Abstract
Objectives:
The primary brain tumors are the second most common cause of death due to malignancies in children. This study was done to analyze the histological spectrum of primary brain tumors in children and also to find out the expression of p53 and Ki67 in some of the common pediatric brain tumors.
Materials and Methods:
This study was done over a period of 2.5 years. The patients were followed up until 6 months to determine the outcome. We examined H and E sections from 61 pediatric brain tumors and also performed immunohistochemical stains with p53 and Ki67 on 52 of these samples.
Results:
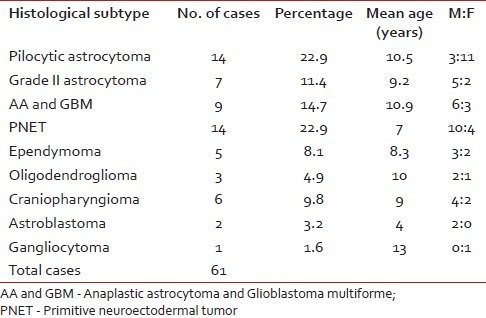
Of the 61 cases of pediatric brain tumors the commonest were pilocytic astrocytomas and medulloblastomas both constituting 22.9% of total cases, followed by high grade gliomas, that is, anaplastic astrocytoma and glioblastoma taken together (14.7%), diffuse astrocytomas (11.4%), ependymomas (8.1%), and oligodendrogliomas (4.9%). Other cases comprised craniopharyngiomas, astroblastomas, and gangliocytoma. The mean age of presentation was 9.3 years, male children being more commonly affected. Ki67 labeling index (LI) and p53 expression in pilocytic astrocytomas and diffuse astrocytomas were significantly lower than that of high-grade astrocytomas. However, there was no significant difference of expression of these two antigens in pilocytic astrocytomas and diffuse astrocytomas. It was found that Ki67 LI was a better marker for distinguishing between grades of astrocytoma than p53 (P=0.000 and P=0.002, respectively). The survival in cases of pilocytic astrocytomas was far better than high-grade gliomas. However, there was no significant difference in survival between pilocytic astrocytoma and diffuse infiltrating astrocytoma. There was significant positive correlation between expression of p53 and Ki67 LI in cases of medulloblastomas. Both p53 (P=0.002) and Ki67 LI (P=0.000) taken individually correlated well with survival in these cases. Also, Ki67 LI is better predictor of outcome than p53.
Conclusion:
From this study, it can be concluded that Ki67 and p53 score correlated well with the grade of astrocytoma; however, Ki67 is a better marker for differentiating between the grades of astrocytoma than p53. Also, Ki67 LI is a better prognostic factor than p53 in case of medulloblastomas.
Keywords: Pediatric brain tumors, p53, Ki67
INTRODUCTION
The primary brain tumors account for less than 2% of all human cancers, but cause a disproportionate burden of cancer-related morbidity and mortality. They are the most common solid tumors of childhood and after leukemia are the leading cause of death in children. They account for 20% of all pediatric malignancies. Unfortunately, the incidence of brain tumor is on the rise and about 15000 new cases of pediatric brain tumors are diagnosed each year in USA. The overall annual incidence in USA of pediatric malignant brain tumor is about 28 per million children <14 years.[1] But data regarding the incidence of pediatric brain tumors in India and especially in our setting are lacking. The most prevalent primary brain tumors among the pediatric population are astrocytomas, ependymomas, medulloblastomas, craniopharyngiomas, choroid plexus neoplasms, etc. Seventy percent of these pediatric brain tumors arise in the posterior fossa. In the infratentorial region, the common tumors are medulloblastoma, pilocytic astrocytoma, brain stem glioma, and ependymoma. Among astrocytomas, pilocytic astrocytoma is the most common glioma in children, and represents 10% of cerebral and 85% of cerebellar astrocytomas.[2] There is enormous heterogeneity in the pathogenesis of brain tumors. Many studies have been undertaken so far to analyze the role of Ki67 and p53 in adult astrocytomas and also assess their significance as prognostic factors in adult glioma. Both p53 and Ki67 have been implicated in gliomagenesis in the case of adults. However, pediatric gliomagenesis seem to follow different molecular pathways than the adults. Not only studies regarding pediatric brain tumors are rare, different studies have given varied propositions regarding the role of p53 and Ki67 in pediatric brain tumors. This work was done to analyze the histopathological spectrum of pediatric brain tumors in our setting and to study the possible role of p53 and Ki67 in their prognosis.
