Abstract
Background:
Dexmedetomidine, is a selective α2-adrenoceptor agonist that is used as an adjuvant mixed with local anesthetics during regional anesthesia. This study was designed to test the efficacy of adding dexmedetomidine to bupivacaine during placement of infraclavicular brachial plexus blockade (ICB).
Methods:
Sixty adult patients were divided into 2 equal groups of 30 subjects each. Patients in Group I received an ICB using 30 mL of 0.33% bupivacaine and Group II patients received 30 mL of 0.33% bupivacaine mixed with 0.75 μg/kg of dexmedetomidine. The following brachial plexus nerve block parameters were assessed: block success rate, sensory onset time and duration, motor block onset time and duration, analgesic pain scores using the verbal rating scale (VRS) for pain, duration of analgesia, and amount of supplemental intravenous (IV) morphine required.
Results:
There was a statistically significant shorter time to onset of sensory blockade (13.2 vs 19.4 min, P=0.003), longer duration of sensory block (179.4 vs 122.7 min, P=0.002), shorter onset time to achieve motor block (15.3 vs 22.2 min, P=0.003), longer duration of motor block (155.5 vs 105.7 min, P=0.002), lower VRS pain scores, prolonged analgesia (403 vs 233 min, P=0.002), and lower morphine rescue requirements for 48 h after surgery (4.9 (0–8.0) vs 13.6 mg (4.0–16.0) mg, P=0.005). All patients recovered without evidence of sensory or motor deficit.
Conclusion:
Adding dexmedetomidine to bupivacaine during the placement of an ICB provides: (1) enhancement of onset of sensory and motor blockade, (2) prolonged duration of analgesia, (3) increases duration of sensory and motor block, (4) yields lower VRS pain scores, and (5) reduces supplemental opioid requirements.
Keywords: Analgesia, dexmedetomidine, infraclavicular block
INTRODUCTION
Infraclavicular brachial plexus blockade (ICB) provides anesthesia for surgery of the hand, forearm, elbow, and distal humerus. Both the axillary and musculocutaneous nerves are affected/blocked at the level of the cords before they branch from the brachial plexus sheath. In contrast to interscalene and supraclavicular brachial plexus blockade, an infraclavicular block has the advantage of minimal risk to intravertebral, intrathecal, or epidural injection, as well as reduced incidence of phrenic nerve paralysis or stellate ganglion block.[1] However, an ICB has a small risk of pneumothorax, hematoma, and nerve injury.[1–3]
Clonidine, an α2-adrenergic agonist, has been added to local anesthetics used during regional anesthesia. A recent meta-analysis has demonstrated that adding clonidine to intermediate and long-acting local anesthetics during a single-shot peripheral nerve or nerve plexus block provides a longer duration of analgesia and motor blockade by approximately 2 h.[4] Dexmedetomidine, a selective α2-adrenoceptor agonist, has been used as an adjuvant during regional and local anesthesia.[5–7] Animal and human studies have shown safety and efficacy of adding dexmedetomidine to local anesthetics in various regional anesthetic procedures, such as subarachnoid, epidural, and caudal injections, yet other investigations have reported reduced or negative analgesic effects when using dexmedetomidine.[8–12] However, there remains limited knowledge on the analgesic efficacy and clinical utility of adding dexmedetomidine to local anesthetics during peripheral nerve and nerve plexus blockade in humans.[13,14] Therefore, this study was designed to investigate the efficacy of dexmedetomidine as an adjuvant in combination with local anesthetic solutions during an ICB for upper extremity surgery. So it has been hypothesized that dexmedetomidine may improve overall efficacy during an ICB.
