), New Delhi, India, and Ranbaxy Research Laboratories, Gurgaon, for providing amoxicillin.
References
- 1.The wealth of India: raw materials. VI. Delhi, India: NISCAIR (PID); 2001. Council for Scientific and Industrial Research; pp. 63–5. [Google Scholar]
- 2.Burdock GA. Encyclopedia of food and color additives. Boca Raton, Floroda: CRC Press; 1997. pp. 230–1. [Google Scholar]
- 3.Chi-Yuan W, Leslie ZB. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji A, Nakashima E, Kagami I, Yamana T. Intestinal absorption mechanism of amphoteric, β-lactam antibiotics I. Comparative absorption and evidence for saturable transportof amino-, β-lactam antibiotics by in situ rat small intestine. J Pharm Sci. 1981;70:768–72. doi: 10.1002/jps.2600700714. [DOI] [PubMed] [Google Scholar]
- 5.Jesus CJ, Jose EP, Francisca TM, Luis G. Low bioavailability of amoxicillin in rats as a consequence of presystemic degradation in the intestine. Antimicrob Agents Chemother. 1994;38:842–7. doi: 10.1128/aac.38.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lennernas H, Knutson L, Knutson T, Hussain A, Lesko L, Salmonson T, et al. The effect of amiloride on the in-vivo effective permeability of amoxicillin in human jejunum: experience from a regional perfusion technique. Eur J Pharm Sci. 2002;15:271–7. doi: 10.1016/s0928-0987(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 7.Rao JP. Influence of dietary calcium content on intestinal permeability in rat. Indian J Med Res. 2009;129:681–4. [PubMed] [Google Scholar]
- 8.Barthe L, Woodley JF, Kenworthy S, Houin G. An improved everted gut sac as a simple and accurate technique to measure paracellular transport across the small intestine. Eur J Drug Metab Pharmacokinet. 1998;23:313–23. doi: 10.1007/BF03189357. [DOI] [PubMed] [Google Scholar]
- 9.Wilson TH, Wiseman G. The use sacs of everted small intestine for the study of transference of substances from the mucosal to the serosal surface. J Physiol. 1954;123:116–25. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilling RJ, Mitra AK. Intestinal mucosal transport of insulin. Int J Pharm. 1990;62:53–64. [Google Scholar]
- 11.Juan AQ, Santiago VJ, Segundo P. Penicillin-binding proteins of Bacteroides fragilis and their role in the resistance to imipenem of clinical isolates. J Med Microbiol. 2005;54:1055–64. doi: 10.1099/jmm.0.45930-0. [DOI] [PubMed] [Google Scholar]
- 12.Wen A, Hang T, Chen S, Wang Z, Ding L, Tian Y, et al. Simultaneous determination of amoxicillin and ambroxol in human plasma by LC-MS/MS: validation and application to pharmacokinetic study. J Pharm Biomed Anal. 2008;48:829–34. doi: 10.1016/j.jpba.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Yoon KH, Lee SY, Kim W, Park JS, Kim HJ. Simultaneous determination of amoxicillin and clavulanic acid in human plasma by HPLC–ESI mass spectrometry. J Chromatogr B. 2004;813:121–7. doi: 10.1016/j.jchromb.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Kang MJ, Cho JY, Shim BH, Kim DK, Lee J. Bioavailability enhancing activities of natural compounds from medicinal plants. J Med Plants Res. 2009;3:1204–11. [Google Scholar]
- 15.Tokumura T, Tsushima Y, Tatsuishi K, Kayano M, Machida Y, Nagai T. Enhancement of the oral bioavailability of cinnarizine in oleic acid in beagle dogs. J Pharm Sci. 1987;76:286–8. doi: 10.1002/jps.2600760404. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Yonebayashi S, Yoshida M, Shimizu K, Aotsuka T, Takayama K. Improvement in the bioavailability of poorly absorbed glycyrrhizin via various non-vascular administration routes in rats. Int J Pharm. 2003;265:95–102. doi: 10.1016/s0378-5173(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 17.Dos-Santos I, Fawaz F, Lagueny AM, Bonini F. Improvement of norfloxacin oral bioavailability by EDTA and sodium caprate. Int J Pharm. 2003;260:1–4. doi: 10.1016/s0378-5173(03)00257-6. [DOI] [PubMed] [Google Scholar]
- 18.Chi SC, Park ES, Kim H. Effect of penetration enhancers on flurbiprofen permeation through rat skin. Int J Pharm. 1995;126:267–74. [Google Scholar]
- 19.Sinha VR, Kaur MP. Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 2000;26:1131–40. doi: 10.1081/ddc-100100984. [DOI] [PubMed] [Google Scholar]
- 20.Amin S, Kohli K, Khar RK, Mir SR, Pillai KK. Mechanism of in-vitro percutaneous absorption enhancement of carvedilol by penetration enhancers. Pharm Dev Tech. 2008;13:533–9. doi: 10.1080/10837450802309646. [DOI] [PubMed] [Google Scholar]
- 21.Amin S, Mir SR, Kohli K, Ali B, Ali M. A study of chemical composition of Nigella oil and its effect on penetration enhancement from transdermal formulation. Nat Prod Res. 2010;24:1151–7. doi: 10.1080/14786410902940909. [DOI] [PubMed] [Google Scholar]