MATERIALS AND METHODS
Sixty-one pediatric brain tumor specimens of patients aged 0 to 15 years were included in this study over a period of 2 years. The case selection was random with no bias. Clinical details, namely age and site were recorded in each case. The haematoxylin and eosin (H and E) stained slides of all 61 cases were studied and grading was done according to WHO criteria. One or two representative blocks of formalin-fixed paraffin-embedded tissue in 52 cases out of 61 were selected and serial 5 μm thick sections were cut and taken on poly-L-lysine coated slides for immunohistochemical stains with p53 and Ki67 using standard immunohistochemical procedure. Antibody used was ready to use pre diluted antibody from Bio-Genex (USA). Result of Ki67 immunostaining was interpreted as labeling index (Ki67 LI) – as percentage of positively stained nuclei. P53 scoring was calculated by studying a minimum of five fields in the highest labeled areas and the scoring was assessed as follows: 0 = no positive nuclei, 1 = <5% positive, 2 = 5–30% positive, 3 = >30% positive.[3] Survival was defined as the interval between biopsy proven diagnosis and death or date of last follow up, up to 6 months. The software used for this purpose was software version 6.0. Tulsa Oklahoma: Statsoft, Inc; 2001 and SPSS 17.1 August 2007.
RESULTS
Of 61 observed cases, most common were medulloblastomas (22.9%) and pilocytic astrocytomas (22.9%). The histopathological spectrum, mean age, sex distribution of these 61 cases of pediatric brain tumors are shown in Table 1.
Table 1.
Distribution of histological types, sex ratio, and mean age of all the cases
Regarding distribution of these tumors, it was found that 24 were infratentorial tumors out of 52 cases that accounted for 46.1% of total case. Remaining 28 (53.8%) were supratentorial tumors.
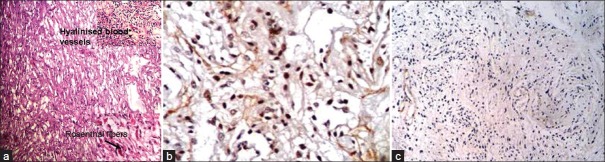
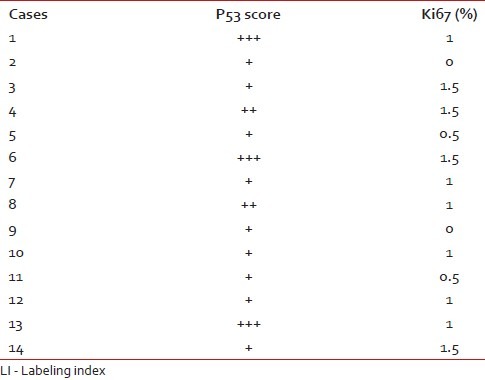
The mean age of presentation of 14 pilocytic astrocytoma cases was 10.5 years. The mean Ki67 LI of pilocytic astrocytoma (PA) was 0.9%. About 35.7% cases showed high p53 score. Nine cases (64.2%) had low or absent expression of p53. Ki67 and p53 were significantly lower than high-grade glioma cases (P<0.001 and P<0.01, respectively) [Figure 1a–c]. We lost follow up of three patients. 81.8% cases were alive after 6 months. The survival of PAs was significantly longer than that of high-grade gliomas (P=0.03).
Figure 1.
(a) Photomicrograph showing microcystic areas in a case of pilocytic astrocytoma. The upper-right inset picture shows hyalinized blood vessels and the one at the lower right corner shows Rosenthal fibers (H and E); (b) Photomicrograph showing high p53 expression in a case of pilocytic astrocytoma (original ×400); (c) Photomicrograph showing low Ki67 LI in pilocytic astrocytoma (original ×100)
The Ki67 LI and p53 expression of the pilocytic astrocytomas are shown in Table 2.
