Abstract
Waardenburg syndrome (WS) is a rare autosomally inherited and genetically heterogeneous disorder of neural crest cell development. Literature regarding the anesthetic management of these cases is limited. We present 2 cases of Shah–Waardenburg syndrome and discuss them in the context of review of previously published cases.
Keywords: Anesthesia, Shah–Waardenburg syndrome, white forelock,
INTRODUCTION
A neonate with white forelock and/or pigmentation abnormalities should alert anesthesiologist to the possibility of Waardenburg syndrome (WS) or Piebaldism or other genetic syndromes.
We present 2 cases of Shah–Waardenburg syndrome and discuss them in the context of review of previously published cases. The authors searched PUBMED and ovid MEDLINE, without date restrictions using the key words, namely, Shah–Waardenburg syndrome, WS, Waardenburg, and anaesthesia or anesthesia.
CASE REPORT
Case 1
A 3-day-old male full-term neonate weighing 3.4 kg born to consanguineous parents presented with bilious vomiting and constipation since birth. The child was posted for exploratory laparotomy. On examination, he had a prominent white forelock, heterochromia of both irides, facial dysmorphism, broad nose, and a distended abdomen [Figure 1]. His hemogram and serum electrolytes were within normal limits. Audiologic evaluation (BERA) revealed a complete bilateral sensorineural hearing loss. The abdominal roentgenogram revealed dilated bowel loops but no air-fluid levels. Barium enema showed a microcolon with no obvious transitional zone. A provisional diagnosis of Shah–Waardenburg syndrome was made.
Figure 1.
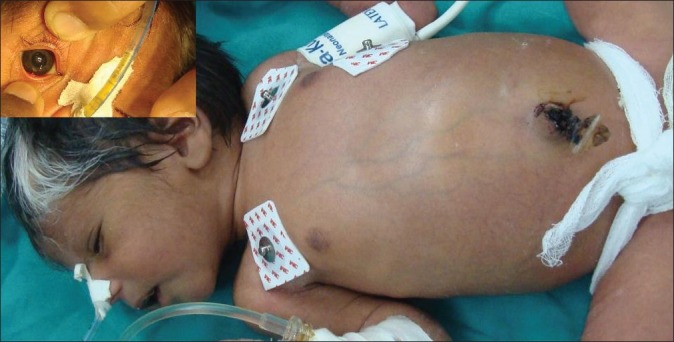
Clinical picture showing white forelock, distended abdomen, and heterochromia irides
After a preoperative parental counseling and consent, anesthesia was induced with 6% sevoflurane and 100% oxygen with spontaneous respiration. A 24-gauge venous access was then secured and intravenous atropine 0.1 mg and fentanyl 6 μg were administered. Tracheal intubation was accomplished with intravenous 1.5 mg atracurium with utmost care, and ventilation was controlled manually. Caudal anesthesia was given—0.125% bupivacaine (4 mL) with 1 μg/kg of clonidine. Pediatric solution (6 mL/kg/h) was infused during the operation. Intraoperative and postoperative course was uneventful.
The child was admitted repeatedly for recurrent gastrointestinal symptoms due to malabsorption. In the last admission, he succumbed to sepsis and expired at the age of 6 months.
Case 2
A 7-day-old female baby, weighing 2.8 kg presented with distension of abdomen and constipation since birth. On examination, she had a prominent white forelock, heterochromia of both irides, facial dysmorphism, partial albinism, and vaginal bleeding [Figure 2]. The child's hemogram and serum electrolytes were within normal limits. She had received intramuscular inj. Vitamin K on first 2 days of life. Radiologic findings were consistent with intestinal obstruction and a provisional diagnosis of Shah–Waardenburg syndrome was done.
Figure 2.