METHODS
This prospective, randomized, double-blinded study was performed on 60 adult patients undergoing minor orthopedic surgery of the hand and forearm. Written informed consent and institutional review board approval were obtained. All patients were evaluated and assessed as American Society of Anesthesiologists (ASA) I or ASA II physical status. Patients had an ultrasound-guided single-shot ICB performed 2 cm caudal and 2 cm medial to the coracoid process on the anterior chest wall targeting the cords of the brachial plexus around the axillary artery.[3]
Patients were randomly chosen to receive either bupivacaine + placebo (group I, n=30) or bupivacaine + dexmedetomidine (group II, n=30) by computer-generated random selection. Randomization was performed by an independent statistician and concealed from patients and investigators until completion of statistical analysis. The local anesthetic mixtures (placebo vs dexmedetomidine) for injection were supplied by the pharmacy and patients and health care providers involved in patient care were blinded to the drug combinations. Exclusion criteria included the following: patient refusal, patients with chronic pain, those using chronic analgesic medications, coagulopathy, history of brachial plexus injury, allergy to the study drugs, patients taking other medications with α-adrenergic blocking effect, hepatic or renal insufficiency, systemic infection or infection at the site of injection, and shoulder surgery. Patients were instructed preoperatively about use of the verbal rating scale (VRS) for pain assessment (VRS: 0 = no pain, 10=worst pain possible).
Patients were given 1–2 mg of midazolam intravenous (IV) as a premedication 10–15 min before beginning each block technique in addition to 50–100 μg of fentanyl just prior to block needle insertion. Subcutaneous injection with 4 mL of 1% lidocaine was administered at the needle insertion site. Patients were continuously monitored throughout the procedure with continuous electrocardiogram, pulse oximetry, capnography by nasal cannula, and noninvasive blood pressure. Patients were placed supine and their head turned away from the side to be blocked. The probe (8–12 MHz) of a Hewlett–Packard 77020A ultrasound machine (Andover, MA, USA) was positioned in the parasagittal plane near the coracoid process to best visualize a cross-sectional view of the axillary artery. A 10 cm 18-ga insulated needle (Plexolong; Pajunk, Geisingen, Germany) attached to a nerve stimulator (Stimuplex®; Braun, Melsungen, Germany) was inserted inplane to the ultrasound probe. Initial stimulating current was set at 0.6–0.8 mA. The brachial plexus was typically reached at 6–9 cm and the stimulating current was then gradually decreased eliciting a motor response at 0.3 mA or less. Muscle twitches from biceps muscle was not accepted (musculocutaneous nerve may have branched from the brachial sheath) and if obtained, the needle would be redirected inferiorly and slightly medially to achieve median, radial, or ulnar nerve stimulation. Stimulation of the axillary nerve (deltoid muscle twitch) was not accepted (axillary nerve is often outside the brachial sheath at this level of the brachial plexus) and if obtained, the needle was redirected more superiorly. Only motor responses (twitches) from the triceps, forearm, and hand muscles were accepted prior to local anesthetic injection. Aspiration was performed to detect an unintentional intravascular needle placement prior to a test dose of 4 mL of 1% lidocaine with epinephrine (5 μg/mL) followed by the loading dose of local anesthetic administered incrementally. The injectate contained either 30 mL of 0.33% bupivacaine + placebo (Group I) or 30 mL of 0.33% bupivacaine + 0.75 μg/kg of dexmedetomidine (Group II). All of the subjects had a brachial plexus catheter placed prior to block needle removal for continuous administration of analgesic medication.
Dexmedetomidine hydrochloride (Precedex®, manufactured by Hospira, Inc. Lake Forest, IL, USA) was supplied in 100 μg/mL and both bupivacaine and dexmedetomidine were diluted in physiological saline to achieve a mean pH of 5.6. The same volume of saline (placebo), corresponding to that of dexmedetomidine, was added to bupivacaine for the bupivacaine + placebo group (Group I). Any evidence of clinical criteria suggesting local anesthetic toxicity (lightheadedness, dizziness, tinnitus, disorientation, drowsiness, generalized muscle twitching, convulsions, respiratory depression, cardiovascular depression, and collapse) in addition to possible systemic effects of dexmedetomidine, such as bradycardia, hypotension, fainting, and somnolence were recorded. Postoperatively, all patients received patient controlled analgesia (PCA) with IV morphine at 1 mg bolus with a 10 min lockout period and a maximum hourly limit of 5 mg. In addition, 1 g of oral paracetamol was prescribed regularly every 6 h starting at 6 h postoperatively.