Table 2.
P53 score and Ki67 LI in pilocytic astrocytomas
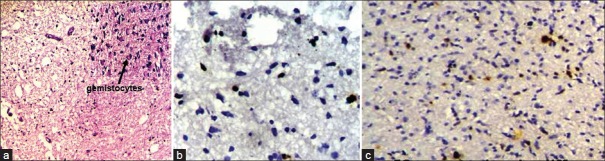
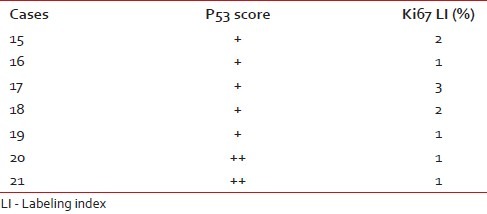
The mean age of presentation of seven diffuse infiltrating astrocytoma patients was 9.2 years. Ki67 LI varied between 1 and 3% with a mean of 1.57%. 71.4% of diffuse infiltrating astrocytomas had a low p53 score, while two cases (28.5%) had high score; however, none showed intense p53 staining. Both p53 and Ki67 expression were significantly lower than high-grade gliomas (P<0.05 in each case) [Figure 2a–c]. Of seven cases, we lost follow up of two cases. Eighty percent cases were alive at the end of 6 months of follow up. No significant difference was found in survival between grade II astrocytoma and pilocytic astrocytoma.
Figure 2.
(a) Photomicrograph showing a case of diffuse infiltrating astrocytoma. The upper-right inset picture shows gemistocytes (H and E, ×100); (b) Photomicrograph showing low Ki67 LI in diffuse infiltrating astrocytoma (original ×400); (c) Photomicrograph showing p53 expression in diffuse astrocytoma (original ×400)
The Ki67 LI and p53 expression of the diffuse infiltrating astrocytoma are shown in Table 3.
Table 3.
P53 score and Ki67 LI in diffuse infiltrating astrocytoma
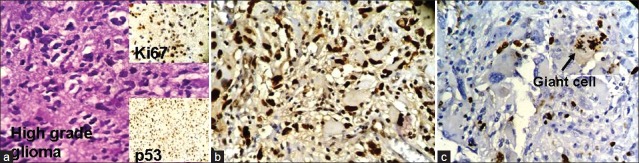
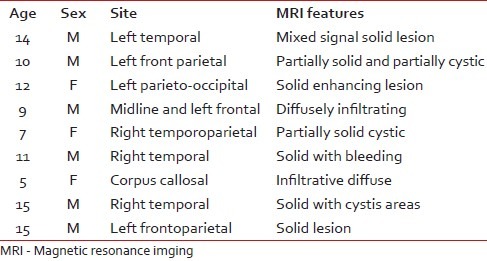
We have taken together anaplastic astrocytomas and glioblastomas as high-grade gliomas as we had very few cases of anaplastic astrocytoma. Ki67 LI ranged from 12–45% with a mean of 28.8%. Around 78% of the high-grade gliomas showed high p53 score while two cases had a low score (22.2%). Both p53 score and Ki67 LI in high-grade gliomas were significantly more than both pilocytic and diffuse infiltrating astrocytoma [Figure 3a–c]. We lost follow up of two cases, but 71.4% cases died within 6 months. Patients with high-grade gliomas lived for significantly shorter period than those with pilocytic astrocytoma. The Ki67 LI and p53 expression of the high-grade gliomas are shown in Table 4. The sites and radiological features of the seven patients with high-grade gliomas are represented in Table 5.
Figure 3.
(a) Photomicrograph showing highly pleomorphic astrocytes in a case of anaplastic astrocytoma. The upper-right inset shows high expression of Ki67 in the same case and the lower right inset picture shows high expression of p53 in the same case; (b) Photomicrograph showing high power view of high p53 expression in glioblastoma (original ×400); (c) Photomicrograph showing Ki67 expression in a dividing giant cell in glioblastoma (original ×400)
Table 4.