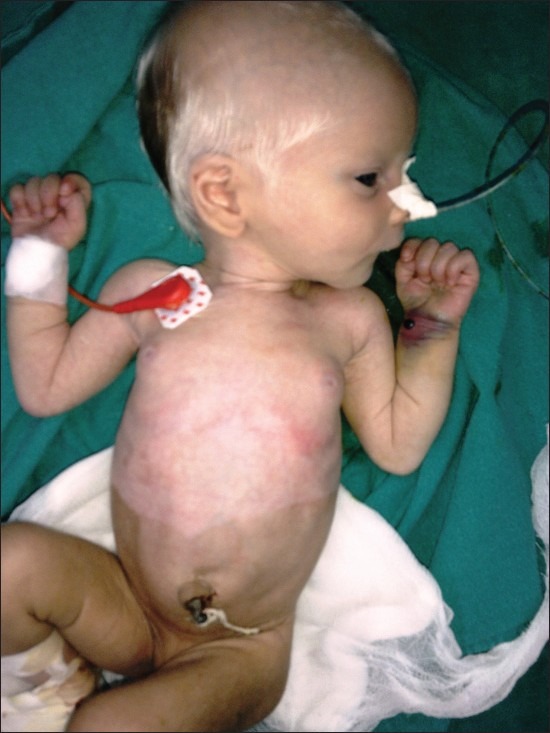
Clinical picture showing white forelock, distended abdomen, heterochromia irides, and partial albinism. (An explicit consent from the parents of the children regarding the possible publication of the material in medical journals was obtained)
Anesthetic management was similar to the previous case. Vaginal bleeding was likely due to maternal hormonal influences and it was considered in calculating intraoperative blood loss. The presence of partial albinism posed difficulty in assessing pallor and capillary refilling time. Postoperative course was uneventful and the child lost followup for definitive surgery.
DISCUSSION
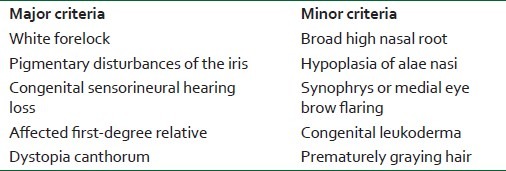
WS is an uncommon autosomally inherited and genetically heterogeneous disorder of neural crest cell development named after the Dutch Ophthalmologist P. J. Waardenburg in 1951. Based on the clinical presentations, 4 subtypes are described.[1] According to the diagnostic criteria proposed by the Waardenburg Consortium, a person must have 2 major or one major plus 2 minor criteria to be diagnosed as WS type I [Table 1].[2]
Table 1.
Diagnostic criteria for Waardenburg syndrome type I
WS type II lacks dystopia canthrum of WS type I. Type III, also called Klein–Waardenburg syndrome, has associated limb abnormalities. Type III is the rarest form of WS. Shah–Waardenburg syndrome, type IV, is an unusual variant of WS associated with long-segment Hirschsprung's disease. Both our patients had features of Shah–Waardenburg syndrome with extended long-segment aganglionosis, which is rare in occurrence. WS IV is a milder form of neurocristopathy.[3] Neurocristopathies are disorders caused by an alteration in the migration of the neural crest cells during the embryonic phase, which causes the association of different anomalies.[4] Other features associated with WS include urinary system abnormalities, neural tube defects, Sprengel shoulder, cleft-lip or palate, facial nerve palsy and plicated tongue, laryngomalacia,[5] and severe cyanotic cardiopathy.[6] [Mutations in PAX gene on chromosome 2 are seen in WS I and III, and MITF mapped on chromosome 3 in type II. Type IV is due to SOX10 or endothelin-B receptor (EDNRB) gene mutations.[1]
Although affected patients require surgical treatment, very little has been published about the anesthetic implications. Michalek et al[7] have described successful management of difficult airway in an adult WS patient using fiberoptic bronchoscope with I-gel as conduit. Mizushima et al[8] have described anesthetic management of a child with type IV WS undergoing multiple surgical procedures. In all the procedures, anesthetic technique was not standardized. They encountered perioperative problems, such as malnutrition, electrolyte imbalance, and communication difficulties due to congenital deafness and blindness. In both our cases we had anticipated multiple future surgeries. Kfoury et al[9] have managed a 5-year-old child with WS, operated previously for cleft palate and spina bifida, having facial dysmorphism, epilepsy with bilateral pudendal nerve block using nerve stimulator. From our experience, no particular anesthetic technique can be recommended. Changes in volatile anesthetics and muscle relaxants had no noticeable effects.