Parameters and recordings
An ICB was considered successful when evidence of dermatomes of the brachial plexus (C5–T1) were blocked by the original injection within 30 min. The block was considered incomplete if any supplemental local anesthetic was needed for complete anesthesia and the block was considered to have failed if a supplementary volume did not provide complete anesthesia. Sensory blockade was assessed every 3 min and motor block was evaluated every 5 min within the first 30 min following completion of drug administration. Sensory block was confirmed by loss to cold sensation using an alcohol swab and pinprick sensation using a 23-ga needle in all dermatomes of the brachial plexus (C5–T1). Sensory block onset was defined as a decrease of sensation to 25% or less by comparison to the contralateral limb as a reference. Sensory block duration was defined as the time from injection of local anesthetic mixture to complete recovery from cold and pain sensation as tested by an alcohol swab and pinprick, respectively, in all dermatomes of the brachial plexus (C5–T1). Motor blockade was evaluated by the ability to flex the elbow and hand against gravity as follows: grade 1 = ability to flex and extend the forearm; grade 2 = ability to flex or extend only the wrist and fingers; grade 3 = ability to flex or extend only the fingers; and grade 4 = inability to move the forearm, wrist, and fingers.[15] Onset of motor block was defined as the time from injection of local anesthetic mixture until achieving a reduction in motor power to grade 3 or less. Motor block duration was described as the time from injection of local anesthetic to complete recovery of motor function in all nerves dermatomes. Sensory and motor blockade duration were assessed every 10 min in the postoperative period. Pain scores were assessed using the VRS (0–10) where pain was evaluated during rest, at 1, 2, 12, 24, 36, and 48 h postoperatively. Duration of analgesia (time interval from completion of local anesthetic administration until first need of rescue analgesia in the form of IV morphine, PCA) and amount of IV morphine consumed during the postoperative 48 h were recorded. Any evidence of neurologic, gastrointestinal, and cardiopulmonary complications were also recorded. Primary outcome measures were duration of analgesia while secondary measures were onset and duration of sensory blockade, pain scores, motor blockade onset and duration, narcotic requirements, and evidence of any adverse drug reactions.
Statistical analysis
A power analysis was performed to determine the necessary number of patients for each group based on duration of analgesia. With a 2-sided type I error of 5% and study power at 80%, it was estimated that 25 patients would be needed in each group in order to detect a difference of 35 min in the duration of analgesia between the 2 groups. Kolmogorov–Smirnov test was used to verify normal distribution of continuous variables. Continuous variables are expressed as mean ± standard deviation or median with interquartile range as appropriate and categorical variables are reported as percentages. Statistical analysis was done using Statistica version 6 (StatSoft Inc.; Tulsa, OK, USA; 2001) and GraphPad Prism version 4 (GraphPad Software Inc.; San Diego, CA, USA; 2005) software. Distributed continuous variables were compared using Student's unpaired t—test, whereas Mann–Whitney U test was used for comparison of VRS pain scores and supplemental morphine requirements. Categorical variables were compared by Chi-square test or Fisher's exact test, as appropriate. All analyses were two-tailed and P<0.05 was considered statistically significant.
RESULTS
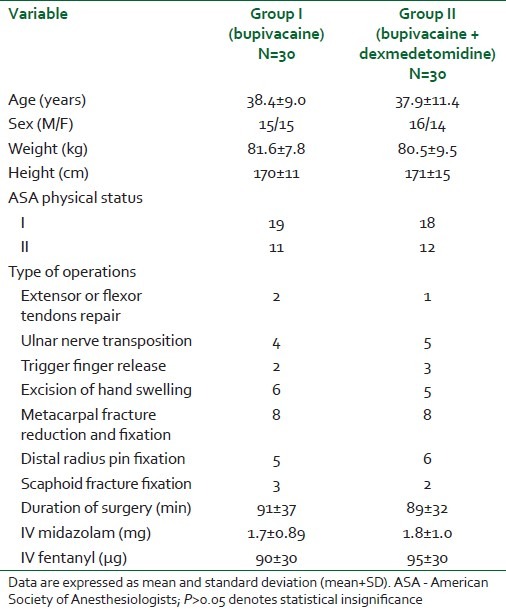
Both groups of patients had comparable demographic variables, duration of surgery, and intravenous sedation for block placement (P>0.05) as shown in Table 1.
Table 1.