P53 score and Ki67 LI in high-grade astrocytomas
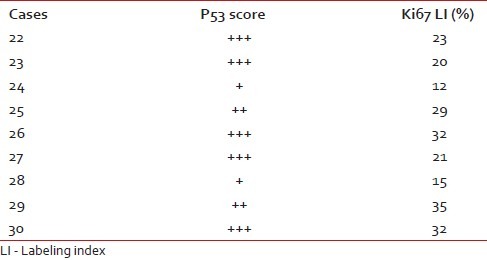
Table 5.
High-grade gliomas
Ki67 and p53 individually showed a significant positive correlation with grade of astrocytoma (P=0.000 and P=0.002, respectively). It was seen Ki67 was a better marker for differentiating between grades of astrocytoma.
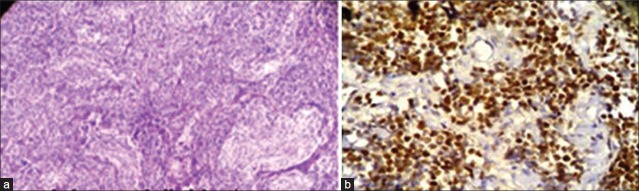
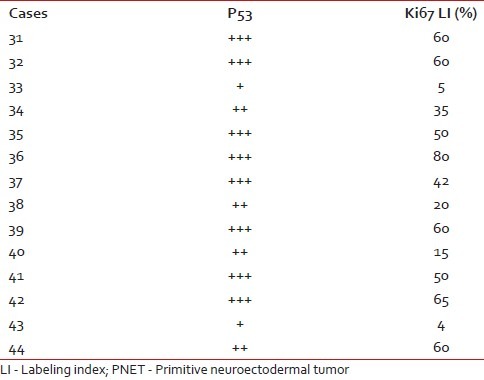
There were 14 cases of medulloblastomas having age ranging from 6 months to 15 years. Ki67 LI ranged from 4 to 80, with a mean of 43.2%. Out of 14 medulloblastoma cases, 12 cases (85.7%) showed a high p53 score, while two (14.2%) had low score of p53. Three cases were lost to follow up and out of the available cases 72.7% died after 6 months of follow up. There was significant positive correlation between expression of p53 and Ki67 LI in cases of medulloblastoma (P=0.002). Both p53 (P=0.002) and Ki67 LI (P=0.000) taken individually as well as taken together (P=0.00) correlated well with survival in these cases [Figure 4a and b]. Ki67 correlated better with survival in medulloblastoma than p53. The Ki67 LI and p53 expression of the medulloblastoma cases are shown in Table 6.
Figure 4.
(a) Photomicrograph showing pale nodular areas in a nodular medulloblastoma with advanced neuronal differentiation (H and E, ×100); (b) Photomicrograph showing high power view of p53 positive cells arranged around blood vessels forming pseudorosettes in medulloblastoma (original ×400)
Table 6.
P53 score and Ki67 LI in PNET
Of the five ependymomas, all were grade-two tumors, located infratentorially [Figure 5a].
Figure 5.
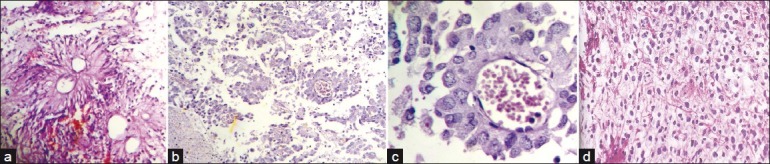
(a) Photomicrograph showing pseudorosettes in a case of ependymoma; (b) Photomicrograph showing anchoring of tumor cells to blood vessels by short stout processes, in a case of astroblastoma (H and E, ×100); (c) Photomicrograph showing high power view of tumor cells with processes arranged around blood vessels (H and E, ×400); (d) Photomicrograph showing cells with clear cytoplasm and well defined plasma membrane in a case of oligodendroglioma (H and E, ×400)
Some of the rarer tumors we encountered were astroblastoma [Figure 5b and c] and oligodendrogliomas [Figure 5d].