Most prominent and obvious feature of WS is white forelock. The differential diagnosis of white forelock are Pie baldism with deafness, Rozychi's syndrome (leukoderma, congenital deafness, muscle wasting, and achalasia), Vogt–Koyanagi–Harada syndrome (uveitis, graying of hair, meningitis, and vitiligo), Tietz's syndrome (deafmutism, blue eyes, and hypomelanosis), and tuberous sclerosis.[10] There is paucity of literature describing the anesthetic management of above syndromes.
In conclusion, although there is no significant anesthetic management in these cases, we would like to emphasize, through this case report, that the anesthesiologist encountering a child with white forelock should keep in mind the above-said differential diagnosis and variants of WS. We would recommend an individualized anesthetic approach in managing these patients. Meticulous attention is required with preoperative evaluation, co-existence of other system abnormalities, airway management, and perioperative nutrition strategies. Anesthesiologists should work with pediatricians and pediatric-surgeons in unison to manage such patients as these patients present for multiple surgeries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ghosh SK, Bandyopadhyay D, Ghosh A, Biswas SK, Mandal RK. Indian J Dermatol Venereol Leprol. 2010;76:550–2. doi: 10.4103/0378-6323.69089. [DOI] [PubMed] [Google Scholar]
- 2.Farrer LA, Grundfast KM, Amos J, Arnos KS, Asher JH, Jr, Beighton P, et al. Waardenburgh syndrome (WS) Type I is caused by defects at multiple loci, one of which is near ALPP on Chromosome 2: First report of the WS Consortium. Am J Hum Genet. 1992;50:902–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue K, Ohyama T, Sakuragi Y, Yamamoto R, Inoue NA, Yu LH, et al. Translation of SOX 10 3’ untranslated region causes a complex severe neurocristopathy by generation of a deleterious functional domain. Hum Mol Genet. 2007;16:3037–46. doi: 10.1093/hmg/ddm262. [DOI] [PubMed] [Google Scholar]
- 4.Yanes-Vidal GJ, Garcia-Perla JL, Alacon-Rubio M, Martinez-Canguelossi S. Apnoea episodes in Hirschsprung's disease and anaesthesia implications of neurocristopathies. Paediatr Anaesth. 2004;14:280–1. doi: 10.1046/j.1460-9592.2003.01183.x. [DOI] [PubMed] [Google Scholar]
- 5.Thapa R, Mallik D, Ghosh A, Ghosh A. Waardenburg syndrome associated with laryngomalacia. Singapore Med J. 2009;50:401. [PubMed] [Google Scholar]
- 6.Mathieu M, Bourges E, Caron F, Piussan C. Waardenburg's syndrome and severe cyanotic cardiopathy. Arch Fr Pediatr. 1990;47:657–9. [PubMed] [Google Scholar]
- 7.Michalek P, Hodgkinson P, Donaldson W. Fiberoptic intubation through an I-gel supraglottic airway in two patients with predicted difficult airway and intellectual disability. Anesth Analg. 2008;106:1501–4. doi: 10.1213/ane.0b013e31816f22f6. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima A, Nitami K, Kikuchi T, Kugimiya T, Ohya T, Miyano T. Anesthetic problems in a child with Waardenburg's syndrome and Hirschsprung's disease. J Anesth. 1996;10:144–6. doi: 10.1007/BF02483352. [DOI] [PubMed] [Google Scholar]
- 9.Kfoury T, Staiti G, Baujard C, Benhamou D. Pudendal nerve block by nerve stimulation in a child with Waardenburg disease. Paediatr Anaesth. 2008;18:1267–8. doi: 10.1111/j.1460-9592.2008.02781.x. [DOI] [PubMed] [Google Scholar]
- 10.Hornyak T. Hypomelanoses and Hypermelanoses. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: McGraw-Hill; 2008. p. 615. [Google Scholar]