Demographic and operative data of the patients receiving bupivacaine or bupivacaine + dexmedetomidine for infraclavicular brachial plexus block
Both groups were similar regarding the motor twitch response where 8, 5, 7, and 10 subjects from Group I developed twitches of the pectoralis, triceps, forearm, and hand muscles, respectively, while 7, 6, 5, and 12 patients of Group II developed twitches of the pectoralis, triceps, forearm, and hand muscles, respectively. Successful blockade was achieved in 96.7% of patients in both groups and all patients recovered uneventfully without sensory or motor deficit and no evidence of respiratory depression, bradycardia, or hypotension reported. The dexmedetomidine group of patients (Group II) showed a statistically significant shorter time to onset of sensory blockade (13.2 vs 19.4 min, P=0.003), longer sensory block duration (179.4 vs 122.7 min, P=0.002), shorter onset time to motor blockade (15.3 vs 22.2 min, P=0.003), longer motor block duration (155.5 vs 105.7 min, P=0.002), longer duration of postoperative analgesia (403 vs 233 min, P=0.002), and lower rescue morphine requirements 48 h after surgery. No patient in the dexmedetomidine group developed somnolence, whereas 4 patients in the control group (Group I) suffered from somnolence (P=0.04) [Table 2, Figure 1].
Table 2.
Characters of the infraclavicular block and postoperative pain in patients receiving bupivacaine or bupivacaine + dexmedetomidine
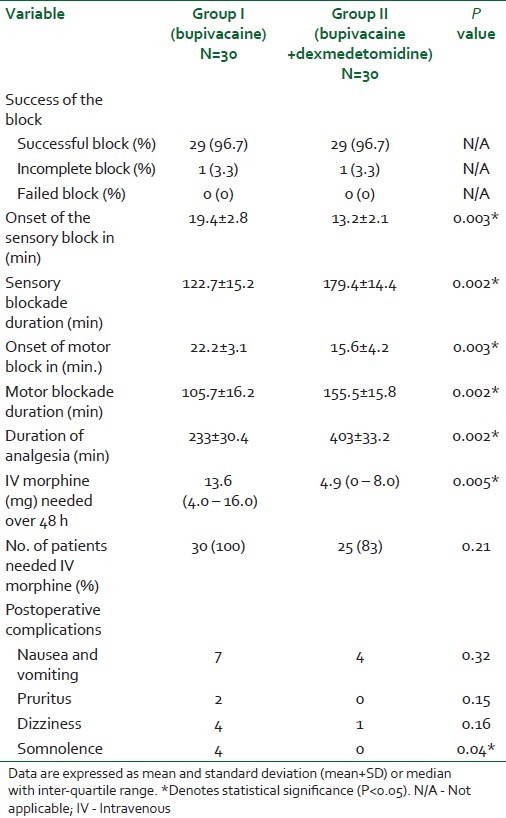
Figure 1.
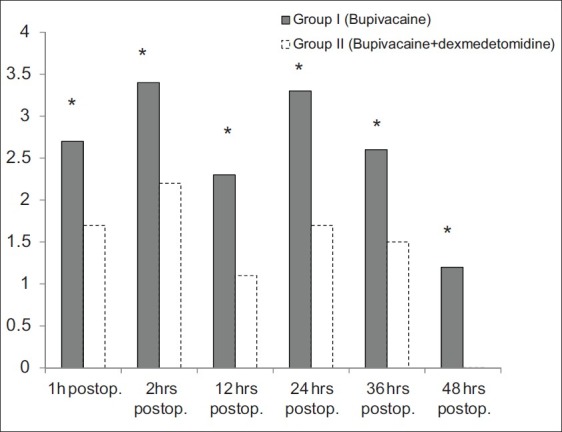
Verbal rating scale at rest over the first 48 postoperative hours. *Indicates significant difference between both groups (P<0.05)
DISCUSSION
Use of dexmedetomidine as an adjuvant mixed with local anesthetics has been performed with neuraxial anesthesia in both adult and pediatric patients. Dexmedetomidine (5 μg) added to intrathecal bupivacaine during gynecologic surgeries has resulted in a longer sensory and motor block duration.[16] In another study, dexmedetomidine (3 μg) in combination with bupivacaine for spinal anesthesia has been shown to provide a shorter onset to motor blockade and prolongation of motor and sensory block along with preservation of hemodynamics and absence of sedation.[9]
Saadawy and coworkers[11] added dexmedetomidine (1 μg/kg) to bupivacaine for caudal anesthesia in pediatrics; achieving longer analgesia, less rescue analgesic consumption, and improved sleep quality with no adverse clinically relevant side effects. El-Hennawy and colleagues[17] added either dexmedetomidine or clonidine to bupivacaine in pediatric caudal anesthesia for lower abdominal surgeries. Both dexmedetomidine and clonidine medications mixed with bupivacaine significantly prolonged analgesia when compared with using bupivacaine alone (16 h (15–19 h) for dexmedetomidine, 12 h (3–21 h) for clonidine, and 5 h (4–6 h) with plain bupivacaine; P<0.001). The study showed no difference with analgesia duration (P=0.796) between either dexmedetomidine or clonidine when added to bupivacaine.