DISCUSSION
Pediatric brain tumors are rare and the management of each child has to be individualized depending upon the clinical presentation, age, histology, tumor location, size, etc. All patients with malignant brain tumors require postoperative radiotherapy. The quality of life of those children who had been treated for brain tumor is typically poor. Very few long-term survivors are expected to live a normal life. However, there is rarity of information regarding the genetic abnormalities underlying the brain tumors in children. Identification of these genetic lesions is the key to develop targeted therapeutics (smart drugs) that can eradicate brain tumors without affecting other organ systems in growing children, thus ensuring a better quality of life.
In our study, the two commonest pediatric tumors were pilocytic astrocytomas and medulloblastomas. This is in keeping with published literatures; however, Kumar et al., had reported a higher incidence of medulloblastomas amongst Indian children.[4] We reported a higher percentage of craniopharyngioma (10.5%) than both Yates et al. and Chan et al.[5] However, Tobias et al. and Kumar had reported similar incidence of craniopharyngiomas as ours.[4,6] In contrast to most studies we had higher percentage of glioblastomas in children, the reason for this is unknown. Also, we did not encounter any germ-cell tumor that is reported quite frequently in many Asian studies specially from far East.
Just over half of our tumors were located supratentorially. PA and medulloblastomas were most common in posterior fossa. Patients with astrocytomas presented at a higher age in this series than that reported in other studies, probably because of greater number of glioblastoma cases. Childhood primitive neuroectodermal tumor (PNETs) presented at a mean age of 7, similar to the observations made by Grotzer et al. and Chan et al.[5,7] There was equal distribution of boys and girls suffering from brain tumor when all tumors were taken together; however, girls outnumbered boys in case of PAs, while male predominance was found in medulloblastomas.
Different series have reported varying ranges of Ki67 LI and p53 immunostaining in pediatric brain tumors. The results of these are sometimes overlapping and even conflicting with no common consensus.
For analyzing the Ki67 LI we chose the “hot spots” or maximal labeling index areas.[8] as randomly chosen areas underestimates the growth fraction by combining active and quiescent areas. The mean Ki-67 in case of PA and diffuse infiltrating astrocytoma (DIA) were typically low similar to data published in the world literature,[9] The LI for DIA and PA were significantly lower than that of high-grade malignant gliomas. This is in keeping with the observation by Ho et al.[8] Though Ki67 correlated significantly with other grades, it was not of much importance in differentiating between pilocytic astrocytomas with diffuse infiltrating variety corroborating the finding of Giannini et al.[10] Matsumoto et al., who worked in Japan also reached to the same conclusion.[11] However, Ki-67 LI among different studies vary considerably in range possibly because of storage, processing, fixation, and different staining protocols.
This study has documented a higher Ki67 LI in case of medulloblastomas than that by Sarkar et al.[12] We also found that there was a significant inverse association of Ki67 LI with survival in case of medulloblastomas similar to the observations made by Grotzer et al.[7] and Shim et al.[13] But other studies by Mirabell et al., had reported different results regarding this association.[14]
The reported frequencies of p53 alterations in pediatric astrocytic tumors vary considerably. Although initial studies indicated that childhood malignant gliomas rarely exhibit p53 mutation,[15,16] more recent studies by Sung et al. and Sure et al., have suggested a role of p53 in pediatric gliomagenesis.[17,18] Unlike other series, we found p53 expression in fair number of cases in PA implying its possible role in gliomagenesis. We observed p53 expression in 35.7% cases of PA in keeping with the observation of Hayes et al.[19] A high percentage of high-grade gliomas strongly expressed p53 in this study, similar to that of Suri et al., who also observed high incidence of p53 alteration in pediatric glioblastoma.[20] Bhattacharjee et al., also found that the overall frequency of p53 immunoreactivity was 47% in pediatric high-grade astrocytomas that was similar to the one reported for adult astrocytomas.[3] However, contrary to our findings, Rasheed et al., showed p53 immunoreactivity in only 16% of pediatric high-grade glioma patients, and concluded that p53 has limited role in pediatric gliomagenesis.[21] Also, p53 score was significantly lower in PA and grade-II astrocytomas than the high-grade ones (anaplastic astrocytoma, glioblastoma multiforme) similar to results obtained by Matsumoto et al.[11] p53 alteration, also correlated inversely with survival amongst medulloblastoma cases in our study. Similar observations were made by Woodburn et al. and Jaros et al.[22,23] Both p53 and Ki67 taken together and individually had significant association with survival in these cases, in the present study. The relation of p53 and Ki67 with medulloblastomas so far reported conflicting results in many series. Ertan et al., showed that the MIB-1 value and p53 immunoreactivity had no relation with prognosis in pediatric medulloblastomas contrary to our findings.[24] Ki67 in this study had better predictive value than p53.
In this study, p53 and Ki67 were not only found to be significantly associated with survival individually, but taken together they also were significantly related to survival in astrocytoma patients in keeping with Tihan et al.[25] However, Ki67 was a better marker than p53 in astrocytoma series.
CONCLUSION
In this study, we feel that both Ki67 and p53 have potential diagnostic and prognostic use in the diagnosis of medulloblastomas, astrocytomas, and differentiating between various grades of astrocytoma. This may be particularly useful in small/stereotactic biopsies where deciding on the grade is a diagnostic problem. We found Ki67 to be a better diagnostic marker than p53 in both medulloblastomas and astrocytomas.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bunin G. What causes childhood brain tumors? Limited knowledge, many clues. Pediatric Neurosurg. 2000;32:321–6. doi: 10.1159/000028961. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Eibl RH, Schwab M, Reichel MB, Mariani L, Gehring M, et al. Mutations of the p53 tumor suppressor gene in neoplasms of the human central nervous system. Mol Carcinog. 1993;8:74–80. doi: 10.1002/mc.2940080203. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee MB, Bruner JM. p53 protein in pediatric malignant astrocytomas: A study of 21 patients. J Neurooncol. 1997;32:225–33. doi: 10.1023/a:1005727902387. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R. Scenario of pediatric CNS tumors in India. JK Sci. 2006;8:190–2. [Google Scholar]
- 5.Chan GC. Recent advances in childhood brain tumours. HK J Paediatr. 2006;11:3–12. [Google Scholar]
- 6.Tobias J, Hayward RD. Brain and spinal cord tumors in children. In: Thomas DG, editor. Neuro-oncology: Primary malignant brain tumors. Baltimore: The Johns Hopkins University Press; 1990. pp. 164–92. [Google Scholar]
- 7.Grotzer MA, Janss AJ, Fung KM, Biegel JA, Sutton LN, Rorke LB, et al. TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol. 2000;18:1027–30. doi: 10.1200/JCO.2000.18.5.1027. [DOI] [PubMed] [Google Scholar]
- 8.Ho DM, Wong TT, Hsu CY, Ting LT, Chiang H. MIB-1 labeling index in nonpilocytic astrocytoma of childhood: A study of 101 cases. Cancer. 1998;82:2459–66. doi: 10.1002/(sici)1097-0142(19980615)82:12<2459::aid-cncr21>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Scheithauer BW, Burger PC, Christensen MR, Wollan PC, Sebo TJ, Forsyth PA, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol. 1999;58:46–53. doi: 10.1097/00005072-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Giannini C, Scheithauer BW, Burger PC, Christensen MR, Wollan PC, Sebo TJ, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol. 1999;58:46–53. doi: 10.1097/00005072-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Fujii T, Yabe M, Oka K, Hoshi T, Sato K. MIB-1 and p53 immunocytochemistry for differentiating pilocytic astrocytomas and astrocytomas from anaplastic astrocytomas and glioblastomas in children and young adults. Histopathology. 1998;33:446–52. doi: 10.1046/j.1365-2559.1998.00503.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar C, Pramanik P, Karak AK, Mukhopadhyay P, Sharma MC, Singh VP, et al. Are childhood and adult medulloblastomas different? A comparative study of clinicopathological features, proliferation index and apoptotic index. J Neuro Oncol. 2002;59:49–61. doi: 10.1023/a:1016357731363. [DOI] [PubMed] [Google Scholar]