Some recent investigations have studied the effects of mixing dexmedetomidine with local anesthetics during peripheral nerve and nerve plexus blockade. A study by Obayah and colleagues[13] added dexmedetomidine to bupivacaine during placement of a greater palatine nerve block for cleft palate repair. The addition of dexmedetomidine to bupivacaine provided lower pain scores and prolonged analgesia (approximately 50%) with no negative effect on hemodynamics when compared with bupivacaine alone. Another study by Esmaoglu et al.[14] mixed dexmedetomidine with levobupivacaine during placement of axillary brachial plexus blockade that resulted in shortening of block onset time and longer block duration resulting in improved postoperative analgesia.
Several animal studies have investigated the analgesic effects of dexmedetomidine as an adjunct. A study performed on a rat model by Brummett and colleagues[10] reported that dexmedetomidine added to ropivacaine during sciatic nerve blockade provided longer analgesia than systemic administration. Local injection of dexmedetomidine alone provided a brief period of analgesia that may suggest a peripherally mediated mechanism for the analgesic action of dexmedetomidine. Another study in guinea pigs[7] added clonidine or dexmedetomidine to lidocaine that showed enhanced local analgesic effect. The authors Yoshitomi et al. postulated that improved analgesic efficacy of clonidine and dexmedetomidine was mediated through α2A-adrenoceptors. Another sciatic nerve rat model investigation[8] (combined with histopathologic evaluation) assessed the efficacy and safety of adding dexmedetomidine to bupivacaine. In this study, Brummett and colleagues discovered that dexmedetomidine added to bupivacaine significantly potentiated sensory and motor blockade duration, however, dexmedetomidine alone failed to show any evidence of significant sensory or motor blockade. Histopathologic assessment of the sciatic nerves from the specimens in the study showed normal structure of axons and myelin. In another study by Brummett et al.,[18] they reported an antinociceptive effect of dexmedetomidine in a rat model with sciatic nerve blockade where perineural injection of dexmedetomidine mixed with ropivacaine resulted in prolonged duration to thermal protection/analgesia in a dose-dependent manner.
Several hypothesized mechanisms of action have been suggested to explain the analgesic effect of α2-adrenoceptor agonists. Some of these include vasoconstriction around the injection site,[6,7,19] direct suppression of impulse propagation through neurons as a result of a complex interaction with axonal ion channels or receptors,[6,7,20] local release of enkephalin-like substances,[21] a decrease in localized inflammatory mediators[22] and an increase in anti-inflammatory cytokines through an α2-adrenoceptor–mediated mechanism.[21–23]
A study conducted by Duma and colleagues[12] found no difference in analgesic efficacy when adding clonidine 0.5 μg/kg to levobupivacaine during placement of axillary brachial plexus blockade. These results may be explained by the intrinsic vasoconstrictive activity of levobupivacaine that could negate or inhibit any advantageous vasoconstrictor action of clonidine or it may be due to a variation of the pharmacokinetic effects resulting from using different concentrations of clonidine.[24,25] In addition, the results from the study may be secondary to anatomic variations of the brachial plexus or different nerve block approaches that could lead to barriers or variations in the spread of local anesthetics within the brachial plexus neurovascular bundle. Another possible reason for lack of efficacy when adding clonidine to local anesthetics may be related to variations in rate and extent of penetration of injected anesthetic solutions through neurons.[26]
The emphasis of this study was to assess clinical utility of adding dexmedetomidine to local anesthetics for brachial plexus blockade. Potential pharmacokinetic effects of adding dexmedetomidine to bupivacaine may explain some of the increased analgesic efficacy of the present investigation that may pose a limitation to the results of this study. Another limitation of this study could be that the beneficial effects of dexmedetomidine are explained by systemic absorption of the injected medication and by including a third control group (perineural bupivacaine + intravenous systemic dexmedetomidine) into the design of the study may help to address this issue. A relatively small dose of dexmedetomidine (0.75 μg/kg) was used in this clinical trial (concern about potential adverse hemodynamics, such as hypotension, bradycardia, fainting, and sedation), so the optimal dose must be furtherly investigated.
CONCLUSION
Improved parameters of analgesic efficacy support the use of dexmedetomidine as an adjunct mixed with local anesthetics for brachial plexus blockade to improve pain management and prolong anesthesia duration of local anesthetics.
ACKNOWLEDGMENT
This study was supported by finance from Minoufiya University, Egypt. We pay great thanks to radiology department staff, Faculty of Medicine, Minoufiya University, for their co-operation and help in using the ultrasound machine.
Footnotes
Source of Support: Minoufiya University, Egypt
Conflict of Interest: None declared.
REFERENCES
- 1.Raj PP, Montgomery SJ, Nettles D, Jenkins MT. Infraclavicular brachial plexus block-a new approach. Anesth Analg. 1973;52:897–904. [PubMed] [Google Scholar]
- 2.Fitzgibbon DR, Debs AD, Erjavec MK. Selective musculocutaneous nerve block and infraclavicular brachial plexus anesthesia. Case report. Reg Anesth. 1995;20:239–41. [PubMed] [Google Scholar]
- 3.Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth. 2002;89:254. doi: 10.1093/bja/aef186. [DOI] [PubMed] [Google Scholar]
- 4.Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology. 2009;111:406–15. doi: 10.1097/ALN.0b013e3181aae897. [DOI] [PubMed] [Google Scholar]
- 5.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specifity and potency of medetomidine as an á2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 6.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 10.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 11.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 12.Duma A, Urbanek B, Sitzwohl C, Kreiger A, Zimpfer M, Kapral S. Clonidine as an adjuvant to local anaesthetic axillary brachial plexus block: A randomised controlled study. Br J Anaesth. 2005;94:112–6. doi: 10.1093/bja/aei009. [DOI] [PubMed] [Google Scholar]
- 13.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 14.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 15.Borgeat A, Ekatodramis G, Dumont C. An evaluation of the infraclavicular block via a modified approach of the Raj technique. Anesth Analg. 2001;93:436–41. doi: 10.1097/00000539-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]
- 17.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 18.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–9. doi: 10.1097/ALN.0b013e3181bbcc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singelyn FJ, Gouverneur JM, Robert A. A minimum dose of clonidine added to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Anesth Analg. 1996;83:1046–50. doi: 10.1097/00000539-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Butterworth JF, Strichartz GR. The á -2-adrenergic agonists clonidine and guanfacine produce tonic and phasic block of conduction in rat sciatic nerve fibers. Anesth Analg. 1993;76:295–301. [PubMed] [Google Scholar]
- 21.Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: Mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol. 1988;146:223–8. doi: 10.1016/0014-2999(88)90296-8. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Sandoval A, Eisenach JC. Perineural clonidine reduces mechanical hypersensitivity and cytokine production in established nerve injury. Anesthesiology. 2006;104:351–5. doi: 10.1097/00000542-200602000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Sandoval A, Eisenach JC. Clonidine reduces hypersensitivity and alters the balance of pro- and anti-inflammatory leukocytes after local injection at the site of inflammatory neuritis. Brain Behav Immun. 2007;21:569–80. doi: 10.1016/j.bbi.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster RH, Markham A. Levobupivacaine: A review of its pharmacology and use as a local anaesthetic. Drugs. 2000;59:551–79. doi: 10.2165/00003495-200059030-00013. [DOI] [PubMed] [Google Scholar]
- 25.Kopacz DJ, Bernards CM. Effect of clonidine on lidocaine clearance in vivo: A microdialysis study in humans. Anesthesiology. 2001;95:1371–6. doi: 10.1097/00000542-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Vester-Andersen T, Eriksen C, Christiansen C. Perivascular axillary block III: Blockade following 40 ml of 0.5%, 1% or 1.5% mepivacaine with adrenaline. Acta Anaesthesiol Scand. 1984;28:95–8. doi: 10.1111/j.1399-6576.1984.tb02019.x. [DOI] [PubMed] [Google Scholar]