- 13.Shim KW, Joo SY, Kim SH, Choi JU, Kim DS. Prediction of prognosis in children with medulloblastoma by using immunohistochemical analysis and tissue microarray. J Neurosurg Pediatr. 2008;1:196–205. doi: 10.3171/PED/2008/1/3/196. [DOI] [PubMed] [Google Scholar]
- 14.Miralbell R, Tolnay M, Bieri S, Probst A, Sappino AP, Berchtold W, et al. Pediatric medulloblastoma: Prognostic value of p53, bcl-2, Mib-1, and microvessel density. J Neuro Oncol. 1999;45:103–10. doi: 10.1023/a:1006330324991. [DOI] [PubMed] [Google Scholar]
- 15.Litofsky NS, Hinton D, Raffel C. The lack of a role for p53 in astrocytomas in pediatric patients. Neurosurgery. 1994;34:967–73. doi: 10.1227/00006123-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Lang FF, Miller DC, Koslow M, Newcomb EW. Pathways leading to glioblastoma multiforme: A molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg. 1994;81:427–36. doi: 10.3171/jns.1994.81.3.0427. [DOI] [PubMed] [Google Scholar]
- 17.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–59. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sure U, Rüedi D, Tachibana O, Yonekawa Y, Ohgaki H, Kleihues P, et al. Determination of p53 Mutations, EGFR overexpression, and loss of p16 expression in pediatric glioblastomas. J Neuropathol Exp Neurol. 1997;56:7829. [PubMed] [Google Scholar]
- 19.Hayes VM, Dirven CM, Dam A, Verlind E, Molenaar WM, Mooij JJ, et al. High frequency of TP53 mutations in juvenile pilocytic astrocytomas indicates role of TP53 in the development of these tumors. Brain Pathol. 1999;9:463–7. doi: 10.1111/j.1750-3639.1999.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri V, Das P P, Jain A, Sharma MC, Borkar SA, et al. Pediatric glioblastomas: A histopathological and molecular genetic study. Neuro Oncol. 2009;11:274–80. doi: 10.1215/15228517-2008-092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed R, McLendon RE, Herndon JE, Friedman HS, Friedman AH, Bigner DD, et al. Alteration of the p53 gene in human gliomas. Cancer Res. 1994;54:1324–30. [PubMed] [Google Scholar]
- 22.Woodburn RT, Azzarelli B, Montebello JF, Goss IE. Intense p53 staining is a valuable prognostic indicator for poor prognosis in medulloblastoma/central nervous system primitive neuroectodermal tumors. J Neurooncol. 2001;52:57–62. doi: 10.1023/a:1010691330670. [DOI] [PubMed] [Google Scholar]
- 23.Jaros E, Lunec J, Perry RH, Kelly PJ, Pearson AD. P53 protein overexpression identifies a group of central primitive neuroectodermal tumours with poor prognosis. Br J Cancer. 1993;68:801–7. doi: 10.1038/bjc.1993.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertan Y, Sezak M, Demirağ B, Kantar M, Cetingül N, Turhan T, et al. Medulloblastoma: Clinicopathologic evaluation of 42 pediatric cases. Child Nerv Syst. 2009;25:353–6. doi: 10.1007/s00381-008-0784-4. [DOI] [PubMed] [Google Scholar]
- 25.Tihan T, Davis R, Elowitz E, DiCostanzo D, Moll U. Practical value of Ki-67 and p53 labeling indexes in stereotactic biopsies of diffuse and pilocytic astrocytomas. Arch Pathol Lab Med. 2000;124:108–13. doi: 10.5858/2000-124-0108-PVOKAP. [DOI] [PubMed] [Google Scholar]